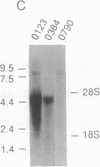
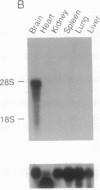
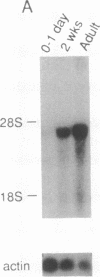
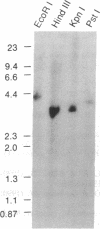
Abstract
In an attempt to define the molecular basis of the functional diversity of K+ channels, we have isolated overlapping rat brain cDNAs that encoded a neuronal delayed rectifier K+ channel, K,4, that is structurally related to the Drosophila Shaw protein. Unlike previously characterized mammalian K+ channel genes, which each contain a single protein-coding exon, K,4 arises from alternative exon usage at a locus that also encodes another mammalian Shaw homolog, NGK2. Thus, the enormous diversity of K+ channels in mammals can be generated not just through gene duplication and divergence but also through alternative splicing of RNA.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auld V. J., Goldin A. L., Krafte D. S., Catterall W. A., Lester H. A., Davidson N., Dunn R. J. A neutral amino acid change in segment IIS4 dramatically alters the gating properties of the voltage-dependent sodium channel. Proc Natl Acad Sci U S A. 1990 Jan;87(1):323–327. doi: 10.1073/pnas.87.1.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann A., Grupe A., Ackermann A., Pongs O. Structure of the voltage-dependent potassium channel is highly conserved from Drosophila to vertebrate central nervous systems. EMBO J. 1988 Aug;7(8):2457–2463. doi: 10.1002/j.1460-2075.1988.tb03092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckh S., Pongs O. Members of the RCK potassium channel family are differentially expressed in the rat nervous system. EMBO J. 1990 Mar;9(3):777–782. doi: 10.1002/j.1460-2075.1990.tb08173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler A., Wei A. G., Baker K., Salkoff L. A family of putative potassium channel genes in Drosophila. Science. 1989 Feb 17;243(4893):943–947. doi: 10.1126/science.2493160. [DOI] [PubMed] [Google Scholar]
- Butler A., Wei A., Salkoff L. Shal, Shab, and Shaw: three genes encoding potassium channels in Drosophila. Nucleic Acids Res. 1990 Apr 25;18(8):2173–2174. doi: 10.1093/nar/18.8.2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandy K. G., Williams C. B., Spencer R. H., Aguilar B. A., Ghanshani S., Tempel B. L., Gutman G. A. A family of three mouse potassium channel genes with intronless coding regions. Science. 1990 Feb 23;247(4945):973–975. doi: 10.1126/science.2305265. [DOI] [PubMed] [Google Scholar]
- Christie M. J., Adelman J. P., Douglass J., North R. A. Expression of a cloned rat brain potassium channel in Xenopus oocytes. Science. 1989 Apr 14;244(4901):221–224. doi: 10.1126/science.2539643. [DOI] [PubMed] [Google Scholar]
- Christie M. J., North R. A., Osborne P. B., Douglass J., Adelman J. P. Heteropolymeric potassium channels expressed in Xenopus oocytes from cloned subunits. Neuron. 1990 Mar;4(3):405–411. doi: 10.1016/0896-6273(90)90052-h. [DOI] [PubMed] [Google Scholar]
- Douglass J., Osborne P. B., Cai Y. C., Wilkinson M., Christie M. J., Adelman J. P. Characterization and functional expression of a rat genomic DNA clone encoding a lymphocyte potassium channel. J Immunol. 1990 Jun 15;144(12):4841–4850. [PubMed] [Google Scholar]
- Early P., Rogers J., Davis M., Calame K., Bond M., Wall R., Hood L. Two mRNAs can be produced from a single immunoglobulin mu gene by alternative RNA processing pathways. Cell. 1980 Jun;20(2):313–319. doi: 10.1016/0092-8674(80)90617-0. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Frech G. C., VanDongen A. M., Schuster G., Brown A. M., Joho R. H. A novel potassium channel with delayed rectifier properties isolated from rat brain by expression cloning. Nature. 1989 Aug 24;340(6235):642–645. doi: 10.1038/340642a0. [DOI] [PubMed] [Google Scholar]
