Abstract
The extremophilic microbes of the Berkeley Pit Lake are a valuable source of new and interesting secondary metabolites. It is of particular interest that these acidophilic microbes produce small molecule inhibitors of pathways associated with low pH and high Eh. These same small molecules also inhibit molecular pathways induced by reactive oxygen species (ROS) and inflammation in mammalian cells. Low pH is a hallmark of inflammation and high Eh is one of ROS, so the suitability of this collection as a source of bioactive metabolites is actually quite biorational. Compound isolation was guided by inhibition of caspase-1 and matrix metalloproteinase-3, and active compounds were sent to the National Cancer Institute-Developmental Therapeutics Program and Memorial Sloan Kettering Cancer center for evaluation as either antiproliferative or cytotoxic agents.
Keywords: Extremophilic, Acidophilic, Caspase-1, Matrix metalloproteinase-3, Inflammasome, Small molecule inhibitors
The earth is rich in hostile and inhospitable environments. These include natural systems like deep-sea vents and thermal pools, and anthropogenic systems that result from extractive hardrock, oil or coal mining. Nobel laureate Paul Crutzen suggested that this is the “anthropocene era”, an era in which humans and human activities have become a major geological force on the planet [1]. Whether man-made or natural, extreme environments can harbor unique life forms called extremophiles. Bacteriologist Thomas Brock demonstrated the importance of extremophiles in the 1960s when he isolated bacteria from a 70°C thermal pool in Yellowstone National Park [2]. Brock’s Thermus aquaticus owes its survival to its unusually thermostable enzymes, including DNA polymerase. DNA polymerases catalyze the synthesis of DNA in a template-dependent process and are necessary to propagate, maintain, and manipulate the genetic code of living organisms. Although usually not heat stable, the T. aquaticus DNA polymerase variant (Taq) remains operational at high temperatures. It is now a key ingredient in the polymerase chain reaction (PCR). The use of thermostable Taq eliminates the need for adding new enzyme to the PCR reaction during the thermocycling process. In the forty years following Brock’s discovery, scientists have explored ecological niches as varied as deep sea vents and Antarctic ice sheets and have found unusual life forms in unlikely places. Many of these organisms have yielded new enzymes or small molecules that have not been found in other, more hospitable environments [3-11].
For the natural products chemist, unusual microbes represent novel chemistry with potential medicinal, industrial and agrochemical applications. Although the rapidly developing tools of molecular biology have focused attention on population genomics and proteomics, the direct study of secondary metabolites is still an important means to the discovery of new chemotherapeutic agents. Berkeley Pit Lake (BP Lake) is our extreme environment and our studies have been confined to the microbial life in the Pit for the simple reason that microbes are the predominant, and, perhaps, sole inhabitants of this toxic lake [12]. These microbes include bacteria, fungi, and both heterotrophic and autotrophic protists (green algae) [12]. Unlike most rivers and lakes in Montana, BP Lake does not harbor trout, grayling or other blue ribbon fish species. Aside from a single water bug photographed resting on the surface of the Pit Lake, and a flock of snow geese that landed on the water and subsequently died, no evidence of macrobial life exists.
The BP Lake system in Butte, Montana is part of the largest EPA Superfund site in North America. This abandoned open-pit copper mine was gouged out of the Boulder Batholith in 1955 and gradually developed into a 1.6 kilometer wide, 400 meter deep pit that sits in the shadow of the Continental Divide – and at the headwaters of the Clark Fork and Columbia River ecosystems. The Pit was dewatered by constant pumping, but when the pumps were decommissioned in 1982, it began its inexorable evolution into an acid mine waste lake. Thirty years later there are over 150 billion L of water in the Pit, with an inflow rate of 15 million L/day. Iron pyrite plays a dominant role in the geology of the area, and ultimately determines the nature of BP Lake. Even if all of the influent waters were pristine, the pyrite walls of the Pit continually react with air and water to generate sulfuric acid [13, 14], which helps to dissolve the minerals in the surrounding rocky overburden. As oxygen concentration decreases with depth, pyrite oxidation and resulting acid generation should also decrease. However, oxidation of pyrite by dissolved ferric iron can take place at a rapid rate in acidic waters, even in the absence of oxygen. The rate of ferrous iron oxidation by O2 is known to increase by many orders of magnitude in the presence of certain acidophilic bacteria, chiefly Acidothiobacillus ferrooxidans [14]. Because of this dynamic interplay, the water is acidic (pH 2.5 - 2.7) and contaminated with high concentrations of metal sulfates, including iron, copper, aluminum, cadmium and zinc [13, 14].
With its low pH and high metal content, BP Lake was considered too toxic to support life. In 1995, however, three filamentous fungi isolated from a surface water sample provided by the Montana Bureau of Mines and Geology launched our investigation of acid mine waste microbes and their secondary metabolites [15, 16]. Although conditions within the Pit Lake System were toxic for “normal” aquatic biota, these same conditions provided an ideal environment for extremophiles. Extremophiles can be more than just an interesting scientific oddity. They can provide a new untapped reservoir of bioactive secondary metabolites waiting to be discovered. Our ongoing research efforts have generated a collection of culturable acidophilic microbes from the surface waters to the bottom of the Pit Lake, and from the basal sediments. A subset of these organisms has been identified by Microbial ID, Inc. to the genus and species level, although several microbes could not be matched in any sequence databases.
Thirty-six of the seventy organisms have been grown in small pilot cultures (100 mL) and six have been studied thoroughly. Microbes were grown using 12-14 different physicochemical conditions. At time of harvest, these cultures were extracted with CHCl3. The remaining aqueous fraction was lyophilized, then sequentially extracted with CHCl3-MeOH (1:1) and MeOH. Each of the organic extracts and the aqueous residue were then evaluated in a series of bioassays. In the earliest phase of this study, extracts were tested for antimicrobial activity in a standard disk assay and in the brine shrimp lethality assay to determine cytotoxicity.
Ten years ago we introduced signal transduction enzyme inhibition to the study, and our microbial extracts are currently tested as inhibitors of caspase-1, matrix metalloproteinase-3 (MMP-3) and, most recently, caspase-3. Organisms are prioritized for further investigation: those that yield extracts with the most potent bioactivity are studied first. They are grown in larger volume cultures using the same physicochemical conditions that yielded the most bioactive extracts. Enzyme inhibition assays guide compound purification and the structures of pure compounds are determined using spectroscopic methodologies. Active enzyme inhibitors are submitted for further study to the National Cancer Institute-Developmental Therapeutics Program for testing against 60 human cancer cell lines (NCI-DTP 60) and to Memorial Sloan Kettering Cancer Center (MSKCC) for evaluation as antiproliferative agents against selected and established cancer cell lines. Secondary metabolites from six of the extremophilic fungi have been reported to date and these are the focus of this review. The remainder of the microbe collection is the subject of ongoing investigation.
