Abstract
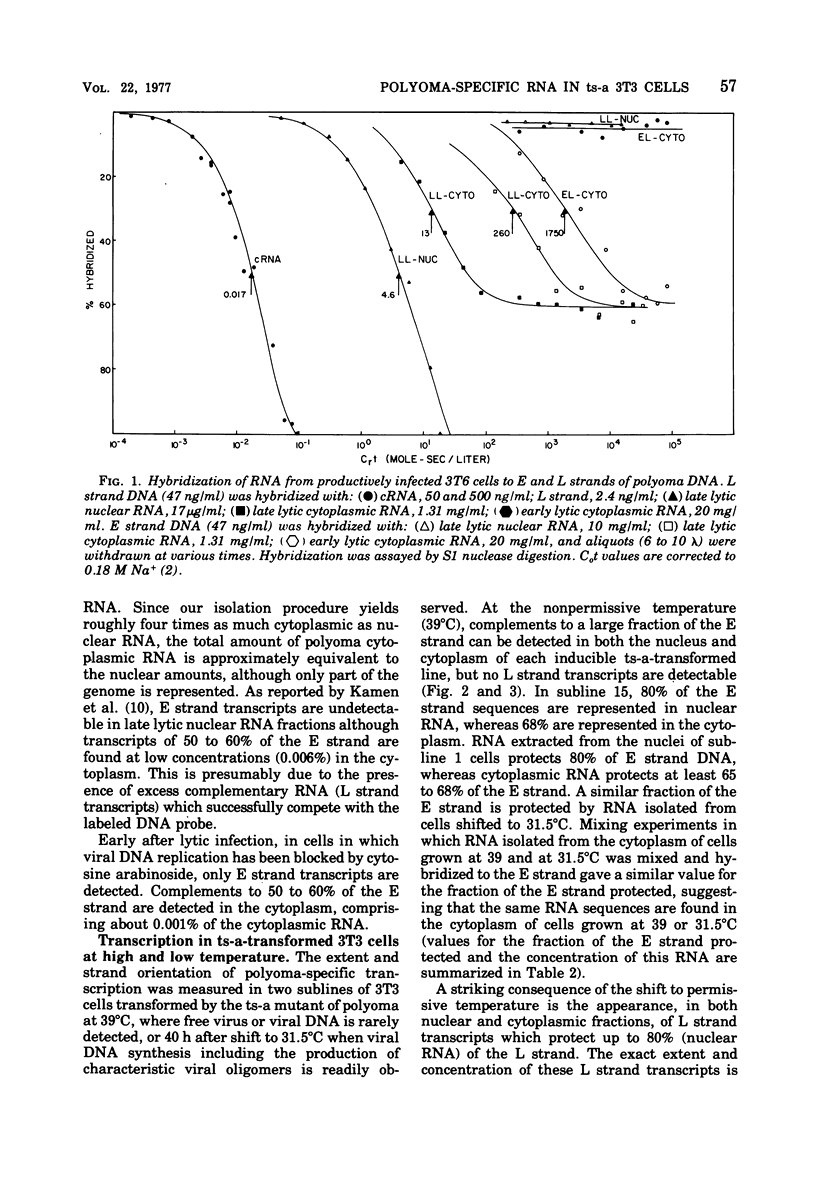
