Abstract
Malignant tumors display remarkable heterogeneity to the extent that even at the same tissue site different types of cells with varying genetic background may be found. In contrast, a relatively consistent marker the scavenger receptor type B1 (SR-B1) has been found to be consistently overexpressed by most tumor cells. Scavenger Receptor Class B Type I (SR-BI) is a high density lipoprotein (HDL) receptor that facilitates the uptake of cholesterol esters from circulating lipoproteins. Additional findings suggest a critical role for SR-BI in cholesterol metabolism, signaling, motility, and proliferation of cancer cells and thus a potential major impact in carcinogenesis and metastasis. Recent findings indicate that the level of SR-BI expression correlate with aggressiveness and poor survival in breast and prostate cancer. Moreover, genomic data show that depending on the type of cancer, high or low SR-BI expression may promote poor survival. This review discusses the importance of SR-BI as a diagnostic as well as prognostic indicator of cancer to help elucidate the contributions of this protein to cancer development, progression, and survival. In addition, the SR-B1 receptor has been shown to serve as a potential gateway for the delivery of therapeutic agents when reconstituted high density lipoprotein nanoparticles are used for their transport to cancer cells and tumors. Opportunities for the development of new technologies, particularly in the areas of cancer therapy and tumor imaging are discussed.
Keywords: SR-B1, Drug Delivery Systems, tumor imaging, gateway for theranostics, individual therapy
Background
High density lipoproteins (HDLs) have been designated as “the good cholesterol” carriers due to their athero-protective effects. The beneficial impact of elevated HDL levels have been attributed to several factors, including participation in reverse cholesterol transport, anti-oxidant effects and anti-inflammatory functions (Miller et al., 1985; Navab et al., 1991, 2000a,b, 2001). During reverse cholesterol transport, HDL removes excess peripheral cholesterol and channels it toward the liver for disposal (or re-cycling), facilitated, at least in part, by the Scavenger Receptor, Class B, Type 1 (SR-B1; Figure 1A). Decreased plasma cholesterol levels (especially, lower HDL cholesterol levels) have been found in several groups of cancer patients representing a broad range of malignancies (Rose et al., 1974; Fiorenza et al., 2000; Shah et al., 2008; Muntoni et al., 2009). Regarding the mechanism of the lowering of HDL cholesterol levels in cancer patients, several reports found over-expression of the SR-B1 receptors as the potential cause (Lacko et al., 2002; Mooberry et al., 2010;
FIGURE 1.
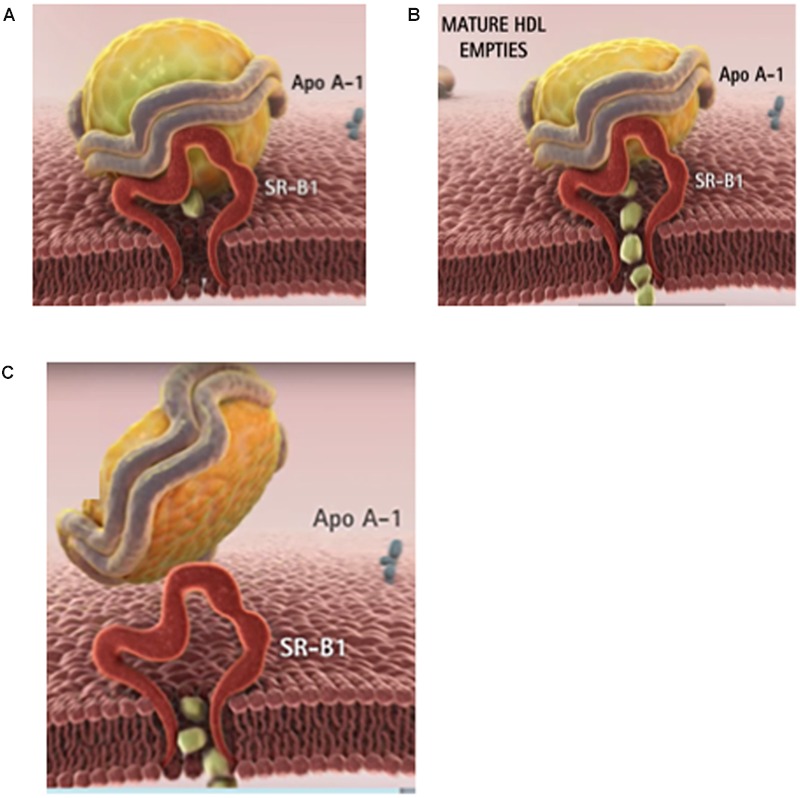
The physiological role of HDL is to clear the excess tissue cholesterol through the liver. (A) In phase 1, the cholesterol-rich HDL is captured by the SR-B1 receptor on the surface of the cell. (B) In phase 2, HDL delivers its cholesteryl ester cargo through SR-B1 directly into the cytoplasm. (C) In phase 3, the depleted HDL particle is released into the blood. The majority of cancer patients have lower than normal blood cholesterol especially HDL (or good) cholesterol. Images courtesy of Andras Lacko; full animation video is available at https://www.youtube.com/watch?v=q0YiPqmsXRg.
Shahzad et al., 2011; de Gonzalo-Calvo et al., 2015; Li et al., 2016; Yuan et al., 2016). Consequently, one of the key issues to be resolved is the extent to which the increased functionality of the SR-B1 receptor may be responsible for the markedly lower HDL cholesterol levels found in most cancer patients as the precise role of the SR-B1 receptor in the development of malignant and metastatic growth is yet to be delineated. Findings from a number of studies (Cruz et al., 2013; Yang et al., 2013) suggest that the delivery of cholesterol to rapidly growing malignant cells, facilitated by the overexpressed SR-B1 receptor, is essential for at least some tumor types. Cao et al. (2004) on the other hand showed that breast cancer xenografts could not develop when the carboxy terminal of the CLA-1 (SR-B1) receptor was mutated while other studies indicated that the carboxy terminal region of the SR-B1 receptor was not directly involved in cholesterol transport (Connelly et al., 2001; Cao et al., 2004). While the mechanism whereby the SR-B1 receptor contributes to carcinogenesis is not yet clear, expression of the SR-B1 receptor may signal the prognosis and survival prospects of cancer patients and thus could have a role as an important diagnostic biomarker for specific malignancies.
HDL Metabolism and Receptors with Special Emphasis on Scavenger Receptors
The precursor to circulating HDL is produced in the liver and subsequently released into the circulation. These are cholesterol-poor, discoidal structures, composed primarily of apolipoprotein A-I (apo A-I) and phospholipid (Hamilton et al., 1976). Next the ATP-binding cassette protein, ABCA-1, facilitates the loading of the nascent discoidal HDL with excess cholesterol from peripheral tissues. The cholesterol progressively accumulated in HDL is then esterified by Lecithin-Cholesterol Acyltransferase (LCAT) to produce mature spherical HDL particles with an expanded core region, containing primarily cholesteryl esters. Although apo A-I is the main protein component of HDL, it acquires other apolipoproteins including apo A-II, apo A-IV, apo C-III or apo E, from other lipoproteins. The respective apolipoproteins may facilitate the interactions of HDL with several lipoprotein receptors (Alaupovic et al., 1972; Scanu, 1972; Scanu and Wisdom, 1972; Schmitz and Mollers, 1994; Fidge, 1999).
Unlike the delivery of Low density lipoprotein (LDL) cholesterol that involves endocytic uptake of the entire lipoprotein particle, most of the cholesterol from HDL undergoes a process termed selective lipid uptake, facilitated by the SR-B1 receptor, involving only the removal of cholesteryl esters from the HDL complex (Figure 1B). The subsequent steps involve the release of the now cholesterol-depleted HDL via a process called retroendocytosis (Pagler et al., 2006; Sun et al., 2006). The protein component of HDL is thus not internalized, but remains (at least transiently) bound to the plasma membrane (Figure 1C).
A plasma membrane protein, SR-B1, is the murine HDL receptor (Acton et al., 1996). The human homolog is termed CD36-and-LIMPII analogous-1 (CLA-1). The human HDL receptor is composed of 509 amino acids and has an apparent molecular weight of 85 kDa including carbohydrate moieties that are attached to the polypeptide portion via N-linked glycosylation (Calvo et al., 1997). Expression of SR-B1 is most prominent in tissues involved in cholesterol metabolism or steroid hormone synthesis, including the liver, adrenal glands, ovaries, and testes (Landschulz et al., 1996; Calvo et al., 1997; Arenas et al., 2004; Shahzad et al., 2011). However, besides acting as an HDL receptor, facilitating selective lipid uptake from HDL, SR-B1 has also been shown to be a high-affinity receptor for LDL, VLDL, oxidized LDL, and acetylated LDL (Calvo et al., 1997).
