Abstract
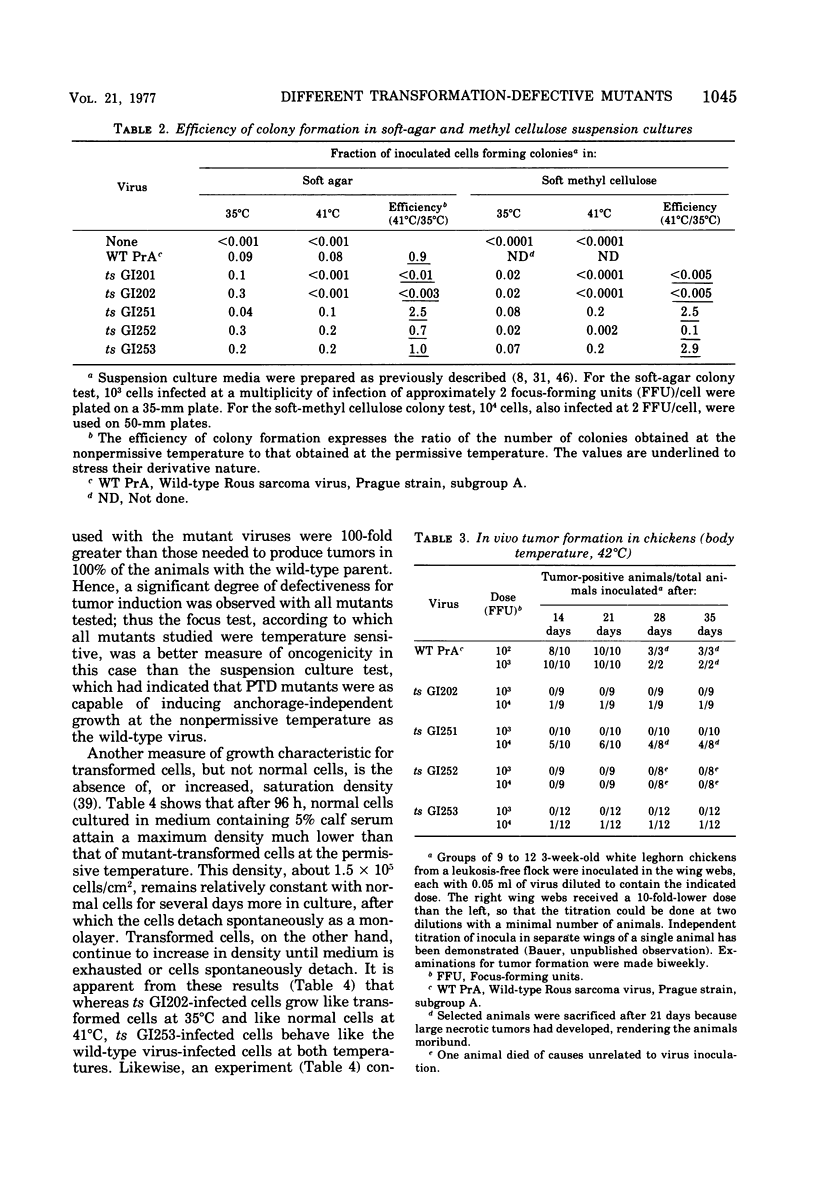
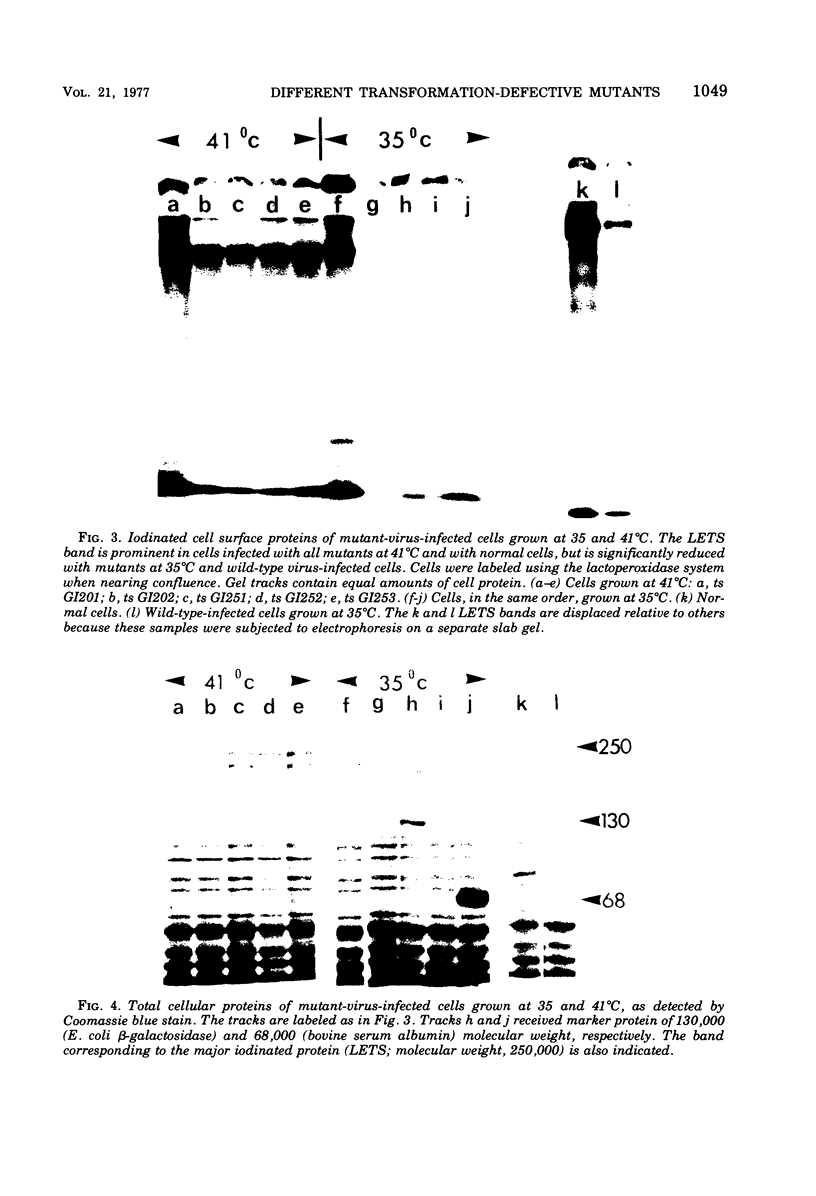
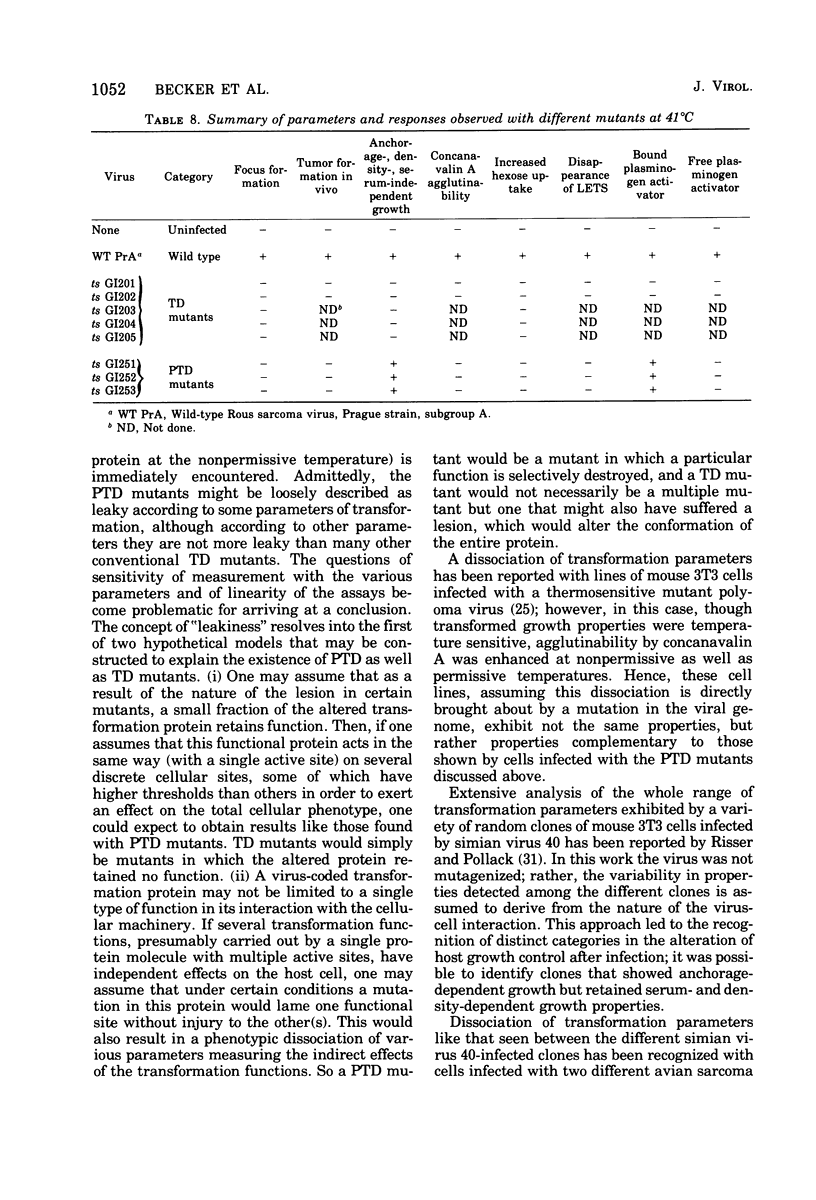
Eight transformation-defective, temperature-sensitive (ts) mutants of the Prague strain of Rous sarcoma virus, subgroup A, have been isolated after mutagenesis with 5-bromodeoxyuridine followed by selection on the basis of focus tests. Five of these mutants, ts GI201, GI202, GI203, GI204, and GI205, exhibit properties like most previously reported isolates in that they show a temperature-sensitive response to each of a variety of transformation-specific parameters tested. Interestingly, GI201, in addition to the temperature-sensitive defect, carries a lesion that was observed as a nonconditional loss of expression of plasminogen activator protease. Three mutants, ts GI251, GI252, and GI253 have been disignated partial transformation-defective (PTD) mutants since they behave as ts mutants according to some tests for transformation and as wild type according to others. These three mutants fail to form foci at the nonpermissive temperature (41 degrees C) and art nontumorigenic in 3-week-old chickens (body temperature, 42 degrees C). The agglutinability by concanavalin A of cells infected with these mutants shows a definite temperature sensitivity, as do the rate of 2-deoxyglucose uptake and the disappearance of the 250, 000-dalton normal cell glycoprotein (large, external, transformation sensitive [LETS]). Although the PTD mutant-infected cells, unlike cells infected with other transformation mutants, exhibit a cell-bound plasminogen activator protease at the nonpermissive temperature, this activator is not detectable as a free protease in the medium, as it is with wild-type, virus-infected cells. The PTD mutants behave like the wild-type parent in their ability to induce transformed growth properties in the infected cells, i.e., growth beyond normal cell saturation density with or without serum-supplemented medium and growth leading to colony formation in soft-agar- or methyl cellulose-containing suspension media.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bader J. P., Brown N. R. Induction of mutations in an RNA tumour virus by an analogue of a DNA precursor. Nat New Biol. 1971 Nov 3;234(44):11–12. doi: 10.1038/newbio234011a0. [DOI] [PubMed] [Google Scholar]
- Bader J. P., Ray D. A., Brown N. R. Accumulation of water during transformation of cells by an avian sarcoma virus. Cell. 1974 Nov;3(3):307–313. doi: 10.1016/0092-8674(74)90146-9. [DOI] [PubMed] [Google Scholar]