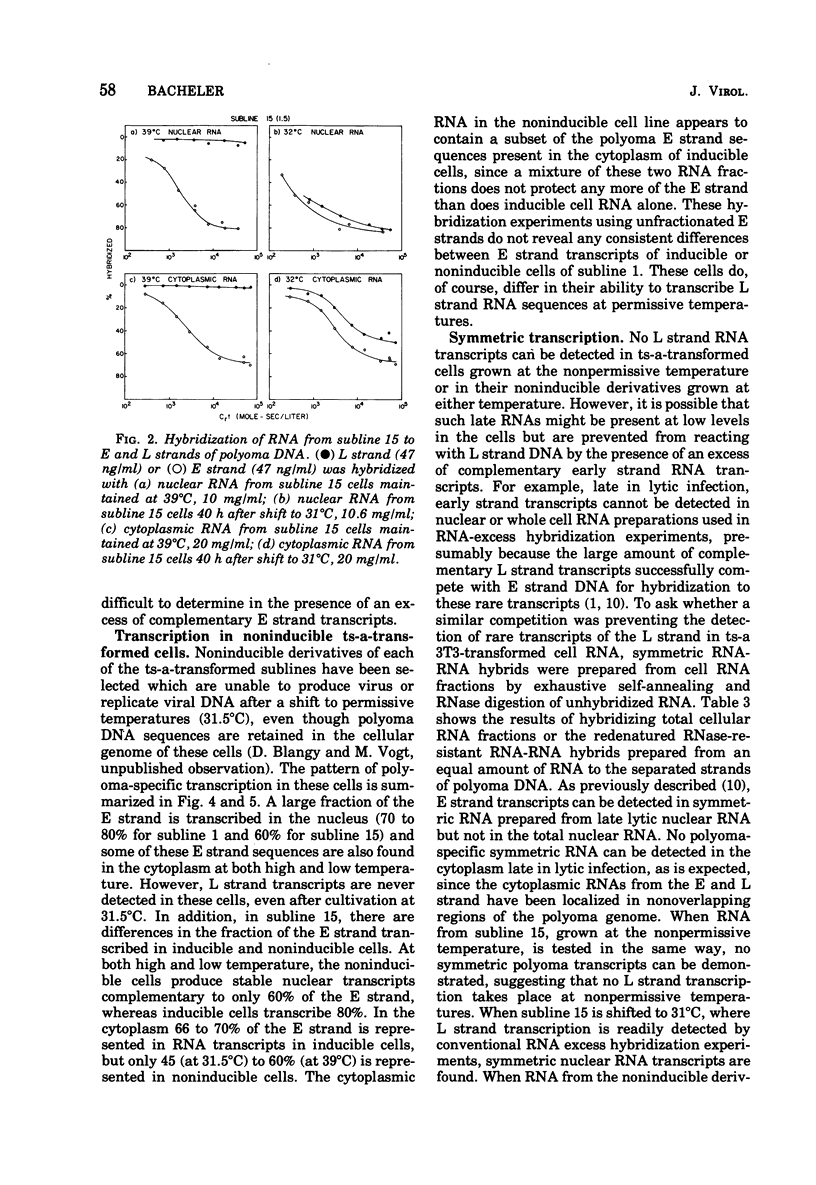