The mechanism of selective lipid uptake by SR-B1 has yet to be described in detail. The known domains of SR-B1 consist of a short N-terminal intracellular region, an N-terminal transmembrane domain, a cysteine-rich region that forms an extracellular loop, a C-terminal transmembrane domain, and a C-terminal intracellular domain (Krieger, 1999). It has been postulated that SR-B1 forms a “hydrophobic channel” to allow passage of lipophilic HDL components into the cell (Rodrigueza et al., 1999). Reports in the literature have attempted to delineate the respective functional domains and their specific contributions to HDL binding, and selective lipid uptake. Studies creating chimeric receptors between domains of CD36 and SR-B1 have shown that neither the N-terminal nor C-terminal transmembrane and cytoplasmic domains of SR-B1 are necessary for selective lipid uptake. The function of selective lipid uptake was found to be the task of the extracellular domain of SR-B1 (Connelly et al., 2001). The extracellular domain of SR-B1 contains six cysteine residues highly conserved among mouse, rat, hamster, rabbit, pig, cow, dog, and human isoforms (Hu et al., 2011). In a mutational analysis, some of these cysteine residues were found to interfere with cell surface localization and selective lipid uptake, possibly due to the formation of aberrant disulfide bonds and protein misfolding (Hu et al., 2011).
Expression and Potential Roles of the SR-B1 Receptor in Cancer Cells and Tumors
Cholesterol Metabolism
A breast cancer cell line HBL-100, was shown to take up cholesteryl esters (CE) selectively through SR-B1. The cells internalized CE in a dose-dependent manner in response to the HDL3 concentration. No increase in uptake of CE was observed with adrenocorticotropic hormone, but down-regulation of SR-B1 expression and decreased CE uptake were seen with phorbol 12-myristate 13-acetate (PMA). The cholesteryl esters were subsequently hydrolyzed by hormone sensitive lipase (Pussinen et al., 2000). A study in patients with three subtypes of breast cancer found that CE accumulation corresponded with increases in cholesterol esterification and the expression of the LDL receptor and SR-B1 (de Gonzalo-Calvo et al., 2015). In patients with Her-2 breast cancer (ER-/PR-/HER2+) or triple negative breast cancer (ER-/PR-/HER2-), tumor samples with enriched cholesteryl ester accumulation were associated with increased expression of the cell proliferation marker, Ki-67, thus indicating the potential for enhanced tumor proliferation and progression. However, the third subtype, estrogen and progesterone receptor positive luminal-A breast cancer (ER+/PR+/HER2-) did not show increased intracellular cholesteryl esters or increased Ki-67 expression (de Gonzalo-Calvo et al., 2015). Because of the differential cholesteryl ester accumulation and expression of SR-B1 in varying grades and subtypes of breast cancer that links to proliferation and progression, more research into cholesterol metabolism in cancer subtypes is warranted.
In a study involving high grade metastatic prostate cancer increased accumulation of CE into lipid droplets was observed. The CE accumulation was found to be in response to the loss of the tumor suppressor PTEN, activation of the mTOR pathway and downstream activation of SREBP, and the up-regulation of LDL receptor (Yue et al., 2014). This study examined only uptake of LDL and the LDL receptor, not selective CE uptake through SR-B1. However, the SR-B1 gene does contain two Sterol Regulatory Elements (SREs) that are activated by SREBP (Lopez and McLean, 1999). In a report on high Gleason grade primary prostate cancer, SR-B1 mRNA and protein expression were found to be high, unlike the LDL receptor (Schörghofer et al., 2015). The link between SR-B1 expression and cholesteryl ester accumulation could provide the link between androgen independence and advanced prostate cancer (Twiddy et al., 2012; Schörghofer et al., 2015).
Signaling Mechanisms
High density lipoprotein, beyond binding to SR-B1 and completing reverse cholesterol transport has also been found to subsequently activate the PI3K/Akt and MAPK pathways (Danilo et al., 2013). SR-B1 has been investigated as a mediator of cell signaling events in atherosclerosis (Al-Jarallah and Trigatti, 2010; Saddar et al., 2010). SR-B1 has been found to be highly expressed in several different types of tumors including breast, ovarian, colorectal, pancreatic cancer (Shahzad et al., 2011) and prostate cancer (Schörghofer et al., 2015) with relatively low expression in most healthy tissue (Rhainds and Brissette, 2004). Although SRB1 has been found to have a protective role in atherosclerosis, high expression of SR-B1 has also been linked to the rapid progression and aggressiveness of certain types of cancer, while the exact underlying mechanisms and downstream cell signaling events of SR-B1 are presently not fully understood.
Hypercholesterolemia and excessive consumption of a cholesterol or fat rich diet have been recognized as risk factors for prostate cancer. Additional studies have also shown that the aggressive form of the disease is likely to be associated with the intracellular accumulation of esterified cholesterol (Shimizu et al., 1991; Bravi et al., 2006; Yue et al., 2014). While cellular cholesterol uptake is known to be facilitated by the SR-B1 and the LDL receptor (Acton et al., 1996; Krieger, 1999; Chen and Hughes-Fulford, 2001; Rohrl and Stangl, 2013). SR-B1 has been found to be highly expressed in prostate cancer cells (Connelly and Williams, 2004; Kraemer, 2007; Connelly, 2009; Hoekstra et al., 2009). Twiddy et al. (2012) have found that protein expression of SR-B1 was significantly increased upon progression to castration-resistance stage in the LNCaP xenograft model (Leon et al., 2010). Even higher expression of SR-B1 has been reported in C4-2 cells compared to LNCaP cells, suggesting a role for SR-B1 in the development and progression of advanced prostate cancer (Twiddy et al., 2012). These findings indicate that the castration resistant prostate cancer cells might be able to survive in a low androgen environment by increasing their demand for cholesterol influx for cellular processes (Wu et al., 1994; Leon et al., 2010).
The Akt signaling pathway is activated in 40% of breast cancers (Bellacosa et al., 2005). Studies conducted by Danilo et al. (2013) showed that HDL (100 μg/ml) can trigger the activation of Erk1/2 and Akt pathways in breast cancer cells. Their studies in MDA-MB-231 cells have shown that knockdown of the SR-B1 receptor (via lentiviral transduction of SR-B1 shRNA or pharmacological inhibition of SR-B1 in the presence of HDL) mitigated the activation of the expression of the Akt signaling molecule. Whereas, the knockdown of SR-B1 receptor in MCF-7 cells showed a marked decrease in the activation of Akt and the Erk1/2 pathway. These findings support the hypothesis that the binding of HDL to SR-B1 may be involved in the regulation of these signaling pathways. Additionally, the studies of Danilo et al. (2013) have also shown that knockdown of SR-B1 in MDA-MB-231 cells triggered a reduction in the intracellular cholesterol levels, in concurrence with the studies of Assanasen et al. (2005) showing that SR-B1 modulated signal transduction could be linked to cellular cholesterol flux (Danilo et al., 2013).
Proliferation
Cancer cells are considered to be dependent on cholesterol for their proliferation and rapid progression (Gospodarowicz et al., 1982; Johnson et al., 1991; Cruz et al., 2013). Comparative analysis of SR-B1 expression in breast tissues revealed a high expression of SR-B1 in breast tumors compared to the healthy surrounding tissue (Cao et al., 2004; Shahzad et al., 2011). Additionally, reports from multiple studies, conducted with several cancer cells revealed that HDL can enhance their capacity for proliferation and migration (Gospodarowicz et al., 1982; Jozan et al., 1985; Rotheneder and Kostner, 1989; Pussinen et al., 2000; Pan et al., 2012a,b). Studies conducted by Danilo et al. (2013) in MDA-MB-231 (triple negative breast cancer cells) have shown that pharmacological inhibition of SR-B1 (through BLT-1) or knockdown of SR-B1 resulted in a reduction in cell proliferation perhaps via a decreased activation of the Akt signaling pathway.
Migration
High density lipoprotein, a carrier of plasma cholesterol has also been found to be a signaling molecule inducing the migration of endothelial cells by activating the Akt and MAPK pathways (Danilo et al., 2013). Similarly, Danilo et al. (2013) have shown that HDL can induce the migration of MCF-7 and MDA-MB-231 cells. In addition, Danilo et al. (2013) performed SR-B1 knockdown in MDA-MB-231 cells and found a significant decrease in their potential for migration (1.65-fold) compared to control cells. These findings are in accordance with studies conducted in nasopharyngeal carcinoma cells, where knockdown of SR-B1 resulted in reduced migration in 5-8F shSR-B1-treated cells compared to control cells (Zheng et al., 2013).
Angiogenesis
High density lipoprotein was shown to induce endothelial tube formation and angiogenesis in human coronary artery endothelial cells through a MAPK pathway (Miura et al., 2003). Seetharam et al. (2006) showed that endothelial cell migration was activated by PI3K/Akt/MAPK pathways mediated by SR-B1. This effect was impaired in SR-B1 knockout mice (Seetharam et al., 2006). In hypoxic conditions, HDL stimulated the PI3K/Akt pathway through SR-B1, resulting in stabilization of hypoxic inducible factor-1α (HIF-1α; Figure 2). The result is activation of transcription of VEGF and induction of angiogenesis, which could be inhibited by transduction with a lentivirus containing a shRNA against SR-B1 (Tan et al., 2014). Mutational analysis of SR-B1 found that changing a critical glutamine residue in one of the transmembrane domains disrupted p38/MAPK signaling. In a mouse model system, the mutant SR-B1 was not capable of increasing endothelial cell abundance or stimulating angiogenesis, even with HDL activation (Saddar et al., 2013). In inflammatory-mediated angiogenesis, HDL and SR-B1 appear to play an inhibitory role (McGrath et al., 2009).
FIGURE 2.