Extremophilic fungal secondary metabolites isolated by bioassay-guided fractionation
All of the compounds reported from the Berkeley Pit to date have been isolated from six fungi out of the entire collection. Many of the other microbes have been grown in small pilot cultures under various physicochemical conditions [16], and the resulting mature cultures have been thoroughly extracted, evaluated for biological activity, and await further investigation. All of the studies were driven by the search for bioactive metabolites and specific assays guided these efforts. We were initially looking for antimicrobial agents, but were surprised to find only weak antibacterial or antifungal activity. The microbial population density in BP Lake is low and organisms may not require such agents for their survival.
In 2003 we focused our search on small molecule inhibitors of the specific signal transduction enzymes matrix metalloproteinase-3 (MMP-3) and caspase-1. MMP-3 activity is normally associated with epithelial mesenchymal transition (EMT), which is a requisite component of wound healing and angiogenesis. When up-regulated however, MMP-3 promotes tumor cell invasion through the loss of cell-cell adhesion both through the cleavage of E-cadherin (a calcium-dependent cell-cell adhesion molecule) and the repression of E-cad synthesis [17]. These are characteristic features of cells undergoing proliferation. The tumor microenvironment itself can be a potent carcinogen, not only by facilitating cancer progression and activating dormant cancer cells, but also by stimulating tumor formation through induction of enzymes like MMP-3 [17].
Caspase-1 plays an important role in chronic inflammation through the production of specific cytokines. Caspase-1 is activated upon binding to the inflammasome, a multiprotein complex that plays a key role in innate immunity. Once caspase-1 is activated, it cleaves (activates) the precursors of interleukin-1β (IL-1β), IL-18, and IL-33 [18]. Up-regulation of caspase-1 and concomitant chronic inflammation has been associated with a number of different pathologies. High levels of caspase-1 and/or IL-1β have been found in certain cancers: acute myelogenous leukemia [19], melanoma [20,21], certain glioblastomas [22], pancreatic cancers [23a-b] and certain breast cancers [24], all of which are exacerbated by chronic inflammation associated with activation of the inflammasome.
The production of pro-IL-1β and IL-18 are regulated by nuclear factor-kappa B (NF-κB). NF-κB can be activated by exposure of the cell to tumor necrosis factor (TNF), and pathogen-associated or danger-associated molecular patterns (PAMPS or DAMPS), including bacterial lipopolysaccharides (LPS). These same factors also stimulate the assemblage of the inflammasome [18]. Pro-inflammatory cytokines can lead to chronic inflammation and the production of reactive oxygen species that can induce oxidative damage to DNA, and consequently lead to the initiation and progression of carcinogenesis.
Of the six microbes thoroughly studied to date, five have yielded all of the specific enzyme inhibitors that we have reported. Each of these compounds has demonstrated activity against specific cancer cell lines. Potency against cancer cell lines shows a positive correlation to the degree of enzyme inhibitory activity. Some of these compounds will be described below.
Penicillium sp.: berkelic acid and berkebisabolanes A-C
Penicillium rubrum: berkeleydione, berkeleytrione, berkeleyones A-C, preaustinoid A, berkazaphilones A-C, berkeleyamides A-D, berkeleyacetals A-C, and the vermistatin analogs.
Penicillium solitum: berkedrimanes A and B
Penicillium clavigerum: phomopsolides A-F
Chaetomium funicola: berkchaetoazaphilones A-C and berk-chaetorubramine
Pithomyces sp.: prenylated tyrosines
1. Chlorella mutabilis-associated Penicillium sp.
Berkelic acid from a Chlorella mutabilis associated Penicillium
The CHCl3 extract of a Chlorella mutabilis (green-alga)-associated Penicillium sp. inhibited both MMP-3 and caspase-1 in the assay systems. These activities guided isolation of the active metabolites of the fungus and yielded berkelic acid, an unusual oxo-spirane (1), as well as the γ-pyrone spiciferone A (2) and spicifernin (3) [25]. Berkelic acid (1) was active in the NCI-DTP60 against OVCAR-3.
| Berkelic acid (1): | IC50 | MMP-3: 1.87 μM | Caspase-1: 98.0 μM |
| GI50 | NCI-DTP: OVCAR-3: 91 nM | ||
Novel bisabolane MMP-3 inhibitors from a Chlorella mutabilis associated Penicillium sp.
Penicillium sp. also produced a series of sesquiterpenes when grown in the same broth at pH 2.7 [26]. The CHCl3 extract of this organism was active in both the MMP-3 and caspase-1 tests and ultimately yielded berkebisabolanes 4, 5, and 6, and coumarin analogue 7.
Bisabolane sesquiterpenes are unusual microbial metabolites, and have typically been isolated from terrestrial plants, a basidiomycete [27], sponges [28, 29], octocoral [30, 31], and a red alga [32]. There have only been two other reports of bisabolanes from fungi [33, 34]. All four compounds showed moderate inhibitory activity against both MMP-3 and caspase-1.
| Berkebisabolane A (4) | IC50 | MMP-3: 280 nM | Caspase-1: 23 μM |
| Berkebisabolane B (5) | IC50 | MMP-3: 315 nM | Caspase-1: 23 μM |
| Berkebisabolane C (6) | IC50 | MMP-3: 340 nM | Caspase-1: 23 μM |
2. Penicillium rubrum
Berkeleydione and berkeleytrione
The CHCl3 extract of a Penicillium rubrum isolate collected from a depth of 270 m. and grown in acidified potato dextrose broth (PDBH+, pH 2.7) inhibited both MMP-3 and caspase-1.
Assay-guided fractionation yielded two novel meroterpenes, berkeleydione (8) and berkeleytrione (9) [35]. Berkeleydione (8) was tested in the NCI-DTP 60. It showed selective activity towards non-small cell lung cancer NCI-H460. This extreme selectivity is noteworthy in a natural product that has not been modified towards a particular cancer type [35].
| Berkeleydione (8) | IC50 | MMP-3: 22 μM | Caspase-1: 100 μM |
| GI50 | NCI-H460: log10 GI50 −6.40. | ||
| Berkeleytrione (9) | IC50 | MMP-3: 180 μM | Caspase-1: 20 μM |
Berkeleyones
Several additional meroterpenes were isolated from P. rubrum [36]. These included preaustinoid A (10) and A1 (11) and the new meroterpenes, berkeleyones A-C, (12-14), all of which inhibited caspase-1 activity. Of the numerous meroterpenoids reported to date compounds 10-14 appear most closely related biosynthetically to andrastin A (15) and citreohybridone D (16) [37]. This was an excellent opportunity to compare the activity of this series of related compounds to determine how subtle differences in structure could affect biological activity.
The growing awareness of the importance of the inflammasome as a key component in the development of inflammation-associated pathologies provided the next logical step in the investigation of these compounds. Compounds 8 and 10-14 were re-evaluated for their ability to inhibit caspase-1 in vitro. Caspase-1 inhibition was determined in a fluorometric assay and percent enzyme inhibition for each compound was determined at a concentration of 100 μg/mL. Each compound was evaluated for its ability to mitigate the production of IL-1β in THP-1 cells. LPS and multi-walled carbon nanotubes-induced THP-1 cells were exposed to compounds 8 and 10-14 and the resulting concentrations of IL-1β were determined to establish IC50 values for each compound (Table 1) [36].