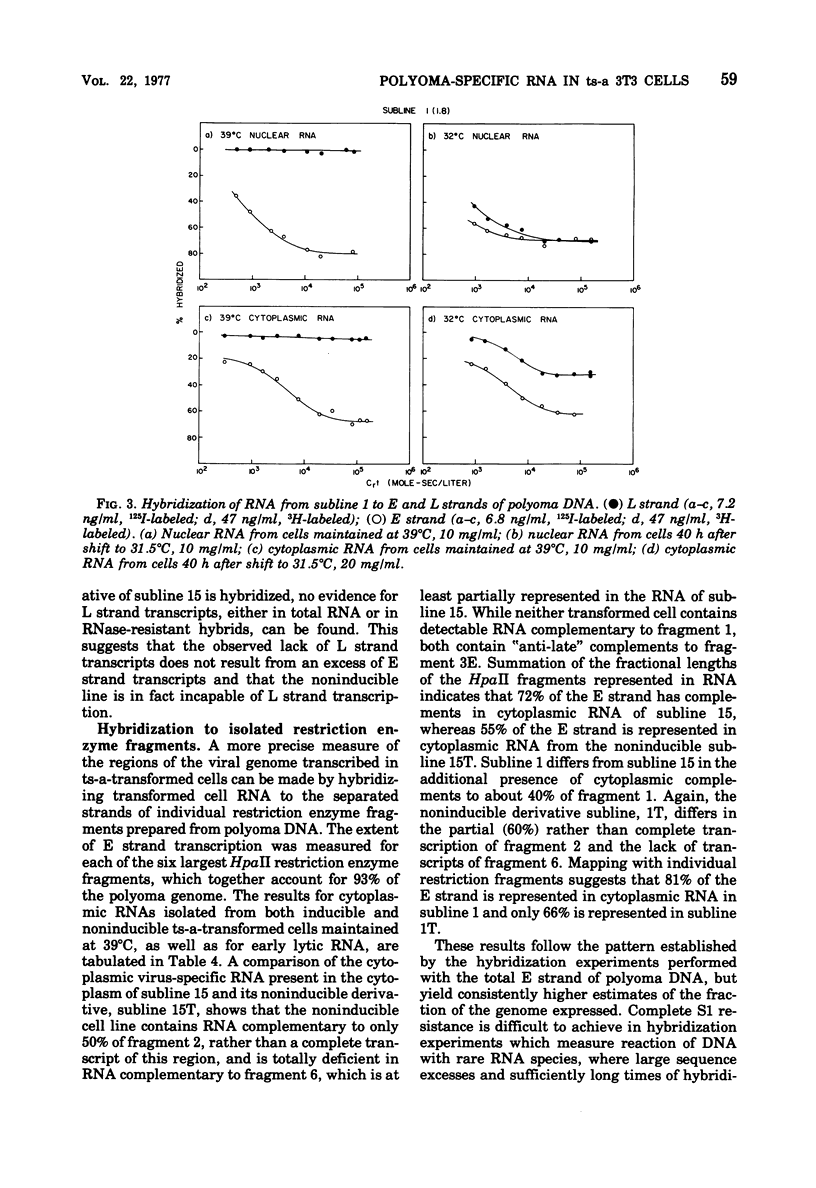
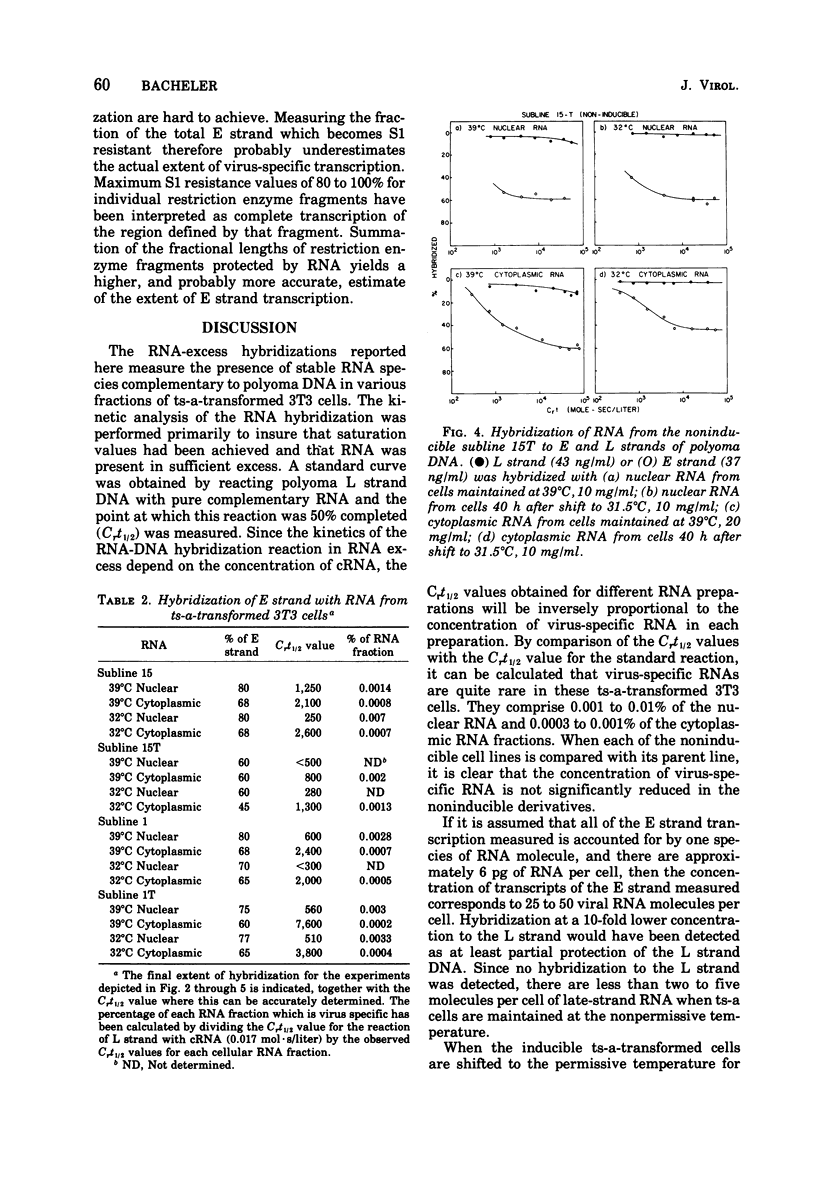