- Balduzzi P. C., Murphy H. Plaque assay of avian sarcoma viruses using casein. J Virol. 1975 Sep;16(3):707–711. doi: 10.1128/jvi.16.3.707-711.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biquard J. M., Vigier P. Characteristics of a conditional mutant of Rous sarcoma virus defective in ability to transform cells at high temperature. Virology. 1972 Feb;47(2):444–455. doi: 10.1016/0042-6822(72)90280-2. [DOI] [PubMed] [Google Scholar]
- Burger M. M., Goldberg A. R. Identification of a tumor-specific determinant on neoplastic cell surfaces. Proc Natl Acad Sci U S A. 1967 Feb;57(2):359–366. doi: 10.1073/pnas.57.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger M. M., Martin G. S. Agglutination of cells transformed by Rous sarcoma virus by wheat germ agglutinin and concanavalin A. Nat New Biol. 1972 May 3;237(70):9–12. doi: 10.1038/newbio237009a0. [DOI] [PubMed] [Google Scholar]
- Chen L. B., Buchanan J. M. Plasminogen-independent fibrinolysis by proteases produced by transformed chick embryo fibroblasts. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1132–1136. doi: 10.1073/pnas.72.3.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friis R. R. Abortive infection of Japanese quail cells with avian sarcoma viruses. Virology. 1972 Dec;50(3):701–712. doi: 10.1016/0042-6822(72)90424-2. [DOI] [PubMed] [Google Scholar]
- Friis R. R., Toyoshima K., Vogt P. K. Conditional lethal mutants of avian sarcoma viruses. I. Physiology of ts 75 and ts 149. Virology. 1971 Feb;43(2):375–389. doi: 10.1016/0042-6822(71)90310-2. [DOI] [PubMed] [Google Scholar]
- Goldberg A. R. Increased protease levels in transformed cells: a casein overlay assay for the detection of plasminogen activator production. Cell. 1974 Jun;2(2):95–102. doi: 10.1016/0092-8674(74)90097-x. [DOI] [PubMed] [Google Scholar]
- Hatanaka M., Hanafusa H. Analysis of a functional change in membrane in the process of cell transformation by Rous sarcoma virus; alteration in the characteristics of sugar transport. Virology. 1970 Aug;41(4):647–652. doi: 10.1016/0042-6822(70)90429-0. [DOI] [PubMed] [Google Scholar]
- Hogg N. M. A comparison of membrane proteins of normal and transformed cells by lactoperoxidase labeling. Proc Natl Acad Sci U S A. 1974 Feb;71(2):489–492. doi: 10.1073/pnas.71.2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollwy R. W., Kiernan J. A. Control of the initiation of DNA synthesis in 3T3 cells: serum factors. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2908–2911. doi: 10.1073/pnas.71.7.2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R. O. Alteration of cell-surface proteins by viral transformation and by proteolysis. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3170–3174. doi: 10.1073/pnas.70.11.3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R. O., Bye J. M. Density and cell cycle dependence of cell surface proteins in hamster fibroblasts. Cell. 1974 Oct;3(2):113–120. doi: 10.1016/0092-8674(74)90114-7. [DOI] [PubMed] [Google Scholar]
- Hynes R. O., Wyke J. A. Alterations in surface proteins in chicken cells transformed by temperature-sensitive mutants of Rous sarcoma virus. Virology. 1975 Apr;64(2):492–504. doi: 10.1016/0042-6822(75)90126-9. [DOI] [PubMed] [Google Scholar]
- Jones P. A., Laug W. E., Benedict W. F. Fibrinolytic activity in a human fibrosarcoma cell line and evidence for the induction of plasminogen activator secretion during tumor formation. Cell. 1975 Oct;6(2):245–252. doi: 10.1016/0092-8674(75)90015-x. [DOI] [PubMed] [Google Scholar]
- Kawai S., Hanafusa H. The effects of reciprocal changes in temperature on the transformed state of cells infected with a rous sarcoma virus mutant. Virology. 1971 Nov;46(2):470–479. doi: 10.1016/0042-6822(71)90047-x. [DOI] [PubMed] [Google Scholar]
- Kurth R., Bauer H. Avian oncornavirus-induced tumor antigens of embryonic and unknown origin. Virology. 1973 Dec;56(2):496–504. doi: 10.1016/0042-6822(73)90052-4. [DOI] [PubMed] [Google Scholar]
- Kurth R., Friis R. R., Wyke J. A., Bauer H. Expression of tumor-specific surface antigens on cells infected with temperature-sensitive mutants of avian sarcoma virus. Virology. 1975 Apr;64(2):400–408. doi: 10.1016/0042-6822(75)90116-6. [DOI] [PubMed] [Google Scholar]
- MACPHERSON I., MONTAGNIER L. AGAR SUSPENSION CULTURE FOR THE SELECTIVE ASSAY OF CELLS TRANSFORMED BY POLYOMA VIRUS. Virology. 1964 Jun;23:291–294. doi: 10.1016/0042-6822(64)90301-0. [DOI] [PubMed] [Google Scholar]
- Martin G. S., Venuta S., Weber M., Rubin H. Temperature-dependent alterations in sugar transport in cells infected by a temperature-sensitive mutant of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2739–2741. doi: 10.1073/pnas.68.11.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada Y. S., Hakura A. Temperature-dependent properties of cells transformed by a thermosensitive mutant (TS-121) of polyoma virus. II. Characterization of 121-6 cells. Int J Cancer. 1975 Sep 15;16(3):394–403. doi: 10.1002/ijc.2910160306. [DOI] [PubMed] [Google Scholar]