Table 1.
Inhibition of caspase-1 and Il-1β production in induced THP-1 cells. Induced THP-1 cells produce inflammasomes, which in turn produce the pro-inflammatory cytokines IL-1β and IL-18.
| Compound | IL-1β production (IC50, μM) |
caspase-1a% inhibitiona |
|---|---|---|
| Berkeleydione (8) | 4.4 | 89 |
| Preaustinoid A (10) | 15.5 | 97 |
| Preaustinoid A1(11) | 34.3 | 77 |
| Berkeleyone A (12) | 2.7 | 68 |
| Berkeleyone B (13) | 3.7 | 100 |
| Berkeleyone C (14) | 37.8 | 0 |
test concentration 100 μg,mL
Berkeleyacetals
The CHCl3 extract of the deep water fungus P. rubrum yielded another family of meroterpenoids, the berkeleyacetals, when grown in PDBH+ broth [38]. The most potent enzyme inhibitor, berkeleyacetal C (19), inhibited both MMP-3 and caspase-1 in the micromolar range, while berkeleyacetals A (17) and B (18) inhibited these enzymes in the mM range.
In the NCI-DTP 60, berkeleyacetal C (19) inhibited the growth of non-small cell lung cancer NCI H460, the same cell line targeted by berkeleydione, as well as all leukemia cell lines. There were only two reports in the literature of the six-seven A-B ring system found in berkeleydione and the berkeleyacetals: paraherquonin (20) [39] and citreonigrin A, which was reported in a conference abstract [40]. Paraherquonin also possesses a similar acetal moiety, as do the citreonigrins [41].
Berkeleyamides
Penicillium rubrum yielded yet another family of compounds, berkeleyamides A-D (21-24), when grown in acidified PDB broth (pH 2.7) [42].
All four compounds were active against both caspase-1 and MMP-3 in the low micromolar range. Berkeleyamide A (21) and D (24) were evaluated in the NCI-DTP 60.
| Berkeleyamide A (21): | non-small cell lung cancer NCI-H522: |
| log10 GI100: 10 μM; | |
| renal carcinoma UO-31: log10GI50: 10 μM | |
| Berkeleyamide D (24): | renal carcinoma UO-31: log10 GI50: 10 μM |
Berkazaphilones
P. rubrum also produced berkazaphilones A-C (25-27), vermistatin (28), dihydrovermistatin (29), and penisimplicissin (30) [43]. Of the azaphilone analogs reviewed, only one other compound, pseudohalonectrin, was not oxygenated at C-7 [44]. All of the compounds were evaluated as caspase-1 inhibitors (100 μg/mL and 10 μg/mL) and the most active compounds were evaluated in the NCI-DTP60. Activity was selective with cytotoxicity only towards specific leukemia cell lines.
Vermistatin (28) and dihydrovermistatin (29) were not inhibitory at test concentrations. Compounds 25-30 also inhibited the production of IL-1β in THP-1 cells at a concentration of 200 μg/mL. However, berkazaphilone B (26) was completely inhibitory at 2.0 μg/mL, and berkazaphilone C (27) at 20 μg/mL [43]. These data will be shown in a later section. Microarray experiments available in the NCI Molecular Target database demonstrated that caspase-1 was up-regulated almost exclusively in different leukemia cell lines [45-48].
| Berkazaphilone A (25): | IC100 | Caspase-1: 100 μg/mL |
| Berkazaphilone B (26): | IC100 | Caspase-1: 10 μg/mL |
| leukemia RPMI-8226: log10 GI50 (−5.67) | ||
| Berkazaphilone C (27): | IC100 | Caspase-1: 10 μg/mL |
| leukemia SR: log10 GI50 (−6.42) | ||
| Penisimplicissimum (30): | IC100 | Caspase-1: 100 μg/mL |
| leukemia CCRFCEM: log10 GI50 (−6.70) | ||
| leukemia HL-60: log10 GI50 (−5.83) | ||
3. Penicillium solitum
Drimane derivatives from a biofilm-associated Penicillium solitum
Penicillium solitum was isolated from the secreted slime of an unusual acidophilic yeast found at a depth of 15 m. in the Pit Lake. The yeast slime itself is a rich mixture of lipopolysaccharides which harbored a complex population of filamentous fungi. Each of these was isolated from the slime and established in pure culture. The CHCl3 extract of P. solitum grown in mycological broth demonstrated strong inhibitory activity against both MMP-3 and caspase-1. Flash silica gel chromatography, followed by HPLC gave the new drimane derivatives 31 and 32 [49].
Berkedrimane A (31) and B (32) were evaluated as caspase-1 inhibitors. They exhibited IC50 values of 60 μg/mL and 50 μg/mL, respectively, against caspase-1. The two compounds were evaluated for their ability to inhibit the production of IL-1β in THP-1 cells. Induced THP-1 cells were exposed to 31 and 32 at concentrations of 50.0, 5.0, and 0.5 μg/mL and the concentrations of IL-1β post-exposure were determined as shown in Figure 11.
Figure 11.
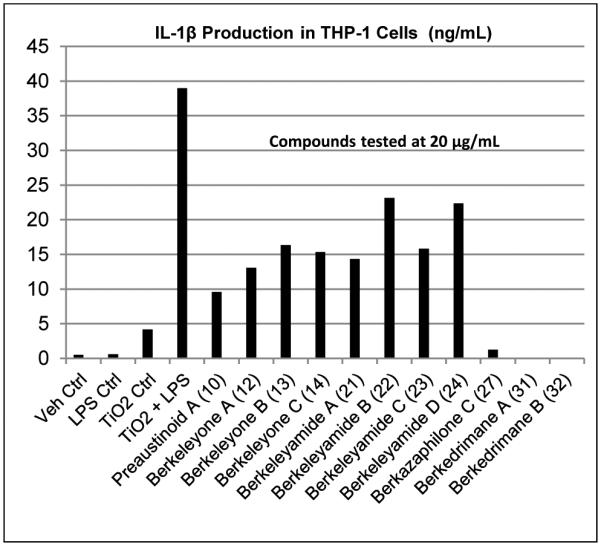
Induced inflammasome assay in THP-1 cells.
4. Penicillium clavigerum
Phomopsolides
Penicillium clavigerum was also isolated from the green-alga Chlorella mutabilis. When grown in PDB broth, the CHCl3 extract of this fungus inhibited MMP-3 and caspase-1. After a series of MMP-3 inhibitors were isolated and purified, 1H NMR spectra of inactive column fractions were examined to identify structurally related analogues and to guide isolation of these metabolites [50].