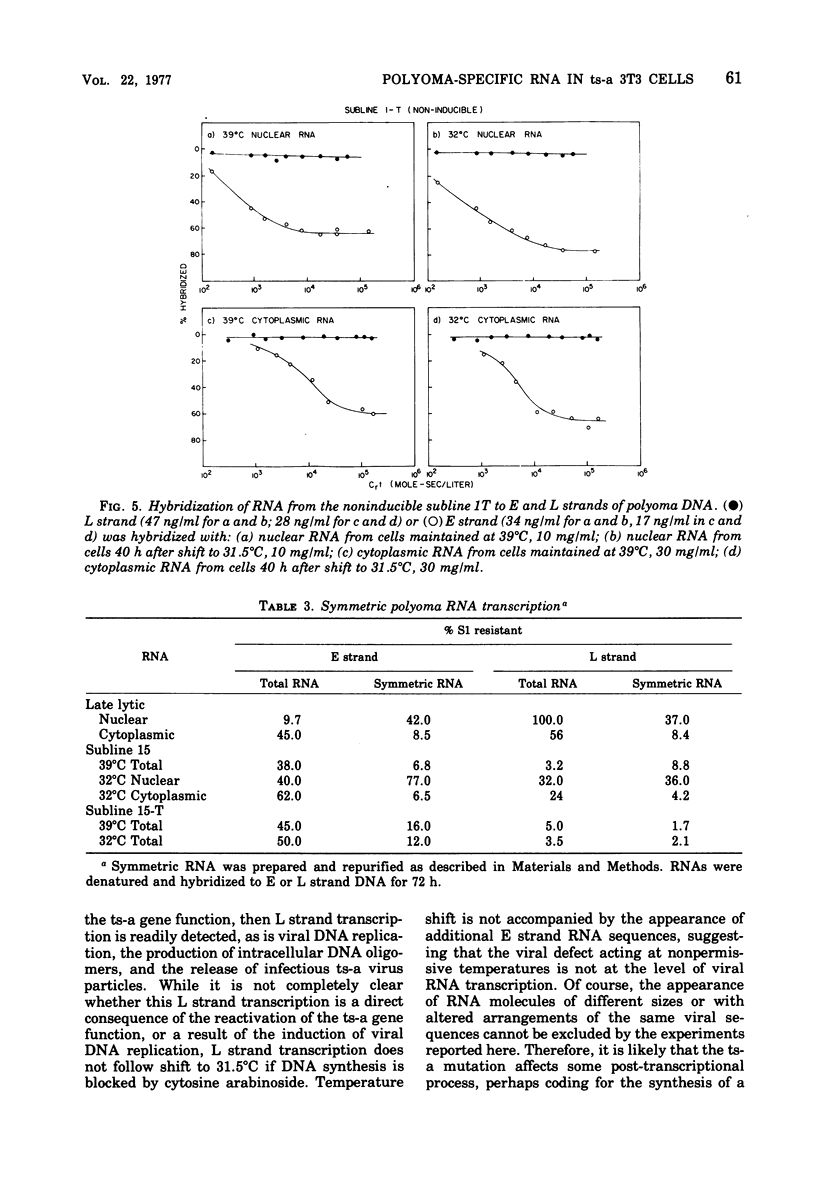