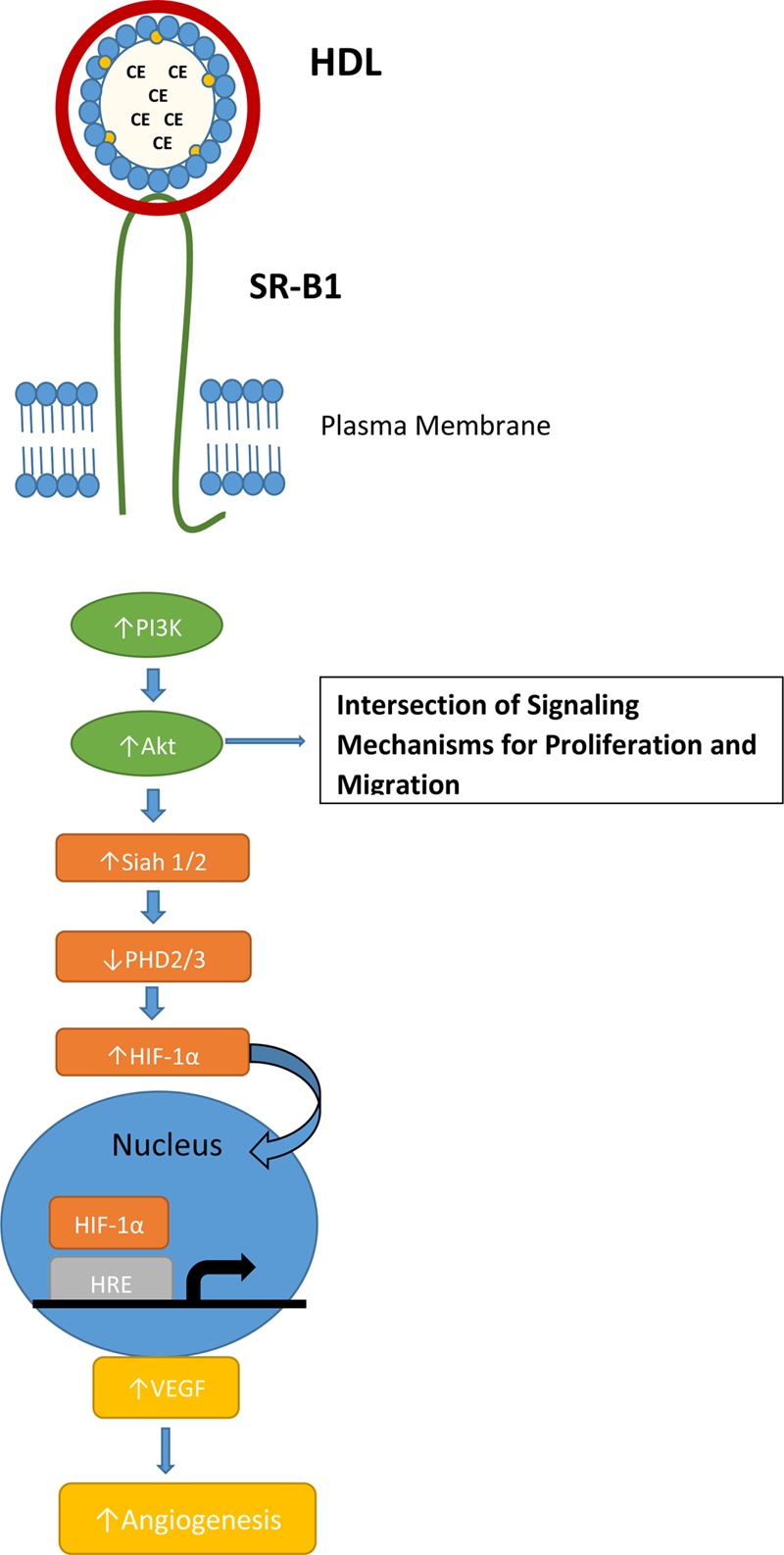
SR-B1 signaling pathway for normal hypoxia-induced angiogenesis. Interaction of HDL and SR-B1 activates the PI3K/Akt pathway leading to HIF-1α translocation to the nucleus and transcription of VEGF. HDL treatment and over-expression of SR-B1 has been shown to activate the PI3K/Akt pathway in tumor cells and stimulate proliferation and migration.
Like HDL and SR-BI, the signaling mechanism for angiogenesis is often utilized by tumors via PI3K/Akt activation and the HIF-1α pathway (Karar and Maity, 2011). Several studies have identified a link between SR-BI and the PI3K/Akt pathway, but the mechanism where by SR-BI impacts angiogenesis is not clear. Danilo et al. (2013) found that mice bearing a MDA-MB-231 xenografts had significantly increased microvessel density in the control tumor when compared to the SR-B1 knockdown tumor. In the TRAMP mouse model of prostate cancer, mice fed a high-fat, high-cholesterol Western-style diet had decreased plasma HDL cholesterol levels, increased tumor expression of SR-B1 and increased tumor angiogenesis (Llaverias et al., 2010).
These studies in cholesterol metabolism, signaling mech-anism, proliferation, migration, and angiogenesis have been conducted with cancer cells, tumor xenograft in mouse models, and patient tumor samples of prostate and breast cancer. The majority of the findings reported in the literature regarding SR-B1 and cancer focus on breast and prostate cancer. Expanding the focus of these studies to other types of tumors would allow the elucidation of the role of the SR-B1 receptor and the related signaling mechanisms governing cancer proliferation, migration, invasion, and angiogenesis.
SR-B1 as a Predictor of Tumor Prognosis and Survival
Scavenger Receptor Class B Type 1 (SR-B1) is a HDL receptor that facilitates the uptake of cholesterol esters from circulating lipoproteins (primarily from HDL). Recent findings indicate that the level of SR-B1 expression is correlated with aggressiveness and poor survival in breast and prostate cancer (Schörghofer et al., 2015; Yuan et al., 2016). Moreover, genomic data show that depending on the type of cancer, high or low SR-B1 expression may promote poor survival. This section focuses on SR-B1 as a diagnostic as well as prognostic biomarker for specific types of cancer to facilitate better outcome for cancer patients by allowing the tailoring of individual therapy via the assessment of progression and survival.
An elevated expression of SR-B1 has been observed in a wide variety of malignant cell lines such as breast, prostate, ovarian, pancreatic, nasopharyngeal, and colorectal cancers (Martin et al., 1999; Cao et al., 2004; Shahzad et al., 2011; Twiddy et al., 2012; Zheng et al., 2013). In human breast carcinoma, higher SR-B1 protein levels were observed in malignant tissue versus cancer free surrounding tissue in xenograft studies (Cao et al., 2004). Moreover, mutation of the HDL receptor attenuated the proliferation of MCF-7 cells (Cao et al., 2004). Similarly, another study demonstrated that over 50% of all examined breast cancer tissue showed a high expression of SR-B1 which significantly correlated with larger size of the tumor, metastasis to lymph node, and overall reduced survival (Yuan et al., 2016). Additionally, breast carcinomas displaying higher SR-B1 mRNA and protein levels showed enhanced accumulation of intratumor cholesteryl ester which linked to aggressiveness and poor prognosis in patients (de Gonzalo-Calvo et al., 2015). Likewise, knockdown of SR-B1 significantly reduced tumor size in mouse models and reduced cell proliferation and migration in vitro. This study also identified a link between HDL-SR-B1 and Akt where breast cancer cells stimulated with cholesteryl ester from HDL exhibited continued proliferation, migration/invasion and subsequent tumor growth (Danilo et al., 2013). Taken together these studies reveal a critical role for SR-B1 as a prognostic marker and therapeutic target for breast cancer treatment.
The uptake of cholesterol ester from HDL via SR-B1 facilitates many important cellular functions such as the production of androgens. Thus, a knockdown of SR-B1 was shown to diminish prostate-specific antigen (PSA) levels and cell viability in prostate cancer cells (Twiddy et al., 2012). Using mRNA microarray files and immunohistochemical analysis of prostate cancer tissue sections Schörghofer et al. (2015) found that mRNA and protein SR-B1 expression positively correlated with progression and metastasis of prostate cancer. The study revealed a higher SR-B1 expression in higher Gleason grade prostate tumors than in lower Gleason grade prostate tumors (Schörghofer et al., 2015). Additionally, SR-B1 expression and enzymes involved in the synthesis of androgens exhibited a positive correlation indicating that SR-B1 may have a potential role in establishing androgen independence (Schörghofer et al., 2015). Moreover, in nasopharyngeal cancer (NPC) SR-B1 was overexpressed in 75% of clinical NPC samples and all examined NPC cell lines while SR-B1 expression was lower in surrounding non-malignant epithelial cells suggesting SR-B1 as a possible biomarker of NPC (Zheng et al., 2013).