Phomopsolides A-C (33-35), E, F (36 and 37), phompyrone (38), berkbenzofuran thioester (39), phomfuranone (40), patulin (41), and dimethylphthalides 42 and 43 were all isolated from the fungal CHCl3 extract [50]. Phomopsolide A (33) was the most active MMP-3 inhibitor with an IC50 of 100 μM. The other phomopsolides were weak inhibitors with IC50>150 μM. Phompyrone (38) was also a weak inhibitor with IC50>100 μM.
MSKCC evaluated the phomopsolides as antiproliferative agents against selected and established cancer cell lines. Human cell lines included cervical adenocarcinoma HeLa S3; bladder transitional cell carcinoma UM-UC-3; alveolar adenocarcinoma A549; lung adenocarcinoma H2030; non-small cell lung carcinoma H3255; and retinoblastoma Y79. Some of these data are shown in Table 2.
Table 2.
IC50 (μM) data for compounds tested at MSKCC against a series of established human cancer cell lines. NE indicates no effect; (−) - not tested.
| Assays: | Alamar Blue Viability | Nuclei Count Proliferation | ||||
|---|---|---|---|---|---|---|
| Compound | HeLaS3 | UMUC3 | Y79 | HeLaS3 | UMUC3 | H3255 |
| 33 | 6.3 | 6.5 | - | 5.0 | 4.8 | - |
| 34 | NE | NE | - | NE | 6.1 | - |
| 35 | 3.6 | 2.2 | 1.4 | 2.7 | 1.4 | 2.6 |
| 36 | NE | NE | - | 7.3 | 6.6 | - |
| 37 | NE | NE | - | NE | NE | - |
| 38 | NE | NE | - | NE | NE | - |
5. Chaetomium funicola
The CHCl3 extract of Chaetomium funicola inhibited both MMP-3 and caspase-1, and caspase-1 inhibition was used to guide isolation of the berkchaetoazaphilones A-C (44-46), berkchaetorubramine (47) and the known quinoline 48. Compound 45 was the strongest caspase-1 inhibitor [51].
Inhibition of cytokine production in the induced inflammasome assay
The cytokine mitigation potential of the various compounds isolated from Berkeley Pit microbes have been measured in repeated assays. Several compounds were evaluated in a comparative study to assess mitigation of the production of IL-1β by the inflammasome in induced THP-1 cells. In this assay, bacterial LPS and either multi-walled carbon nanotubes (MWCNT) or titanium dioxide (TiO2) are used to stimulate the production of inflammasomes and proinflammatory cytokines in THP-1 cells, macrophage-like human monocytic leukemia cells [52]. The assembling inflammasome activates pro-caspase-1, which in turn activates (cleaves) pro-IL-1β and other proinflammatory cytokines [18]. Test compounds are administered to these induced THP-1 cells and the production of cytokines post-administration is determined [43, 49].
Inhaled MWCNT have been associated with both in vitro and in vivo toxicity, including increased production of inflammatory mediators, increased inflammation and often severe lung pathology. These effects have been attributed to MWCNT length, diameter, aggregation state, contaminants, aspect ratio/rigidity, and release of reactive oxygen species [52]. The induced-inflammasome assay is a useful tool in assessing the direct inhibition of caspase-1 as a means of moderating inflammation in a cellular system and also provides lead compounds that could mitigate the direct toxicity of MWCNT [43, 49].
Berkedrimane A (31) and B (32), and berkazaphilone C (27) are the most potent inhibitors tested to date. They completely inhibited cytokine production at the nm level. Several other compounds had IC50 levels in the low μM concentrations (Figure 11).
6. Pithomyces sp.
5-HT2A receptor ligand from Pithomyces sp
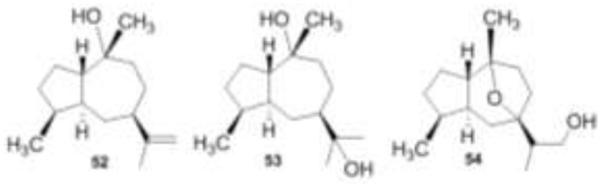
One of the first fungi isolated from the Pit Lake, Pithomyces sp., exhibited good brine shrimp lethality when grown in PDBH+ broth (pH 2.7, 11 days shaken) [15]. Brine shrimp lethality is an indicator of cytotoxicity and has been used to find compounds with anticancer activity [53]. When the fungal culture was harvested and extracted, most of the activity was confined to the CHCl3 extract. Sephadex LH-20 chromatography of this extract followed by RPHPLC gave the three cytotoxic compounds 49-51 and the three inactive sesquiterpenes, 52-54. The structures of all six compounds were determined through the use of mass spectrometry, and 1D and 2D NMR spectroscopic techniques. The brine shrimp lethality assay was used to guide the isolation of compounds 49-51.
Sesquiterpenes 52–54 were isolated by “NMR-guided fractionation”. We often isolate and characterize compounds with interesting NMR spectra even if they are inactive in a particular assay [15].
Sesquiterpenes are more commonly found in higher plants and the three sesquiterpenes produced by this Pithomyces strain are more closely related to plant metabolites. The NMR, IR, and mass spectral data of compound 52 were identical to those of pogostol, which was reported from the oil of the patchouli plant, Pogostemon cablin [54]. The structure of sesquiterpene 53 was also established by extensive spectroscopic analysis. It is structurally similar to 11-hydroxypogostol, which has been reported from the aerial parts of Leuceria floribunda, but it has a trans ring juncture consistent with pogostol [55]. Compound 54 also has a trans ring juncture and was unique to this fungus.
Simple aromatic amino acid derivatives often exhibit neurotransmitter activity. Compounds 49-51 were evaluated using the 5-HT2A receptor assay. Studies suggest that 5-HT2A (serotonin) receptor antagonists might act as migraine preventatives [56], or as antihypertensive agents [57]. In this assay, we assessed the ability of a compound to displace radiolabeled ketanserin from rat 5-HT2A receptor’s ligand binding site [15]. Since 100% of the ketanserin is bound in the control setting, smaller numbers indicated stronger displacement of ketanserin. Compounds were tested at 100 μM concentrations [58, 59]. Compounds 49 and 51 resulted in ketanserin binding of 85%, while compound 50 lowered the ketanserin binding to 11%. Data from this assay suggested that 50 acts as a 5-HT2A receptor ligand, while the other compounds are only marginally active. Drugs that act as 5-HT2A ligands include ketanserin, methysergide, the tricyclic antidepressant amitryptiline and certain calcium channel and beta blockers [56].
CONCLUSIONS
Although only a fraction of this collection has been studied, the results have already demonstrated that the extremophilic microbes of the Berkeley Pit Lake are a valuable source of new and interesting secondary metabolites. It is of particular interest that these acidophilic microbes produce small molecule inhibitors of molecular pathways associated with low pH and high Eh. These same small molecules also inhibit molecular pathways induced by reactive oxygen species (ROS) and inflammation in mammalian cells. Low pH is a hallmark of inflammation and high Eh is a hallmark of ROS, so the suitability of this collection as a source of bioactive metabolites is actually quite biorational.