- Grupe A., Schröter K. H., Ruppersberg J. P., Stocker M., Drewes T., Beckh S., Pongs O. Cloning and expression of a human voltage-gated potassium channel. A novel member of the RCK potassium channel family. EMBO J. 1990 Jun;9(6):1749–1756. doi: 10.1002/j.1460-2075.1990.tb08299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard S. C., Ivatt R. J. Synthesis and processing of asparagine-linked oligosaccharides. Annu Rev Biochem. 1981;50:555–583. doi: 10.1146/annurev.bi.50.070181.003011. [DOI] [PubMed] [Google Scholar]
- Isacoff E. Y., Jan Y. N., Jan L. Y. Evidence for the formation of heteromultimeric potassium channels in Xenopus oocytes. Nature. 1990 Jun 7;345(6275):530–534. doi: 10.1038/345530a0. [DOI] [PubMed] [Google Scholar]
- Iverson L. E., Tanouye M. A., Lester H. A., Davidson N., Rudy B. A-type potassium channels expressed from Shaker locus cDNA. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5723–5727. doi: 10.1073/pnas.85.15.5723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamb A., Tseng-Crank J., Tanouye M. A. Multiple products of the Drosophila Shaker gene may contribute to potassium channel diversity. Neuron. 1988 Jul;1(5):421–430. doi: 10.1016/0896-6273(88)90192-4. [DOI] [PubMed] [Google Scholar]
- Kishimoto A., Nishiyama K., Nakanishi H., Uratsuji Y., Nomura H., Takeyama Y., Nishizuka Y. Studies on the phosphorylation of myelin basic protein by protein kinase C and adenosine 3':5'-monophosphate-dependent protein kinase. J Biol Chem. 1985 Oct 15;260(23):12492–12499. [PubMed] [Google Scholar]
- Kozak M. The scanning model for translation: an update. J Cell Biol. 1989 Feb;108(2):229–241. doi: 10.1083/jcb.108.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuenzel E. A., Mulligan J. A., Sommercorn J., Krebs E. G. Substrate specificity determinants for casein kinase II as deduced from studies with synthetic peptides. J Biol Chem. 1987 Jul 5;262(19):9136–9140. [PubMed] [Google Scholar]
- Leff S. E., Rosenfeld M. G., Evans R. M. Complex transcriptional units: diversity in gene expression by alternative RNA processing. Annu Rev Biochem. 1986;55:1091–1117. doi: 10.1146/annurev.bi.55.070186.005303. [DOI] [PubMed] [Google Scholar]
- Mangin M., Ikeda K., Dreyer B. E., Broadus A. E. Isolation and characterization of the human parathyroid hormone-like peptide gene. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2408–2412. doi: 10.1073/pnas.86.7.2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack K., Lin J. W., Iverson L. E., Rudy B. Shaker K+ channel subunits from heteromultimeric channels with novel functional properties. Biochem Biophys Res Commun. 1990 Sep 28;171(3):1361–1371. doi: 10.1016/0006-291x(90)90836-c. [DOI] [PubMed] [Google Scholar]
- McCormack T., Vega-Saenz de Miera E. C., Rudy B. Molecular cloning of a member of a third class of Shaker-family K+ channel genes in mammals. Proc Natl Acad Sci U S A. 1990 Jul;87(13):5227–5231. doi: 10.1073/pnas.87.13.5227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh K. M., Lessard J. L. The development expression of the rat alpha-vascular and gamma-enteric smooth muscle isoactins: isolation and characterization of a rat gamma-enteric actin cDNA. Mol Cell Biol. 1988 Dec;8(12):5224–5231. doi: 10.1128/mcb.8.12.5224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinnon D. Isolation of a cDNA clone coding for a putative second potassium channel indicates the existence of a gene family. J Biol Chem. 1989 May 15;264(14):8230–8236. [PubMed] [Google Scholar]