Interestingly the generation of Kaplan–Meier survival curves using the R2 Genomics Analysis and Visualization Platform1 along with publicly accessible microarray data confirmed the correlation of high SR-B1 expression and poor prognosis in neuroblastoma, Ewing sarcoma, bladder, breast, and myeloma cancers (Pawitan et al., 2005; Hanamura et al., 2006; Molenaar et al., 2012; Sjodahl et al., 2012). However, a reverse was observed in cancers such ovarian, osteosarcoma, B-Cell lymphoma, and lung (Bild et al., 2006; Kuijjer et al., 2012; Lisowska et al., 2014), specifically low SR-B1 correlated with poor survival. Given the complexities involved in the regulation of SR-B1 expression it is difficult to hypothesize regarding mechanism(s) involved in the observed variations of SR-B1 expression in relation to patient survival. Perhaps transcription factors such as p53, STAT proteins and c-myc could play a role in the regulation of SR-B1 expression (Gutierrez-Pajares et al., 2016) thus impacting carcinogenesis and metastasis. Alternatively, microRNAs such as miR-125 and miR455 may decrease the expression of SR-B1 mRNA and protein levels in malignant tumors as they do in steroidogenic tissue (Hu et al., 2012). Consequently, all these factors (alone or together) could account for the observed variations in SR-B1 expression and its correlation with overall patient survival.
SR-B1 as a Gateway for Drug Delivery
The first definitive role for HDL in reverse cholesterol transport as a promoter of the excess cholesterol from peripheral cells to the liver was proposed by Glomset (1968). In this process, the cholesterol from the circulating HDL particles can be taken up via receptor-mediated mechanisms, either directly from HDL through SR-B1 or by the LDL receptor, subsequent to the transfer of cholesteryl esters from HDL (Besler et al., 2012). The major difference in the uptake of HDL payloads via LDL and SR-B1 receptors is that the LDL mediated operation is endocytotic (non-specific) whereas SR-B1 mediated uptake is non-endocytotic (highly specific). In the later process, the lipoprotein specifically binds to the cell surface and delivers the hydrophobic cargo directly inside the cells, without internalizing the lipoprotein shell. This mechanism bypasses the reticulo endothelial system and lysosomal exposure of the payload (including drugs) and is, therefore, preferred compared with the endocytotic process utilized by the LDL receptors for drug delivery (Bricarello et al., 2011; Ng et al., 2011; Cruz et al., 2013; McMahon et al., 2015).
Due to their unique structure of a phospholipid monolayer stabilized by a protein component, covering a firm inner hydrophobic core, lipoproteins and especially HDL, are considered as natural nanocarriers to harbor and transport hydrophobic compounds, including anti-cancer drugs to cancer cells and tumors via the SR-B1 gateway. (Counsell and Pohland, 1982; Bijsterbosch and van Berkel, 1990; Bijsterbosch et al., 1994; Firestone, 1994; Lacko et al., 2007; Bricarello et al., 2011). Silvente-Poirot and Poirot (2012) have demonstrated that carcinogenesis and tumor development have a dramatic impact on alterations in cholesterol metabolism of the cells. As a repercussion, there is an enhanced expression of SR-B1 receptors in most malignant cells (Shahzad et al., 2011) to meet their cholesterol needs for enhanced proliferative rates (Mooberry et al., 2010; Ng et al., 2011; Shahzad et al., 2011; Zheng et al., 2013). Thus the SR-B1 receptor has a great potential to be a biomarker of different types of cancers. Several studies have shown that malignant cells have an elevated requirement for cholesterol resulting in reduced HDL cholesterol levels in cancer patients, as compared with healthy subjects (Lacko et al., 2007; Ng et al., 2011). Using a quantitative real-time PCR technique, Shahzad et al. (2011) have demonstrated considerable increase in the expression of SR-B1 receptors in most tumor samples of breast, ovarian, colorectal and pancreatic cancer compared with those of normal tissues (Shahzad et al., 2011). The SR-B1 mediated process has also been shown to function in the uptake of anticancer agents from reconstituted high density lipoprotein (rHDL) by cancer cells (McConathy et al., 2008; Mooberry et al., 2010; Sabnis et al., 2012; Shen et al., 2014; Song et al., 2015). An enhanced uptake of paclitaxel from rHDL nanoparticles was demonstrated in SR-B1 overexpressing prostate cancer cells by Mooberry et al. (2010) where the SR-B1 mediated entry of the drug represented 82% of the total paclitaxel incorporation by these cells. In another study, using a construct of a synthetic HDL-gold nanoparticle template, Yang et al. (2013) showed that similar to natural HDLs, biomimetic HDL-NPs can be formulated to target SR-B1 receptors in lymphoma cells. Furthermore, by differential manipulation of SR-B1 binding and restricting cholesterol delivery they showed induction of apoptosis in lymphoma cells (Yang et al., 2013).
SR-B1 as a Conduit for the Delivery of Imaging Agents
Due to their capacity for incorporating preferentially hydrophobic compounds in their core region, lipoproteins have been well-recognized as platform for delivering theranostic agents. rHDL-like particles have been developed for imaging of atherosclerosis by contrast enhanced MRI (Frias et al., 2004). In this study, ApoA-I, extracted from human plasma, was reconstituted with commercially available phospholipids, which was coupled with a gadolinium (Gd)-chelate, making the particle visible for MRI. Furthermore, a green-emitting and amphiphilic fluorophore, 7-nitro-2-1,3-benzoxadiazol-4-yl (NBD-DPPE) was included in the lipid monolayer, to enable its detection with fluorescence techniques. MRI scans of the abdominal aorta of the apo E knock out mice injected with this preparation revealed significant accumulation of rHDL in the aortic wall. Extending this approach, Cormode et al. (2008) developed a synthetic HDL/apoA-I mimicking nanoparticle composed of lipids and an apo A-I derived, amphiphatic α-helical peptide, 37pA. These nanoparticles were formulated with Gd-chelates and a lipid-based fluorophore, rhodamine-PE, into the phospholipid layer, making the particles suitable for MRI and fluorescence imaging.
Skajaa et al. (2010) employed rHDL as a vector for delivering a variety of diagnostic agents to susceptible atherosclerotic plaques in a mouse model. They showed that by loading various types of image-enhancing compounds into either the core or surface of rHDL nanoparticles, they can be visualized by different imaging modalities (MRI, CT, optical). These findings suggest that imaging of pathological processes other than atherosclerosis may also be accomplished using rHDL as a carrier.
Fluorescence based studies reported by Shah et al. (2016) showed that the drug valrubicin has differential optical properties when encapsulated in rHDL. Moreover, valrubicin has low quantum yield in aqueous environment and it increases several fold when incorporated into rHDL nanoparticles. Further, they showed that the rHDL-valrubicin formulation could be used for confocal imaging in a SR-B1-positive prostate cancer cell line.
These studies reveal the potential of HDL type nanoparticles to deliver a cargo of theranostic agents via SR-B1 receptors; thus further emphasizing the role of the SR-B1 receptors in facilitating the delivery of imaging agents.
Cancer Therapeutics: Challenges and Novel Therapies
Many of the currently employed cancer chemotherapeutic drugs are associated with challenges, including solubility, bio-distribution and tissue non-specificity (Farokhzad and Langer, 2009). The majority of anti-cancer drugs are also non-selective in their bio-distribution as they affect all rapidly dividing cells thus disseminating the drug into both healthy as well as malignant tissues. Advances in nanotechnology and using targeted drug delivery vehicles, have shown great potential to overcome these challenges to effective chemotherapy. The therapeutic index of a drug can be significantly improved by increasing the preferential accumulation of the therapeutic agent in malignant cells while protecting healthy cells and thereby reducing toxic side effects (Rigotti et al., 2003; Foit et al., 2015; Lacko et al., 2015).
High density lipoprotein HDL-type nanocarriers exhibit many features of an ideal drug-delivery vehicle. The particles have small size, are biocompatible, non-immunogenic, have long circulating half-life and exhibit selective delivery (Zheng et al., 2005). Thus, HDL particle functionality is a primary tool for HDL-based drug delivery strategies (Kypreos et al., 2013). The potential of HDL-type nanoparticles for drug delivery was proposed by Counsell and Pohland (1982); however, systematic studies on this were first carried out by Kader and Pater (2002) after 20 years (Counsell and Pohland, 1982). They have reported that loading anticancer drugs into HDL as well as LDL had minimal effect on the properties of the complexes, while the encapsulated drugs showed enhanced cytotoxicity toward human carcinoma cells (Kader and Pater, 2002). It has been well documented that tumor tissues are characterized by a leaky vasculature and low lymphatic drainage, leading to unequal interstitial pressure between different parts of the tumor (Wu et al., 2013; Omidi and Barar, 2014). This pressure difference leads to uneven retention of particles between the center of the tumor and its periphery. HDL and HDL-like particles are believed to exhibit active targeting via SR-B1 receptors instead of utilizing passive targeting through the enhanced permeability and retention (EPR) effect (Bricarello et al., 2011; Llaverias et al., 2011; Gorin et al., 2012; Danilo et al., 2013; Huynh and Zheng, 2013).