It is not often that scientists have the opportunity to explore such a unique environment. The Stierle lab is fortunate to have easy access to this dynamic ecosystem. Based on preliminary data, “bioprospecting” in the Berkeley Pit will continue to yield interesting new chemistry. Our methodology has evolved as the research has progressed. New collaborations have provided a means for more in depth investigation of pathways associated with inflammation. The use of caspase-1 and MMP-3 inhibition to guide isolation and to provide a rapid in vitro indicator of anti-inflammatory activity has been validated by the intact inflammasome assay. The compounds produced in this research are already the focus of multiple total synthetic efforts.
It remains for the Stierle lab to isolate a library of compounds from the remainder of these organisms. Only 8% of the culturable microbes isolated from the Berkeley Pit have been fully studied, and even these organisms still have untapped potential. Each fungus has been grown in 14 different physicochemical conditions and each of these methods has yielded bioactive extracts. Most of the compounds, however, have been from a small subset of these conditions.
We continue to be excited by the compounds produced by the hearty and productive denizens of the Berkeley Pit, a once prosperous open pit copper mine that has devolved into a vast acid mine waste lake. These culturable fungi remain a largely untapped gold mine of novel compounds, promising targets for total synthesis and chemical scaffolds for structure/activity studies.
Despite the wealth of copper once pulled from the Berkeley Pit, it is possible that the richest “ore” ever mined from the “Richest Hill on Earth” could be the microbes and secondary metabolites discovered through bioprospecting in the Pit.
Figure 1.
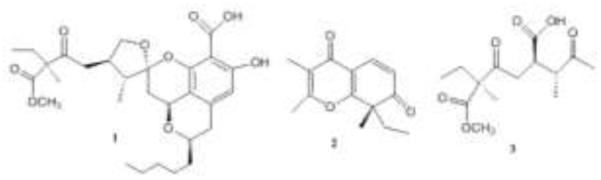
Structures of compounds 1-3.
Figure 2.
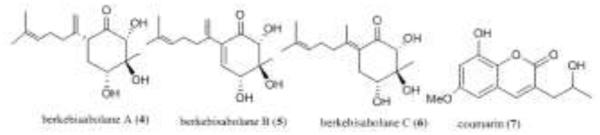
Structures of berkebisabolanes and coumarin analogue 7.
Figure 3.
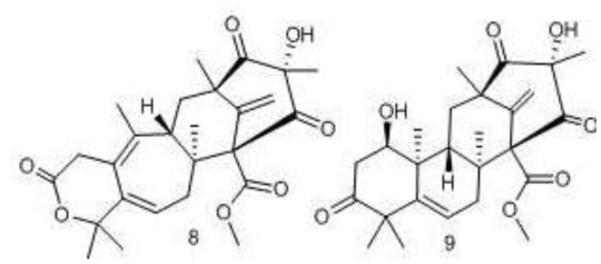
Structures of compounds 8 and 9.
Figure 4.
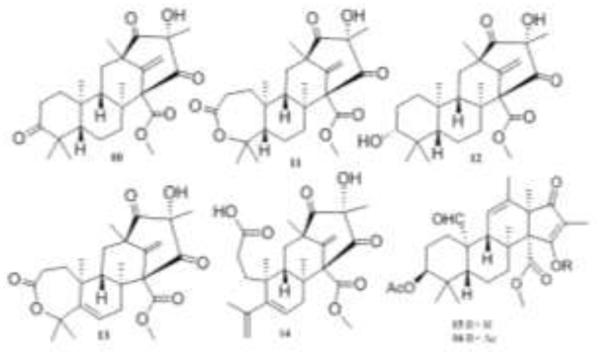
Structures of compounds 10-16.
Figure 5.
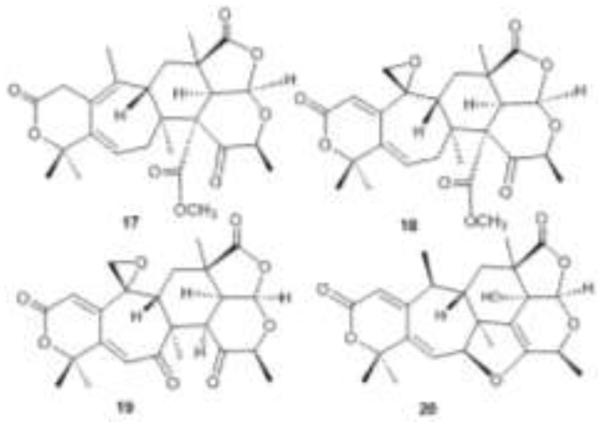
Structures of berkeleyacetals A-C and paraherquonin, 17-20.
Figure 6.
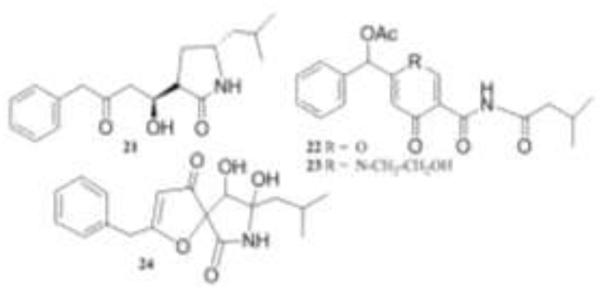
Structures of berkeleyamides 21-24.
Figure 7.
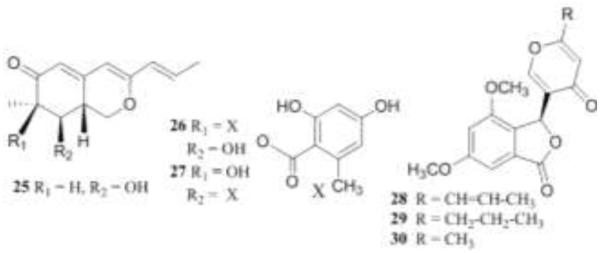
Structures of compounds 25-30.
Figure 8.
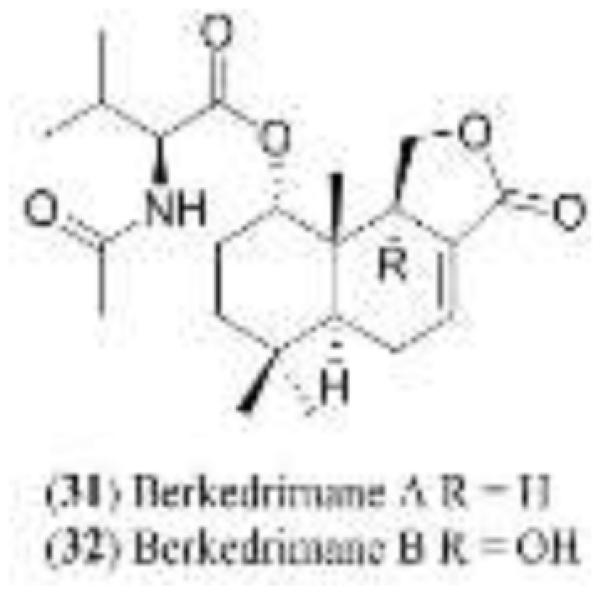
Structures of berkedrimanes 31-32.