- Padgett R. A., Grabowski P. J., Konarska M. M., Seiler S., Sharp P. A. Splicing of messenger RNA precursors. Annu Rev Biochem. 1986;55:1119–1150. doi: 10.1146/annurev.bi.55.070186.005351. [DOI] [PubMed] [Google Scholar]
- Papazian D. M., Timpe L. C., Jan Y. N., Jan L. Y. Alteration of voltage-dependence of Shaker potassium channel by mutations in the S4 sequence. Nature. 1991 Jan 24;349(6307):305–310. doi: 10.1038/349305a0. [DOI] [PubMed] [Google Scholar]
- Pongs O., Kecskemethy N., Müller R., Krah-Jentgens I., Baumann A., Kiltz H. H., Canal I., Llamazares S., Ferrus A. Shaker encodes a family of putative potassium channel proteins in the nervous system of Drosophila. EMBO J. 1988 Apr;7(4):1087–1096. doi: 10.1002/j.1460-2075.1988.tb02917.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proudfoot N. J., Brownlee G. G. 3' non-coding region sequences in eukaryotic messenger RNA. Nature. 1976 Sep 16;263(5574):211–214. doi: 10.1038/263211a0. [DOI] [PubMed] [Google Scholar]
- Ruppersberg J. P., Schröter K. H., Sakmann B., Stocker M., Sewing S., Pongs O. Heteromultimeric channels formed by rat brain potassium-channel proteins. Nature. 1990 Jun 7;345(6275):535–537. doi: 10.1038/345535a0. [DOI] [PubMed] [Google Scholar]
- Schwarz T. L., Tempel B. L., Papazian D. M., Jan Y. N., Jan L. Y. Multiple potassium-channel components are produced by alternative splicing at the Shaker locus in Drosophila. Nature. 1988 Jan 14;331(6152):137–142. doi: 10.1038/331137a0. [DOI] [PubMed] [Google Scholar]
- Stühmer W., Ruppersberg J. P., Schröter K. H., Sakmann B., Stocker M., Giese K. P., Perschke A., Baumann A., Pongs O. Molecular basis of functional diversity of voltage-gated potassium channels in mammalian brain. EMBO J. 1989 Nov;8(11):3235–3244. doi: 10.1002/j.1460-2075.1989.tb08483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson R., Marshall J., Smith J. S., Williams J. B., Boyle M. B., Folander K., Luneau C. J., Antanavage J., Oliva C., Buhrow S. A. Cloning and expression of cDNA and genomic clones encoding three delayed rectifier potassium channels in rat brain. Neuron. 1990 Jun;4(6):929–939. doi: 10.1016/0896-6273(90)90146-7. [DOI] [PubMed] [Google Scholar]
- Tempel B. L., Jan Y. N., Jan L. Y. Cloning of a probable potassium channel gene from mouse brain. Nature. 1988 Apr 28;332(6167):837–839. doi: 10.1038/332837a0. [DOI] [PubMed] [Google Scholar]
- Tempel B. L., Papazian D. M., Schwarz T. L., Jan Y. N., Jan L. Y. Sequence of a probable potassium channel component encoded at Shaker locus of Drosophila. Science. 1987 Aug 14;237(4816):770–775. doi: 10.1126/science.2441471. [DOI] [PubMed] [Google Scholar]
- Timpe L. C., Jan Y. N., Jan L. Y. Four cDNA clones from the Shaker locus of Drosophila induce kinetically distinct A-type potassium currents in Xenopus oocytes. Neuron. 1988 Oct;1(8):659–667. doi: 10.1016/0896-6273(88)90165-1. [DOI] [PubMed] [Google Scholar]
- Wei A., Covarrubias M., Butler A., Baker K., Pak M., Salkoff L. K+ current diversity is produced by an extended gene family conserved in Drosophila and mouse. Science. 1990 May 4;248(4955):599–603. doi: 10.1126/science.2333511. [DOI] [PubMed] [Google Scholar]
- Woodgett J. R., Gould K. L., Hunter T. Substrate specificity of protein kinase C. Use of synthetic peptides corresponding to physiological sites as probes for substrate recognition requirements. Eur J Biochem. 1986 Nov 17;161(1):177–184. doi: 10.1111/j.1432-1033.1986.tb10139.x. [DOI] [PubMed] [Google Scholar]
- Yokoyama S., Imoto K., Kawamura T., Higashida H., Iwabe N., Miyata T., Numa S. Potassium channels from NG108-15 neuroblastoma-glioma hybrid cells. Primary structure and functional expression from cDNAs. FEBS Lett. 1989 Dec 18;259(1):37–42. doi: 10.1016/0014-5793(89)81488-7. [DOI] [PubMed] [Google Scholar]