Although numerous antineoplastic drugs have shown promise during pre-clinical studies, the success rate of these drugs clearing clinical trials is extremely low. This is primarily due to the off target effects that many of these agents exhibit against peripheral (normal) tissues2. The selective delivery of hydrophobic drugs, specifically to malignant tumors is facilitated by SR-B1 receptor, and thus makes the rHDL drug-delivery system unique and a potential enhancer of cancer therapeutics. Lou et al demonstrated a preferential cytotoxicity of the antineoplastic drug aclacinomycin encased in rHDL toward malignant cells (Lou et al., 2005). Sabnis et al. (2012) found that valrubicin when encapsulated into rHDL nanoparticles was effective 1.8 and 2.6 times lower minimum inhibitory concentrations (IC50) than the free drug against prostate and ovarian cells, respectively. The valrubicin/rHDL formulation was also found to have reduced toxicity toward non-malignant cells, demonstrating the selective tumor delivery capabilities of rHDL nanoparticles. In the past 10 years, scientists are realizing the potential of HDL and HDL mimetic drug delivery strategies in cancer theranostics, primarily due to the interaction of these nanoparticles (Figure 1) with the SR-B1 receptor (McMahon et al., 2011; Jia et al., 2012; Rui et al., 2013; Zheng et al., 2013; Dong et al., 2014; Fischer et al., 2014).
Future Opportunities for the Development of Cancer Biomarkers, Therapy and Imaging
Traditional chemotherapy has always been afflicted with long term and short term unpleasant side effects. In some cases the side effects are worse than the disease itself. It is well documented by different research groups now that malignant cells consistently overexpress SR-B1 receptors as compared to normal cells. Lipoprotein-based technologies could address the above concerns via their ability to selectively deliver their payload to cancer cells and tumors. Safety of apoA-1 containing HDL-type formulations for human systemic administration has been confirmed by numerous clinical studies (Lacko et al., 2002; Kuai et al., 2016). Additionally, these formulations have a potential to reposition several drugs that have been considered as anticancer drugs but have been disapproved due to poor solubility and excessive peripheral toxicity. This strategy could be commercially beneficial, in contrast with conventional drug- and target-screening strategies.
If determination of SR-B1 expression levels is included as a pretest before starting the chemotherapy regimen, the HDL based drug delivery could be tailored to respective cancer patients with high SR-B1 expressing tumors. Alternatively for low SR-B1 expressing patients, specific functionalized rHDL nanoparticles could be designed that will target different overexpressing tumor antigens. Thus the oncogenic drugs could be rerouted from the natural lipoproteins receptors thereby personalizing HDL-based therapeutics.
Although the concept of using lipoproteins as targeted drug-delivery agents was introduced back in 1982, and the suitability of their functional characteristics for pharmaceutical formulations was well known, there are currently no lipoprotein-based drug delivery formulations in clinical trials or in clinical use. However, considering their versatility and selective-targeting capability, biofunctional HDL-type nanoparticles are likely to bring a shift in the paradigm of next generation cancer theranostics. The SR-B1 receptor-mediated gateway of lipophilic anticancer drugs or imaging agents via HDL and HDL-mimetic nanoparticles has strengthened the potential for these preparations as theranostic agents.
Author Contributions
All authors listed, have made substantial, direct and intellectual contribution to the work, and approved it for publication.
Conflict of Interest Statement
AL is a co-inventor on two issued patents that involve SR-B1 mediated drug delivery. NS is a co-inventor on one issued patent that involves SR-B1 related drug delivery. All the other authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors’ research is being supported by Cancer Prevention and Research Institute of Texas, Wheels for Wellness. Fort Worth TX and the Rutledge Foundation.
Footnotes
References
- Acton S., Rigotti A., Landschulz K., Xu S., Hobbs H., Krieger M. (1996). Identification of scavenger receptor SR-BI as a high density lipoprotein receptor. Science 271 518–520. 10.1126/science.271.5248.518 [DOI] [PubMed] [Google Scholar]
- Alaupovic P., Lee D., McConathy W. (1972). Studies on the composition and structure of plasma lipoproteins: distribution of lipoprotein families in major density classes of normal human plasma lipoproteins. Biochim. Biophys. Acta 260 689–707. 10.1016/0005-2760(72)90018-5 [DOI] [PubMed] [Google Scholar]
- Al-Jarallah A., Trigatti B. (2010). A role for the scavenger receptor, class B type I in high density lipoprotein dependent activation of cellular signaling pathways. Biochim. Biophys. Acta 1801 1239–1248. 10.1016/j.bbalip.2010.08.006 [DOI] [PubMed] [Google Scholar]
- Arenas M., Lobo M., Caso E., Huerta L., Paniagua R., Martin-Hidalgo M. (2004). Normal and pathological human testes express hormone-sensitive lipase and the lipid receptors CLA-1/SR-BI and CD36. Hum. Pathol. 35 34–42. 10.1016/j.humpath.2003.08.015 [DOI] [PubMed] [Google Scholar]
- Assanasen C., Mineo C., Seetharam D., Yuhanna I., Marcel Y., Connelly M., et al. (2005). Cholesterol binding, efflux, and a PDZ-interacting domain of scavenger receptor–BI mediate HDL-initiated signaling. J. Clin. Invest. 115 969–977. 10.1172/JCI23858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellacosa A., Kumar C., Di Cristofano A., Testa J. (2005). Activation of AKT kinases in cancer: implications for therapeutic targeting. Adv. Cancer Res. 94 29–86. 10.1016/S0065-230X(05)94002-5 [DOI] [PubMed] [Google Scholar]
- Besler C., Lüscher T., Landmesser U. (2012). Molecular mechanisms of vascular effects of High-density lipoprotein: alterations in cardiovascular disease. EMBO Mol. Med. 4 251–268. 10.1002/emmm.201200224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bijsterbosch M., Schouten D., van Berkel T. (1994). Synthesis of the dioleoyl derivative of iodo deoxyuridine and its incorporation in to reconstituted high density lipoprotein particles. Biochemistry 33 14073–14080. 10.1021/bi00251a016 [DOI] [PubMed] [Google Scholar]
- Bijsterbosch M., van Berkel T. (1990). Native and modified lipoproteins as drug delivery systems. Adv.Drug Deliv. Rev. 5 231–251. 10.1016/0169-409X(90)90018-N [DOI] [PubMed] [Google Scholar]
- Bild A., Yao G., Chang J., Wang Q., Potti A., Chasse D., et al. (2006). Oncogenic pathway signatures in human cancers as a guide to targeted therapies. Nature 439 353–357. 10.1038/nature04296 [DOI] [PubMed] [Google Scholar]
- Bravi F., Scotti L., Bosetti C., Talamini R., Negri E., Montella M., et al. (2006). Self-reported history of hypercholesterolaemia and gallstones and the risk of prostate cancer. Ann. Oncol. 17 1014–1017. 10.1093/annonc/mdl080 [DOI] [PubMed] [Google Scholar]
- Bricarello D., Smilowitz J., Zivkovic A., German J., Parikh A. (2011). Reconstituted lipoprotein: a versatile class of biologically inspired nanostructures. ACS Nano 5 42–57. 10.1021/nn103098m [DOI] [PubMed] [Google Scholar]
- Calvo D., Gómez-Coronado D., Lasunción M., Vega M. (1997). CLA-1 is an 85-kD plasma membrane glycoprotein that acts as a high- affinity receptor for both native (HDL, LDL, and VLDL) and modified (OxLDL and AcLDL) lipoproteins. Arterioscler. Thromb. Vasc. Biol. 17 2341–2349. 10.1161/01.ATV.17.11.2341 [DOI] [PubMed] [Google Scholar]
- Cao W., Murao K., Imachi H., Yu X., Abe H., Yamauchi A., et al. (2004). A mutant high-density lipoprotein receptor inhibits proliferation of human breast cancer cells. Cancer Res. 64 1515–1521. 10.1158/0008-5472.CAN-03-0675 [DOI] [PubMed] [Google Scholar]
- Chen Y., Hughes-Fulford M. (2001). Human prostate cancer cells lack feedback regulation of low-density lipoprotein receptor and its regulator, SREBP2. Int. J. Cancer 91 41–45. [DOI] [PubMed] [Google Scholar]
- Connelly M. (2009). SR-BI-mediated HDL cholesteryl ester delivery in the adrenal gland. Mol. Cell Endocrinol. 300 83–88. 10.1016/j.mce.2008.09.011 [DOI] [PubMed] [Google Scholar]
- Connelly M., De la Llera-Moya M., Monzo P., Yancey P., Drazul D., Stoudt G., et al. (2001). Analysis of chimeric receptors shows that multiple distinct functional activities of scavenger receptor, class B, type I (SR-BI), are localized to the extracellular receptor domain. Biochemistry 40 5249–5259. 10.1021/bi002825r [DOI] [PubMed] [Google Scholar]