Figure 9.
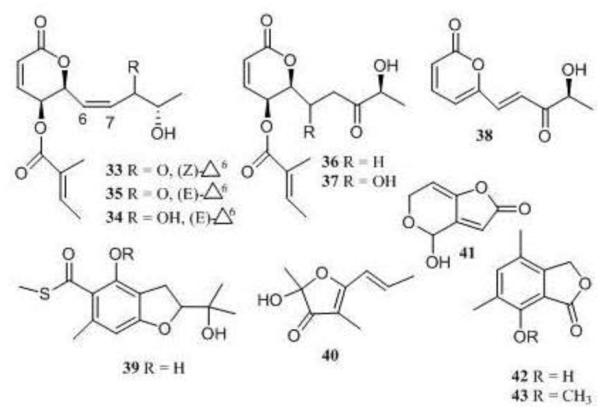
Structures of compounds 33-43.
Figure 10.
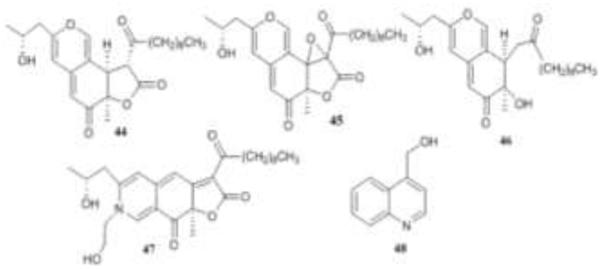
Structures of compounds 44-48.
Figure 12.
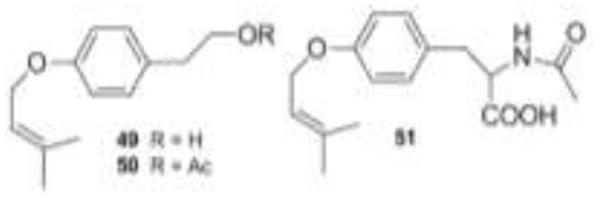
Structures of prenylated tyrosines 49-51.
Figure 13.
Structures of sesquiterpenes 52-54.
Acknowledgments
We thank NIH-NINDS grant #3P30NS055022 and NSF grant #CHE-9977213 for acquisition of the Bruker and Varian NMR spectrometers and the MJ Murdock Charitable Trust #99009:JVZ:11/18/99 for acquisition of the mass spectrometer. The project was supported by NIH grants R01CA139159, P20RR16455-04, P20RR017670, 5P30NS055022, and P20GM103546. The HTS Core Facility is partially supported by Mr William H. Goodwin and Mrs Alice Goodwin and the Commonwealth Foundation for Cancer Research, the Experimental Therapeutics Center of MSKCC, the William Randolph Hearst Fund in Experimental Therapeutics, the Lillian S Wells Foundation and by an NIH/NCI Cancer Center Support Grant 5 P30 CA008748-44.
References
- [1].Crutzen PJ, Stoermer EF. The anthropocene. Global Change Newsletter. 2000;41:17–18. [Google Scholar]
- [2].Brock T, Freeze H. Thermus aquaticus gen. n. and sp. n., a nonsporulating extreme thermophile. Journal of Bacteriology. 1969;98:289–297. doi: 10.1128/jb.98.1.289-297.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ward DM. Microbiology in Yellowstone National Park. American Society for Microbiology News. 1998;64:141–146. [PubMed] [Google Scholar]
- [4].Stetter KO. Extremophiles and their adaptation to hot environments. FEBS Letters. 1999;452:22–25. doi: 10.1016/s0014-5793(99)00663-8. [DOI] [PubMed] [Google Scholar]
- [5].Huber H, Hohn MJ, Rachel R, Fuchs T, Wimmer VC, Stetter KO. A new phylum of Archaea represented by a nanosized hyperthermophilic symbiont. Nature. 2002;417:63–67. doi: 10.1038/417063a. [DOI] [PubMed] [Google Scholar]
- [6].Bult CJ, White O, Olsen GJ, Zhou L, Fleischmann RD, Sutton GG, Blake JA, FitzGerald LM, Clayton RA, Gocayne JD, Kerlavage AR, Dougherty BA, Tomb JF, Adams MD, Reich CI, Overbeek R, Kirkness EF, Weinstock KG, Merrick JM, Glodek A, Scott JL, Geoghagen NS, Venter JC. Complete genome sequence of the methanogenic archaeon, Methanococcus jannaschii. Science. 1996;273:1058–1060. doi: 10.1126/science.273.5278.1058. [DOI] [PubMed] [Google Scholar]
- [7].Tsujii S, Rinehart KL, Gunasekera SP, Kashman Y, Cross SS, Lui MS, Pomponi SA, Diaz MC. Topsentin, bromotopsentin, and dihydrodeoxybromotopsentin: antiviral and antitumor bis(indoly1)imidazoles from Caribbean deep-sea sponges of the family Halichondriidae. Structural and synthetic studies. Journal of Organic Chemistry. 1988;53:5446–5453. [Google Scholar]
- [8].Kohmoto J, Kashman Y, McConnell O, Rinehart KL, Wright A, Koehn F. Dragmacidin, a new cytotoxic bis(indole) alkaloid from a deep water marine sponge, Dragmacidon sp. Journal of Organic Chemistry. 1988;53:3116–3118. [Google Scholar]
- [9].Gunasekera SP, McCarthy PJ, Longley RE, Pomponi SA, Wright AE, Lobkovsky E, Clardy J. Discorhabdin P, a new enzyme inhibitor from a deep-water Caribbean sponge of the genus Batzella. Journal of Natural Products. 1999;62:173–175. doi: 10.1021/np980293y. [DOI] [PubMed] [Google Scholar]
- [10].Gunasekera SP, McCarthy PJ, Longley RE, Pomponi SA, Wright AE. Secobatzellines a and b, two new enzyme inhibitors from a deep-water Caribbean sponge of the genus Batzella. Journal of Natural Products. 1999;62:1208–1211. doi: 10.1021/np990177a. [DOI] [PubMed] [Google Scholar]
- [11].Shin J, Seo Y, Lee HS, Rho JR, Mo SJ. A new cyclic peptide from a marine derived bacterium of the genus Nocardiopsis. Journal of Natural Products. 2003;66:883–884. doi: 10.1021/np030075r. [DOI] [PubMed] [Google Scholar]
- [12].Mitman GG. A Final Report: Biological Survey of the Berkeley Pit Lake System. US EPA-Mine Waste Technology Program: MWTP-132. 1999 http://www.epa.gov/nrmrl/std/mwt/a4/a4132.pdf.
- [13].Montana Bureau of Mines and Geology. http://www.mbmg.mtech.edu/env-berkeley.html. accessed 8/15/11.