- Ossowski L., Unkeless J. C., Tobia A., Quigley J. P., Rifkin D. B., Reich E. An enzymatic function associated with transformation of fibroblasts by oncogenic viruses. II. Mammalian fibroblast cultures transformed by DNA and RNA tumor viruses. J Exp Med. 1973 Jan 1;137(1):112–126. doi: 10.1084/jem.137.1.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack R. E., Burger M. M. Surface-specific characteristics of a contact-inhibited cell line containing the SV40 viral genome. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1074–1076. doi: 10.1073/pnas.62.4.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack R., Risser R., Conlon S., Rifkin D. Plasminogen activator production accompanies loss of anchorage regulation in transformation of primary rat embryo cells by simian virus 40. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4792–4796. doi: 10.1073/pnas.71.12.4792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley J. P., Ossowski L., Reich E. Plasminogen, the serum proenzyme activated by factors from cells transformed by oncogenic viruses. J Biol Chem. 1974 Jul 10;249(13):4306–4311. [PubMed] [Google Scholar]
- RUBIN H. An analysis of the assay of Rous sarcoma cells in vitro by the infective center technique. Virology. 1960 Jan;10:29–49. doi: 10.1016/0042-6822(60)90004-0. [DOI] [PubMed] [Google Scholar]
- Risser R., Pollack R. A nonselective analysis of SV40 transformation of mouse 3T3 cells. Virology. 1974 Jun;59(2):477–489. doi: 10.1016/0042-6822(74)90457-7. [DOI] [PubMed] [Google Scholar]
- Rosenblith J. Z., Ukena T. E., Yin H. H., Berlin R. D., Karnovsky M. J. A comparative evaluation of the distribution of concanavalin A-binding sites on the surfaces of normal, virally-transformed, and protease-treated fibroblasts. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1625–1629. doi: 10.1073/pnas.70.6.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruoslahti E., Vaheri A. Novel human serum protein from fibroblast plasma membrane. Nature. 1974 Apr 26;248(5451):789–791. doi: 10.1038/248789a0. [DOI] [PubMed] [Google Scholar]
- Shin S. I., Freedman V. H., Risser R., Pollack R. Tumorigenicity of virus-transformed cells in nude mice is correlated specifically with anchorage independent growth in vitro. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4435–4439. doi: 10.1073/pnas.72.11.4435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoker M. Abortive transformation by polyoma virus. Nature. 1968 Apr 20;218(5138):234–238. doi: 10.1038/218234a0. [DOI] [PubMed] [Google Scholar]