- Connelly M., Williams D. (2004). SR-BI and HDL cholesteryl ester metabolism. Endocr. Res. 30 697–703. 10.1081/ERC-200043979 [DOI] [PubMed] [Google Scholar]
- Cormode D., Briley-Saebo K., Mulder W., Aguinaldo J., Barazza A., Ma Y., et al. (2008). An ApoA-I mimetic peptide high-density-lipoprotein-based MRI contrast agent for atherosclerotic plaque composition detection. Small 4 1437–1444. 10.1002/smll.200701285 [DOI] [PubMed] [Google Scholar]
- Counsell R., Pohland R. (1982). Lipoproteins as potential site-specific delivery systems for diagnostic and therapeutic agents. J. Med. Chem. 25 111–125. 10.1021/jm00352a001 [DOI] [PubMed] [Google Scholar]
- Cruz P., Mo H., McConathy W., Sabnis N., Lacko A. (2013). The role of cholesterol metabolism and cholesterol transport in carcinogenesis: a review of scientific findings, relevant to future cancer therapeutics. Front. Pharmacol. 4:119 10.3389/fphar.2013.00119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danilo C., Gutierrez-Pajares J., Mainieri M., Mercier I., Lisanti M., Frank P. (2013). Scavenger receptor class B type I regulates cellular cholesterol metabolism and cell signaling associated with breast cancer development. Breast Cancer Res. 15 5 10.1186/bcr3483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Gonzalo-Calvo D., López-Vilaró L., Nasarre L., Perez-Olabarria M., Vázquez T., Escuin D., et al. (2015). Intratumor cholesteryl ester accumulation is associated with human breast cancer proliferation and aggressive potential: a molecular and clinicopathological study. BMC Cancer 15:460 10.1186/s12885-015-1469-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y., Love K., Dorkin J., Sirirungruang S., Zhang Y., Chen D., et al. (2014). Lipopeptide nanoparticles for potent and selective siRNA delivery in rodents and nonhuman primates. Proc. Natl. Acad. Sci. U.S.A. 111 3955–3960. 10.1073/pnas.1322937111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farokhzad O., Langer R. (2009). Impact of nanotechnology on drug delivery. ACS Nano 3 16–20. 10.1021/nn900002m [DOI] [PubMed] [Google Scholar]
- Fidge N. (1999). High density lipoprotein receptors, binding proteins, and ligands. J. Lipid Res. 40 187–201. [PubMed] [Google Scholar]
- Fiorenza A., Branchi A., Sommariva D. (2000). Serum lipoprotein profile in patients with cancer. A comparison with non-cancer subjects. Int. J. Clin. Lab. Res. 30 141–145. 10.1007/s005990070013 [DOI] [PubMed] [Google Scholar]
- Firestone R. (1994). Low-density lipoprotein as a vehicle for targeting antitumor compounds to cancer cells. Bioconjug. Chem. 5 105–113. 10.1021/bc00026a002 [DOI] [PubMed] [Google Scholar]
- Fischer N., Weilhammer D., Dunkle A., Thomas C., Hwang M., Corzet M., et al. (2014). Evaluation of nanolipoprotein particles (NLPs) as an in vivo delivery platform. PLoS ONE 9:3 10.1371/journal.pone.0093342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foit L., Giles F., Gordon L., Thaxton C. (2015). Synthetic high-density lipoprotein-like nanoparticles for cancer therapy. Exp. Rev. Anticancer Ther. 15 27–34. 10.1586/14737140.2015.990889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frias J., Williams K., Fisher E., Fayad Z. (2004). Recombinant HDL-like nanoparticles: a specific contrast agent for MRI of atherosclerotic plaques. J. Am. Chem. Soc. 126 16316–16317. 10.1021/ja044911a [DOI] [PubMed] [Google Scholar]
- Glomset J. (1968). The plasma lecithins: cholesterol acyltransferase reaction. J. Lipid Res. 9 155–167. [PubMed] [Google Scholar]
- Gorin A., Gabitova L., Astsaturov I. (2012). Regulation of cholesterol biosynthesis and cancer signaling. Curr. Opin. Pharmacol. 12 710–716. 10.1016/j.coph.2012.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gospodarowicz D., Lui G., Gonzalez R. (1982). High-density lipoproteins and the proliferation of human tumor cells maintained on extracellular matrix-coated dishes and exposed to defined medium. Cancer Res. 42 3704–3713. [PubMed] [Google Scholar]
- Gutierrez-Pajares J., Ben Hassen C., Chevalier S., Frank P. (2016). SR-BI: linking cholesterol and lipoprotein metabolism with breast and prostate cancer. Front. Pharmacol. 7:338 10.3389/fphar.2016.00338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton R., Williams M., Fielding C., Havel R. (1976). Discoidal bilayer structure of nascent high density lipoproteins from perfused rat liver. J. Clin. Invest. 58 667–680. 10.1172/JCI108513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanamura I., Huang Y., Zhan F., Barlogie B., Shaughnessy J. (2006). Prognostic value of cyclin D2 mRNA expression in newly diagnosed multiple myeloma treated with high-dose chemotherapy and tandem autologous stem cell transplantations. Leukemia 20 1288–1290. 10.1038/sj.leu.2404253 [DOI] [PubMed] [Google Scholar]
- Hoekstra M., Ye D., Hildebrand R., Zhao Y., Lammers B., Stitzinger M., et al. (2009). Scavenger receptor class B type I-mediated uptake of serum cholesterol is essential for optimal adrenal glucocorticoid production. J. Lipid Res. 50 1039–1046. 10.1194/jlr.M800410-JLR200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J., Zhang Z., Shen W., Nomoto A., Azhar S. (2011). Differential roles of cysteine residues in the cellular trafficking, dimerization, and function of the high-density lipoprotein receptor, SR-BI. Biochemistry 50 10860–10875. 10.1021/bi201264y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Z., Shen W., Kraemer F., Azhar S. (2012). MicroRNAs 125a and 455 repress lipoprotein-Supported steroidogenesis by targeting scavenger receptor class B Type I in steroidogenic cells. Mol. Cell. Biol. 32 5035–5045. 10.1128/MCB.01002-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh E., Zheng G. (2013). Engineering multifunctional nanoparticles: all-in-one versus one-for-all. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 5 250–265. 10.1002/wnan.1217 [DOI] [PubMed] [Google Scholar]
- Jia J., Xiao Y., Liu J., Zhang W., He H., Chen L., et al. (2012). Preparation, characterizations, and in vitro metabolic processes of paclitaxel-loaded discoidal recombinant high-density lipoproteins. J. Pharm. Sci. 101 2900–2908. 10.1002/jps.23210 [DOI] [PubMed] [Google Scholar]
- Johnson W., Mahlberg F., Rothblat G., Phillips M. (1991). Cholesterol transport between cells and high-density lipoproteins. Biochim. Biophys. Acta 1085 273–298. 10.1016/0005-2760(91)90132-2 [DOI] [PubMed] [Google Scholar]
- Jozan S., Faye J., Tournier J., Tauber J., David J., Bayard F. (1985). Interaction of estradiol and high density lipoproteins on proliferation of the human breast cancer cell line MCF-7 adapted to grow in serum free conditions. Biochem. Biophys. Res. Commun. 133 105–112. 10.1016/0006-291X(85)91847-9 [DOI] [PubMed] [Google Scholar]
- Kader A., Pater A. (2002). Loading anticancer drugs into HDL as well as LDL has little effect on properties of complexes and enhances cytotoxicity to human carcinoma cells. J. Control. Rel. 80 29–44. 10.1016/S0168-3659(01)00536-3 [DOI] [PubMed] [Google Scholar]
- Karar J., Maity A. (2011). PI3K/AKT/mTOR Pathway in Angiogenesis. Front. Mol. Neurosci. 4:51 10.3389/fnmol.2011.00051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer F. (2007). Adrenal cholesterol utilization. Mol. Cell Endocrinol. 26 42–45. 10.1016/j.mce.2006.12.001 [DOI] [PubMed] [Google Scholar]
- Krieger M. (1999). Charting the fate of the ‘good cholesterol’: identification and characterization of the high-density lipoprotein receptor SR-BI. Annu. Rev. Biochem. 68 523–558. 10.1146/annurev.biochem.68.1.523 [DOI] [PubMed] [Google Scholar]
- Kuai R., Li D., Chen E., Moon J., Schwendeman A. (2016). High-density lipoproteins (HDL)–nature’s multi-functional nanoparticles. ACS Nano 10 3015–3041. 10.1021/acsnano.5b07522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuijjer M., Rydbeck H., Kresse S., Buddingh E., Lid A., Roelofs H., et al. (2012). Identification of osteosarcoma driver genes by integrative analysis of copy number and gene expression data. Genes Chromosomes Cancer 51 696–706. 10.1002/gcc.21956 [DOI] [PubMed] [Google Scholar]
- Kypreos K., Gkizas S., Rallidis L., Karagiannides I. (2013). HDL particle functionality as a primary pharmacological target for HDL-based therapies. Biochem. Pharmacol. 85 1575–1578. 10.1016/j.bcp.2013.03.004 [DOI] [PubMed] [Google Scholar]
- Lacko A., Nair M., Paranjape S., Johnson S., McConathy W. (2002). High density lipoprotein complexes as delivery vehicles for anticancer drugs. Anticancer Res. 22 2045–2049. [PubMed] [Google Scholar]