- [14].Duaime TE. Long term changes in the limnology and geochemistry of the Berkeley Pit Lake, Butte, Montana. Mine Water and the Environment. 2006;25:76–85. [Google Scholar]
- [15].Stierle AA, Stierle DB, Parker K, Goldstein E, Bugni T, Baarson C, Gress J, Blake D. A novel 5-HT receptor ligand and related cytotoxic compounds from an acid mine waste extremophile. Journal of Natural Products. 2003;66:1097–1100. doi: 10.1021/np030044w. [DOI] [PubMed] [Google Scholar]
- [16].Stierle AA, Stierle DB. Bioprospecting in the Berkeley Pit: Bioactive metabolites from acid mine waste extremophiles. In: Atta-Ur-Rahman, editor. Bioactive Natural Products. Vol. 32. Elsevier Science Publishers; Amsterdam: 2005. pp. 1123–1176. [Google Scholar]
- [17].Sternlicht MD, Lochter A, Sympson CJ, Huey B, Rougier J-P, Gray JW, Pinkel D, Bissell MJ. The stromal proteinase MMP3/ stromelysin-1 promotes mammary carcinogenesis. Cell. 1999;98:137–146. doi: 10.1016/s0092-8674(00)81009-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Franchi L, Eigenbrod T, Muñoz-Planillo R, Nuñez G. The inflammasome: a caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nature Immunology. 2009;10:241–256. doi: 10.1038/ni.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Granot T, Milhas D, Carpentier S, Dagan A, Segui B, Gatt S, Levade T. Caspase-dependent and -independent cell death of Jurkat human leukemia cells induced by novel synthetic ceramide analogs. Leukemia. 2006;20:392–399. doi: 10.1038/sj.leu.2404084. [DOI] [PubMed] [Google Scholar]
- [20].Okamoto M, Liu W, Luo Y, Tanaka A, Cai X, Norris DA, Dinarello C, Fujita M. Constitutively active inflammasome in human melanoma cells mediating autoinflammation via caspase-1 processing and secretion of interleukin-1β. Journal of Biological Chemistry. 2010;285:6477–6488. doi: 10.1074/jbc.M109.064907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Voronov E, Shouval DS, Krelin Y, Cagnano E, Benharroch D, Iwakura Y, Dinarello CA, Apte RN. IL-1 is required for tumor invasiveness and angiogenesis. Proceedings of the National Academy of Sciences, USA. 2003;100:264–2650. doi: 10.1073/pnas.0437939100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Paugh BS, Bryan L, Paugh SW, Wilczynska KM, Alvarez SM, Singh SK, Kapitonov D, Rokita H, Wright S, Griswold-Prenner I, Milstien S, Spiegel S, Kordula T. Interleukin-1 regulates the expression of sphingosine kinase 1 in glioblastoma cells. Journal of Biological Chemistry. 2009;284:3408–3417. doi: 10.1074/jbc.M807170200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].(a) Schlosser S, Gansauge F, Ramadani M, Beger H-G, Gansauge S. Inhibition of caspase-1 induces cell death in pancreatic carcinoma cells and potentially modulates expression levels of bcl-2 family proteins. Federation of European Biochemical Societies Letters. 2001;491:104–108. doi: 10.1016/s0014-5793(01)02144-5. [DOI] [PubMed] [Google Scholar]; (b) Muerkoster SS, Lust J, Arlt A, Hasler R, Witt M, Sebens T, Schreiber S, Folsch UR, Schafer H. Acquired chemoresistance in pancreatic carcinoma cells: induced secretion of IL-1β and NO lead to inactivation of caspases. Oncogene. 2006;25:3973–398. doi: 10.1038/sj.onc.1209423. [DOI] [PubMed] [Google Scholar]
- [24].Jin L, Yuan RQ, Fuchs A, Yao Y, Joseph A, Schwall R, Schnitt SJ, Guida A, Hastings HM, Andres J, Turkel G, Polverini PJ, Goldberg ID, Rosen EM. Expression of interleukin-1β in human breast carcinoma. Cancer. 1997;80:421–434. doi: 10.1002/(sici)1097-0142(19970801)80:3<421::aid-cncr10>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- [25].Stierle AA, Stierle DB, Kelly K. Berkelic acid, a novel spiroketal with highly specific anti-tumor activity from an acid-mine waste fungal extremophile. Journal of Organic Chemistry. 2006;71:5357–5360. doi: 10.1021/jo060018d. [DOI] [PubMed] [Google Scholar]
- [26].Stierle AA, Stierle DB, Kemp K. Novel sesquiterpenoid matrix metalloproteinase-3 inhibitors from an acid mine waste extremophile. Journal of Natural Products. 2004;67:1392–1395. doi: 10.1021/np049975d. [DOI] [PubMed] [Google Scholar]
- [27].Stadler M, Anke H, Sterner JJ. New nematicidal and antimicrobial compounds from the basidiomycete Cheimonophyllum candidissimum (Berk & Curt.) sing. I. Producing organism, fermentation, isolation, and biological activities. The Journal of Antibiotics. 1994;47:1284–1289. doi: 10.7164/antibiotics.47.1284. [DOI] [PubMed] [Google Scholar]
- [28].Sullivan BW, Faulkner DJ, Okamoto KT, Chen MHM, Clardy J. (6R,7S)-7-Amino-7,8-dihydro-alpha-bisabolene, an antimicrobial metabolite from the marine sponge Halichondria sp. Journal of Organic Chemistry. 1986;51:5134–5136. [Google Scholar]
- [29].Harrison B, Crews P. The structure and probable biogenesis of helianane, a heterocyclic sesquiterpene, from the Indo-Pacific sponge Haliclona fascigera. Journal of Organic Chemistry. 1997;62:2646–2648. doi: 10.1021/jo962175q. [DOI] [PubMed] [Google Scholar]
- [30].McEnroe F, Fenical W. Structures and synthesis of some new antibacterial sesquiterpenoids from the gorgonian coral Pseudopterogorgia rigida. Tetrahedron. 1978;34:1661–1664. [Google Scholar]
- [31].D’Armas HT, Mootoo BS, Reynolds WF. An unusual sesquiterpene derivative from the Caribbean gorgonian Pseudopterogorgia rigida. Journal of Natural Products. 2000;63:1593–1595. doi: 10.1021/np000229s. [DOI] [PubMed] [Google Scholar]
- [32].Vazquez JT, Chang M, Nakanishi K, Martin JD, Martin VS, Perez R. Puertitols: novel sesquiterpenes from Laurencia obtusa. Structure elucidation and absolute configuration and conformation based on circular dichroism. Journal of Natural Products. 1988;51:1257–1260. doi: 10.1021/np50060a036. [DOI] [PubMed] [Google Scholar]
- [33].Sanson DR, Corley DG, Barnes CL, Searles S, Schlemper EO, Tempesta MS. New mycotoxins from Fusarium sambucinum. Journal of Organic Chemistry. 1989;54:4313–4318. [Google Scholar]