- Stone K. R., Smith R. E., Joklik W. K. Changes in membrane polypeptides that occur when chick embryo fibroblasts and NRK cells are transformed with avian sarcoma viruses. Virology. 1974 Mar;58(1):86–100. doi: 10.1016/0042-6822(74)90143-3. [DOI] [PubMed] [Google Scholar]
- TEMIN H. M., RUBIN H. Characteristics of an assay for Rous sarcoma virus and Rous sarcoma cells in tissue culture. Virology. 1958 Dec;6(3):669–688. doi: 10.1016/0042-6822(58)90114-4. [DOI] [PubMed] [Google Scholar]
- TODARO G. J., GREEN H., GOLDBERG B. D. TRANSFORMATION OF PROPERTIES OF AN ESTABLISHED CELL LINE BY SV40 AND POLYOMA VIRUS. Proc Natl Acad Sci U S A. 1964 Jan;51:66–73. doi: 10.1073/pnas.51.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unkeless J. C., Tobia A., Ossowski L., Quigley J. P., Rifkin D. B., Reich E. An enzymatic function associated with transformation of fibroblasts by oncogenic viruses. I. Chick embryo fibroblast cultures transformed by avian RNA tumor viruses. J Exp Med. 1973 Jan 1;137(1):85–111. doi: 10.1084/jem.137.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unkeless J., Dano K., Kellerman G. M., Reich E. Fibrinolysis associated with oncogenic transformation. Partial purification and characterization of the cell factor, a plasminogen activator. J Biol Chem. 1974 Jul 10;249(13):4295–4305. [PubMed] [Google Scholar]
- Vogt P. K., Weiss R. A., Hanafusa H. Proposal for numbering mutants of avian leukosis and sarcoma viruses. J Virol. 1974 Feb;13(2):551–554. doi: 10.1128/jvi.13.2.551-554.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber M. J. Hexose transport in normal and in Rous sarcoma virus-transformed cells. J Biol Chem. 1973 May 10;248(9):2978–2983. [PubMed] [Google Scholar]
- Weber M. J. Inhibition of protease activity in cultures of rous sarcoma virus-transformed cells: effect on the transformed phenotype. Cell. 1975 Jul;5(3):253–261. doi: 10.1016/0092-8674(75)90100-2. [DOI] [PubMed] [Google Scholar]
- Wyke J. A., Linial M. Temperature-sensitive avian sarcoma viruses: a physiological comparison of twenty mutants. Virology. 1973 May;53(1):152–161. doi: 10.1016/0042-6822(73)90474-1. [DOI] [PubMed] [Google Scholar]
- Wyke J. A method of isolating cells incapable of multiplication in suspension culture. Exp Cell Res. 1971 May;66(1):203–208. doi: 10.1016/s0014-4827(71)80030-7. [DOI] [PubMed] [Google Scholar]
- Yamada K. M., Yamada S. S., Pastan I. Cell surface protein partially restores morphology, adhesiveness, and contact inhibition of movement to transformed fibroblasts. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1217–1221. doi: 10.1073/pnas.73.4.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]