- Lacko A., Nair M., Prokai L., McConathy W. (2007). Prospects and challenges of the development of lipoprotein-based formulations for anti-cancer drugs. Exp. Opin. Drug Deliv. 4 665–675. 10.1517/17425247.4.6.665 [DOI] [PubMed] [Google Scholar]
- Lacko A., Sabnis A., Nagarajan B., McConathy W. (2015). HDL as a drug and nucleic acid delivery vehicle. Front. Pharmacol. 6:247 10.3389/fphar.2015.00247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landschulz K., Pathak R., Rigotti A., Krieger M., Hobbs H. (1996). Regulation of scavenger receptor, class B, Type I, a high density lipoprotein receptor, in liver and steroidogenic tissues of the rat. J. Clin. Invest. 15 984–995. 10.1172/JCI118883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon C., Locke J., Adomat H., Etinger S., Twiddy A., Neumann R., et al. (2010). Alterations in cholesterol regulation contribute to the production of intratumoral androgens during progression to castration-resistant prostate cancer in a mouse xenograft model. Prostate 70 390–400. 10.1002/pros.21072 [DOI] [PubMed] [Google Scholar]
- Li J., Wang J., Li M., Yin L., Li X., Zhang T. (2016). Up-regulated expression of scavenger receptor class B type 1 (SR-B1) is associated with malignant behaviors and poor prognosis of breast cancer. Path. Res. Pract. 212 555–559. 10.1016/j.prp.2016.03.011 [DOI] [PubMed] [Google Scholar]
- Lisowska K., Olbryt M., Dudaladava V., Pamula-Pilat J., Kujawa K., Grzybowska E., et al. (2014). Gene expression analysis in ovarian cancer - faults and hints from DNA microarray study. Front. Oncol. 4:6 10.3389/fonc.2014.00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llaverias G., Danilo C., Mercier I., Daumer K., Capozza F., Williams T., et al. (2011). Role of cholesterol in the development and progression of breast cancer. Am. J. Pathol. 178 402–412. 10.1016/j.ajpath.2010.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llaverias G., Danilo C., Wang Y., Witkiewicz A., Daumer K., Lisanti M., et al. (2010). A western-type diet accelerates tumor progression in an autochthonous mouse model of prostate cancer. Am. J. Pathol. 177 3180–3191. 10.2353/ajpath.2010.100568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez D., McLean M. (1999). Sterol regulatory element-binding protein-1a binds to cis elements in the promoter of the rat high density lipoprotein receptor SR-BI gene. Endocrinology 140 5669–5681. 10.1210/en.140.12.5669 [DOI] [PubMed] [Google Scholar]
- Lou B., Liao X., Wu M., Cheng P., Yin C., Fei Z. (2005). High-density lipoprotein as a potential carrier for delivery of a lipophilic antitumoral drug into hepatoma cells. World J. Gastroenterol. 11 954–959. 10.3748/wjg.v11.i7.954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin G., Pilon A., Albert C., Valle M., Hum D. W., Fruchart J., et al. (1999). Comparison of expression and regulation of the high-density lipoprotein receptor SR-BI and the low-density lipoprotein receptor in human adrenocortical carcinoma NCI-H295 cells. Eur. J. Biochem. 261 481–491. 10.1046/j.1432-1327.1999.00296.x [DOI] [PubMed] [Google Scholar]
- McConathy W., Nair M., Paranjape S., Mooberry L., Lacko A. (2008). Evaluation of synthetic/reconstituted high-density lipoproteins as delivery vehicles for paclitaxel. Anticancer Drugs 19 183–188. 10.1097/CAD.0b013e3282f1da86 [DOI] [PubMed] [Google Scholar]
- McGrath K., Li X., Puranik R., Liong E., Tan J., Dy V., et al. (2009). Role of 3β-hydroxysteroid-Δ24 reductase in mediating antiinflammatory effects of high-density lipoproteins in endothelial cells. Arterioscler. Thromb. Vasc. Biol. 29 877–882. 10.1161/ATVBAHA.109.184663 [DOI] [PubMed] [Google Scholar]
- McMahon K., Foit L., Angeloni N., Giles F., Gordon L., Thaxton C. (2015). Synthetic high-density lipoprotein-like nanoparticles as cancer therapy. Cancer Treat. Res. 166 129–150. 10.1007/978-3-319-16555-4_6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon K. M., Mutharasan R. K., Tripathy S., Veliceasa D., Bobeica M., Shumaker D. K., et al. (2011). Biomimetic high density lipoprotein nanoparticles for nucleic acid delivery. Nano Lett. 11 1208–1214. 10.1021/nl1041947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller N., La Ville A., Crook D. (1985). Direct evidence that reverse cholesterol transport is mediated by high-density lipoprotein in rabbit. Nature 314 109–111. 10.1038/314109a0 [DOI] [PubMed] [Google Scholar]
- Miura S., Fujino M., Matsuo Y., Kawamura A., Tanigawa H., Nishikawa H., et al. (2003). High density lipoprotein-induced angiogenesis requires the activation of Ras/MAP kinase in human coronary artery endothelial cells. Arterioscler. Thromb. Vasc. Biol. 23 802–808. 10.1161/01.ATV.0000066134.79956.58 [DOI] [PubMed] [Google Scholar]
- Molenaar J., Koster J., Zwijnenburg D., van Sluis P., Valentijn L. J., van der Ploeg I., et al. (2012). Sequencing of neuroblastoma identifies chromothripsis and defects in neuritogenesis genes. Nature 483 589–593. 10.1038/nature10910 [DOI] [PubMed] [Google Scholar]
- Mooberry L., Nair M., Paranjape S., McConathy W., Lacko A. (2010). Receptor mediated uptake of paclitaxel from a synthetic high density lipoprotein nanocarrier. J. Drug Target. 18 53–58. 10.3109/10611860903156419 [DOI] [PubMed] [Google Scholar]
- Muntoni S., Atzori L., Mereu R., Satta G., Macis M., Congia M., et al. (2009). Serum lipoproteins and cancer. Nutr. Metab. Cardiovasc. Dis. 19 218–225. 10.1016/j.numecd.2008.06.002 [DOI] [PubMed] [Google Scholar]
- Navab M., Berliner J., Subbanagounder G., Hama S., Lusis A., Castellani L. W., et al. (2001). HDL and the inflammatory response induced by LDL-derived oxidized phospholipids. Arterioscler. Thromb. Vasc. Biol. 21 481–488. 10.1161/01.ATV.21.4.481 [DOI] [PubMed] [Google Scholar]
- Navab M., Hama S., Anantharamaiah G., Hassan K., Hough G. P., Watson A. D., et al. (2000a). Normal high density lipoprotein inhibits three steps in the formation of mildly oxidized low density lipoprotein: steps 2 and 3. J. Lipid Res. 41 1495–1508. [PubMed] [Google Scholar]
- Navab M., Hama S., Cooke C., Anantharamaiah G., Chaddha M., Jin L., et al. (2000b). Normal high density lipoprotein inhibits three steps in the formation of mildly oxidized low density lipoprotein: step 1. J. Lipid Res. 41 1481–1494. [PubMed] [Google Scholar]
- Navab M., Imes S., Hama S., Hough G., Ross L., Bork R., et al. (1991). Monocyte transmigration induced by modification of low density lipoprotein in cocultures of human aortic wall cells is due to induction of monocyte chemotactic protein 1 synthesis and is abolished by high density lipoprotein. J. Clin. Invest. 88 2039–2046. 10.1172/JCI115532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng K., Lovell J., Zheng G. (2011). Lipoprotein inspired nanoparticles for cancer theranostics. Acc. Chem. Res. 44 1105–1113. 10.1021/ar200017e [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omidi Y., Barar J. (2014). Targeting tumor microenvironment: crossing tumor interstitial fluid by multifunctional nanomedicines. Bioimpacts 4 55–67. 10.5681/bi.2014.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagler T., Rhode S., Neuhofer A., Laggner H., Strobl W., Hinterndorfer C., et al. (2006). SR-BI-mediated high density lipoprotein (HDL) endocytosis leads to HDL resecretion facilitating cholesterol efflux. J. Biol. Chem. 281 11193–11204. 10.1074/jbc.M510261200 [DOI] [PubMed] [Google Scholar]
- Pan B., Ren H., He Y., Lv X., Ma Y., Li J., et al. (2012a). HDL of patients with type 2 diabetes mellitus elevates the capability of promoting breast cancer metastasis. Clin. Cancer Res. 18 1246–1256. 10.1158/1078-0432.CCR-11.0817 [DOI] [PubMed] [Google Scholar]
- Pan B., Ren H., Lv X., Zhao Y., Yu B., He Y., et al. (2012b). Hypochlorite-induced oxidative stress elevates the capability of HDL in promoting breast cancer metastasis. J. Transl. Med. 10 65 10.1186/1479-5876-10-65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawitan Y., Bjohle J., Amler L., Borg A., Egyhazi S., Hall P., et al. (2005). Gene expression profiling spares early breast cancer patients from adjuvant therapy: derived and validated in two population-based cohorts. Breast Cancer Res. 7 6 10.1186/bcr1325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pussinen P., Karten B., Wintersperger A., Reicher H., McLean M., Malle E., et al. (2000). The human breast carcinoma cell line HBL-100 acquires exogenous cholesterol from high-density lipoprotein via CLA-1 (CD-36 and LIMPII analogous 1)-mediated selective cholesteryl ester uptake. Biochem. J. 349 559–566. 10.1042/0264-6021:3490559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhainds D., Brissette L. (2004). The role of scavenger receptor class B type I (SR-BI) in lipid trafficking: defining the rules for lipid traders. Int. J. Biochem. Cell Biol. 36 39–77. 10.1016/S1357-2725(03)00173-0 [DOI] [PubMed] [Google Scholar]