- [34].Jelen HH. Volatile sesquiterpene hydrocarbons characteristic for Penicillium roqueforti strains producing PR toxin. Journal of Agriculture and Food Chemistry. 2002;50:6569–6574. doi: 10.1021/jf020311o. [DOI] [PubMed] [Google Scholar]
- [35].Stierle DB, Stierle AA, Hobbs JD, Stokken J, Clardy J. Berkeleydione and berkeleytrione, new bioactive metabolites from an acid mine organism. Organic Letters. 2004;6:1049–1052. doi: 10.1021/ol049852k. [DOI] [PubMed] [Google Scholar]
- [36].Stierle DB, Stierle AA, Patacini B, McIntyre K, Girtsman T, Bolstad E. Berkeleyones and related meroterpenes from a deep water acid mine waste fungus that inhibits the production of interleukin 1-β from induced inflammasomes. Journal of Natural Products. 2011;74:2273–2277. doi: 10.1021/np2003066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].(a) Geris R, Simpson TJ. Meroterpenoids produced by fungi. Natural Product Reports. 2009;26:1063–1094. doi: 10.1039/b820413f. [DOI] [PubMed] [Google Scholar]; (b) Kosemura S. Structure and absolute configuration of citreohybridones isolated from Penicillium species. Tetrahedron Letters. 2002;43:1253–1256. [Google Scholar]; (c) Kosemura S, Yamamura S. Isolation and biosynthetic pathway for citreohybridones from the hybrid strain KO 0031 derived from Penicillium species. Tetrahedron Letters. 1997;38:6221–6224. [Google Scholar]
- [38].Stierle DB, Stierle AA, Patacini B. The berkeleyacetals, three meroterpenes from a deep water acid mine waste Penicillium. Journal of Natural Products. 2007;70:1820–1823. doi: 10.1021/np070329z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Okuyama E, Yamazaki M, Kobayashi K, Sakurai T. Paraherquonin, a new meroterpenoid from Penicillium paraherquei. Tetrahedron Letters. 1983;24:3113–3114. [Google Scholar]
- [40].Ebel R, Rusman Y, Brauers G, Proksch P, Frank W, Wray V. Novel oxygenated meroterpenoids and drimane sesquiterpenoids from the sponge-derived fungus Penicillium citreonigrum. Planta Medica; Abstracts of 54th Annual Congress on Medicinal Plant Research.2006. p. S-027. [Google Scholar]
- [41].Rusman Y. Ph.D. Thesis. University of Dusseldorf; Dusseldorf, Germany: 2006. Isolation of new secondary metabolites from sponge-associated and plant-derived endophytic fungi; p. 99. [Google Scholar]
- [42].Stierle AA, Stierle DB, Patacini B. The berkeleyamides, amides from the acid lake fungus Penicillium rubrum. Journal of Natural Products. 2008;71:856–860. doi: 10.1021/np0705054. [DOI] [PubMed] [Google Scholar]
- [43].Stierle AA, Stierle DB, Girtsman T. Caspase-1 inhibitors from an extremophilic fungus that target specific leukemia cell lines. Journal of Natural Products. 2012;75:344–350. doi: 10.1021/np200414c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Osmanova N, Schultze W, Ayoub N. Azaphilones: a class of fungal metabolites with diverse biological activities. Phytochemistry Reviews. 2010;9:315–342. [Google Scholar]
- [45]. http://dtp.nci.nih.gov/mtweb/targetinfo?moltid=GC19100&moltnbr=9698 accessed 5/12/11.
- [46]. http://dtp.nci.nih.gov/mtweb/targetinfo?moltid=GC29740&moltnbr=29739 accessed 5/12/11.
- [47]. http://dtp.nci.nih.gov/mtweb/targetinfo?moltid=GC29740&moltnbr=29739 accessed 5/12/11.
- [48]. http://dtp.nci.nih.gov/mtweb/targetinfo?moltid=GC226538&moltnbr=131271 accessed 5/12/11.
- [49].Stierle DB, Stierle AA, Girtsman T, McIntyre K, Nichols J. Caspase-1 and -3 inhibiting drimane sesquiterpenoids from the extremophilic fungus, Penicillium solitum. Journal of Natural Products. 2012;75:262–266. doi: 10.1021/np200528n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Stierle AA, Stierle DB, Mitman GG, Snyder S, Antczak C, Djaballah H. Phomopsolides and related compounds from the alga-associated fungus, Penicillium clavigerum. Natural Product Communications. 2014 In press. [PubMed] [Google Scholar]
- [51].Stierle AA, Stierle DB, Antczak C, Djaballah H. Berkchaetoazaphilones from the yeast-slime associated fungus Chaetomium funicola. Journal of Natural Products. 2014 manuscript submitted. [Google Scholar]
- [52].Hamilton RH, Wu Z, Mitra S, Shaw PK, Holian A. Effect of MWCNT size, carboxylation, and purification on in vitro and in vivo toxicity, inflammation and lung pathology. Particle and Fibre Toxicology. 2013;10:57–74. doi: 10.1186/1743-8977-10-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Meyer BN, Ferrigni NR, Putnam JE, Jacobsen LB, Nichols DE, McLaughlin JL. Brine shrimp: a convenient general bioassay for active plant constituents. Planta Medica. 1982;45:31–34. [PubMed] [Google Scholar]
- [54].Hikino H, Ito K, Takemoto T. Structure of pogostol. Chemical and Pharmaceutical Bulletin. 1968;16:1609–1610. [Google Scholar]
- [55].Bittner M, Silva M, Rozas Z, Papastergiou F, Jakupovic J. Sesquiterpenes and other constituents from Chilean Mutisieae. Phytochemistry. 1994;36:695–698. [Google Scholar]
- [56].Tfelt-Hansen P. Neurologic Clinics: Advances in Headache. Vol. 15. WB Saunders Co; Philadelphia: 1997. Prophylactic pharmacotherapy of migraine: some practical guidelines; pp. 153–165. [DOI] [PubMed] [Google Scholar]
- [57].Van Schie DL, de Jeu RM, Steyn DW, Odendaal HJ, van Geijn HP. The optimal dosage of ketanserin for patients with severe hypertension in pregnancy. European Journal of Obstetrics & Gynecology & Reproductive Biology. 2002;102:161–166. doi: 10.1016/s0301-2115(01)00611-x. [DOI] [PubMed] [Google Scholar]
- [58].Weber JT, O’Connor MF, Hayataka K, Colson N, Medora R, Russo EB, Parker KK. Activity of parthenolide at 5HT-2A receptors. Journal of Natural Products. 1997;60:651–653. doi: 10.1021/np960644d. [DOI] [PubMed] [Google Scholar]
- [59].Ortiz TC, Devereaux MC, Parker KK. Structural variants of a human 5-HT1a receptor intracellular loop 3 peptide. Pharmacology. 2000;60:195–202. doi: 10.1159/000028369. [DOI] [PubMed] [Google Scholar]