- Rigotti A., Miettinen H., Krieger M. (2003). The role of the high-density lipoprotein receptor SR-BI in the lipid metabolism of endocrine and other tissues. Endocr. Rev. 24 357–387. 10.1210/er.2001-0037 [DOI] [PubMed] [Google Scholar]
- Rodrigueza W., Thuahnai S., Temel R., Lund-Katz S., Phillips M., Williams D. (1999). Mechanism of scavenger receptor class B type I-mediated selective uptake of cholesteryl esters from high density lipoprotein to adrenal cells. J. Biol. Chem. 274 20344–20350. 10.1074/jbc.274.29.20344 [DOI] [PubMed] [Google Scholar]
- Rohrl C., Stangl H. (2013). HDL endocytosis and resecretion. Biochim. Biophys. Acta 1831 1626–1633. 10.1016/j.bbalip.2013.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose G., Blackburn H., Keys A., Taylor H., Kannel W., Paul O. (1974). Colon cancer and blood-cholesterol. Lancet 303 181–183. 10.1016/S0140-6736(74)92492-1 [DOI] [PubMed] [Google Scholar]
- Rotheneder M., Kostner G. (1989). Effects of low- and high-density lipoproteins on the proliferation of human breast cancer cells in vitro: differences between hormone-dependent and hormone-independent cell lines. Int. J. Cancer 43 875–879. 10.1002/ijc.2910430523 [DOI] [PubMed] [Google Scholar]
- Rui M., Tang H., Li Y., Wei X., Xu Y. (2013). Recombinant High Density Lipoprotein Nanoparticles for Target-Specific Delivery of siRNA. Pharm. Res. 30 1203–1214. 10.1007/s11095-012-0957-4 [DOI] [PubMed] [Google Scholar]
- Sabnis N., Nair M., Israel M., McConathy W., Lacko A. (2012). Enhanced solubility and functionality of valrubicin (AD-32) against cancer cells upon encapsulation into biocompatible nanoparticles. Int. J. Nanomed. 7 1–9. 10.2147/IJN.S28029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saddar S., Carriere V., Lee W., Tanigaki K., Yuhanna I., Parathath S., et al. (2013). Scavenger receptor class B type I is a plasma membrane cholesterol sensor. Circ. Res. 112 140–151. 10.1161/CIRCRESAHA.112.280081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saddar S., Mineo C., Shaul P. (2010). Signaling by the high-affinity HDL receptor scavenger receptor B type I. Arterioscler. Thromb. Vasc. Biol. 30 144–150. 10.1161/ATVBAHA.109.196170 [DOI] [PubMed] [Google Scholar]
- Scanu A. (1972). Structural studies on serum lipoproteins. Biochim. Biophys. Acta Rev. Biomembr. 265 471–508. 10.1016/0304-4157(72)90006-8 [DOI] [Google Scholar]
- Scanu A., Wisdom C. (1972). Serum lipoproteins structure and function. Annu. Rev. Biochem. 41 703–730. 10.1146/annurev.bi.41.070172.003415 [DOI] [PubMed] [Google Scholar]
- Schmitz G., Mollers C. (1994). Analysis of lipoproteins with analytical capillary isotachophoresis. Electrophoresis 15 31–39. 10.1002/elps.1150150106 [DOI] [PubMed] [Google Scholar]
- Schörghofer D., Kinslechner K., Preitschopf A., Schütz B., Röhrl C., Hengstschläger M., et al. (2015). The HDL receptor SR-BI is associated with human prostate cancer progression and plays a possible role in establishing androgen independence. Reprod. Biol. Endocrinol. 13 88 10.1186/s12958-015-0087-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seetharam D., Mineo C., Gormley A., Gibson L., Vongpatanasin W., Chambliss K., et al. (2006). High-density lipoprotein promotes endothelial cell migration and reendothelialization via scavenger receptor-B type I. Circ. Res. 98 63–72. 10.1161/01.RES.0000199272.59432.5b [DOI] [PubMed] [Google Scholar]
- Shah F., Shukla S., Shah P., Patel H., Patel P. (2008). Significance of alterations in plasma lipid profile levels in breast cancer. Integr. Cancer Ther. 7 33–41. 10.1177/1534735407313883 [DOI] [PubMed] [Google Scholar]
- Shah S., Chib R., Raut S., Bermudez J., Sabnis N., Duggal D., et al. (2016). Photophysical characterization of anticancer drug valrubicin in rHDL nanoparticles and its use as an imaging agent. J. Photochem. Photobiol. B. 155 60–65. 10.1016/j.jphotobiol.2015.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahzad M., Mangala L., Han H., Lu C., Bottsford-Miller J., Nishimura M., et al. (2011). Targeted delivery of small interfering RNA using reconstituted high-density lipoprotein nanoparticles. Neoplasia 13 309–319. 10.1593/neo.101372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W., Hua J., Hua Z., Kraemer F., Azhar S. (2014). Scavenger receptor class B type I (SR-BI): a versatile receptor with multiple functions and actions. Metabolism 63 875–886. 10.1016/j.metabol.2014.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu H., Ross R., Bernstein L., Yatani R., Henderson B., Mack T. (1991). Cancers of the prostate and breast among Japanese and white immigrants in Los Angeles County. Br. J. Cancer 63 963–966. 10.1038/bjc.1991.210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvente-Poirot S., Poirot M. (2012). Cholesterol metabolism and cancer: the good, the bad and the ugly. Curr. Opin. Pharmacol. 12 673–676. 10.1016/j.coph.2012.10.004 [DOI] [PubMed] [Google Scholar]
- Sjodahl G., Lauss M., Lovgren K., Chebil G., Gudjonsson S., Veerla S., et al. (2012). A molecular taxonomy for urothelial carcinoma. Clin. Cancer Res. 18 3377–3386. 10.1158/1078-0432.CCR-12-0077-T [DOI] [PubMed] [Google Scholar]
- Skajaa T., Cormode D., Falk E., Mulder W., Fisher E., Fayad Z. (2010). High density lipoprotein-based contrast agents for multimodal imaging of atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 30 169–176. 10.1161/ATVBAHA.108.179275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song G., Kim S., Park K., Kim J., Choi I., Cho K. (2015). SR-B1 mediates high density lipoprotein (HDL)-induced anti-inflammatory effect in macrophages. Biochem. Biophys. Res. Commun. 457 112–118. 10.1016/j.bbrc.2014.12.028 [DOI] [PubMed] [Google Scholar]
- Sun B., Eckhardt E., Shetty S., Van Der Westhuyzen D., Webb N. (2006). Quantitative analysis of SR-BI-dependent HDL retroendocytosis in hepatocytes and fibroblasts. J. Lipid Res. 47 1700–1713. 10.1194/jlr.M500450-JLR200 [DOI] [PubMed] [Google Scholar]
- Tan J., Prosser H., Vanags L., Monger S., Ng M., Bursill C. (2014). High-density lipoproteins augment hypoxia-induced angiogenesis via regulation of post-translational modulation of hypoxia-inducible factor 1α. FASEB J. 28 206–217. 10.1096/fj.13-233874 [DOI] [PubMed] [Google Scholar]
- Twiddy A., Cox M., Wasan K. (2012). Knockdown of scavenger receptor class B type I reduces prostate specific antigen secretion and viability of prostate cancer cells. Prostate 72 955–965. 10.1002/pros.21499 [DOI] [PubMed] [Google Scholar]
- Wu H., Hsieh J., Gleave M., Brown N., Pathak S., Chung L. (1994). Derivation of androgen-independent human LNCaP prostatic cancer cell sublines: role of bone stromal cells. Int. J. Cancer 57 406–412. 10.1002/ijc.2910570319 [DOI] [PubMed] [Google Scholar]
- Wu M., Frieboes H., McDougall S., Chaplain M., Cristini V., Lowengrub J. (2013). The effect of interstitial pressure on tumor growth: coupling with the blood and lymphatic vascular systems. J. Theor. Biol. 320 131–151. 10.1016/j.jtbi.2012.11.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S., Damiano M., Zhang H., Tripathy S., Luthi A., Tink J., et al. (2013). Biomimetic, synthetic HDL nanostructures for lymphoma. Proc. Natl. Acad. Sci. U.S.A. 110 2511–2516. 10.1073/pnas.1213657110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan B., Wu C., Wang X., Wang D., Liu H., Guo L., et al. (2016). High scavenger receptor class B type I expression is related to tumor aggressiveness and poor prognosis in breast cancer. Tumour Biol. 37 3581–3588. 10.1007/s13277-015-4141-4 [DOI] [PubMed] [Google Scholar]
- Yue S., Li J., Lee S., Lee H., Shao T., Song B., et al. (2014). Cholesteryl ester accumulation induced by PTEN loss and PI3K/AKT activation underlies human prostate cancer aggressiveness. Cell. Metab. 19 393–406. 10.1016/j.cmet.2014.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng G., Chen J., Li H., Glickson J. (2005). Rerouting lipoprotein nanoparticles to selected alternate receptors for the targeted delivery of cancer diagnostic and therapeutic agents. Proc. Natl Acad. Sci. U.S.A. 102 17757–17762. 10.1073/pnas.0508677102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y., Liu Y., Jin H., Pan S., Qian Y., Huang C., et al. (2013). Scavenger receptor B1 is a potential biomarker of human nasopharyngeal carcinoma and its growth is inhibited by HDL-mimetic nanoparticles. Theranostics 3 477–486. 10.7150/thno.6617 [DOI] [PMC free article] [PubMed] [Google Scholar]