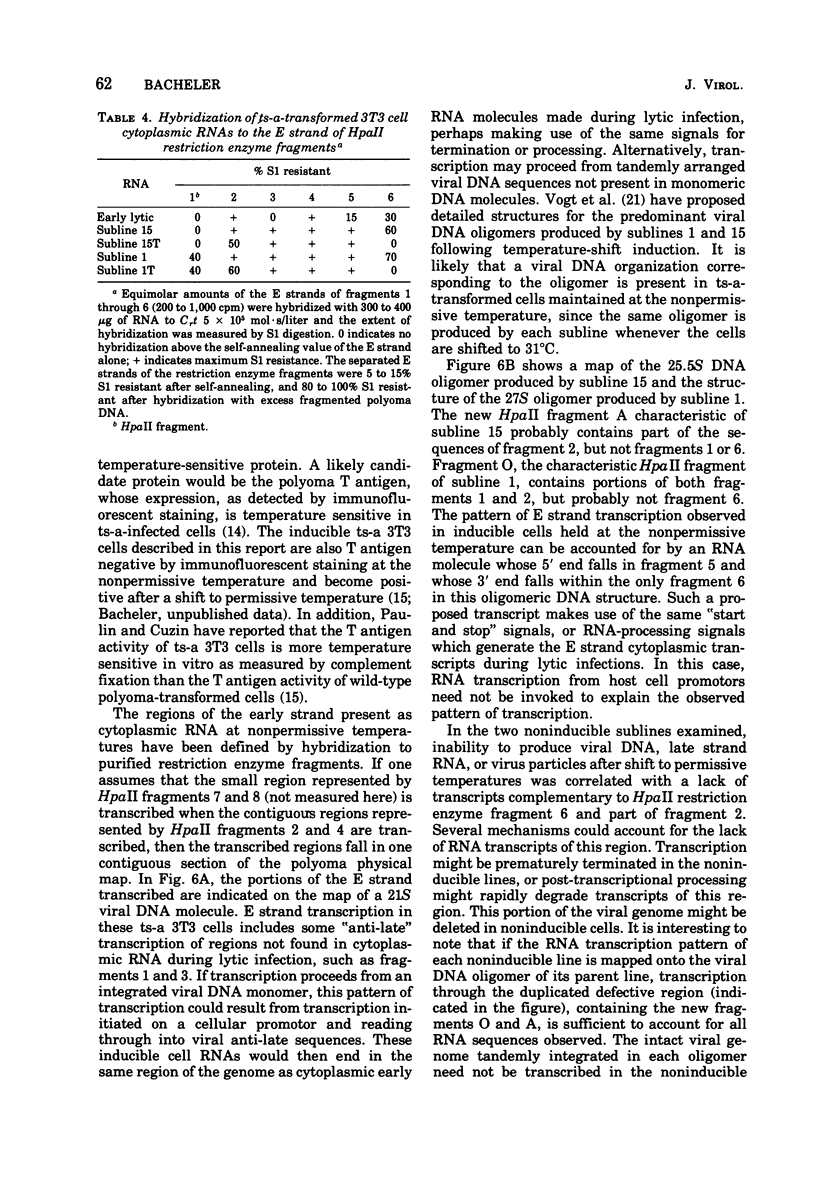
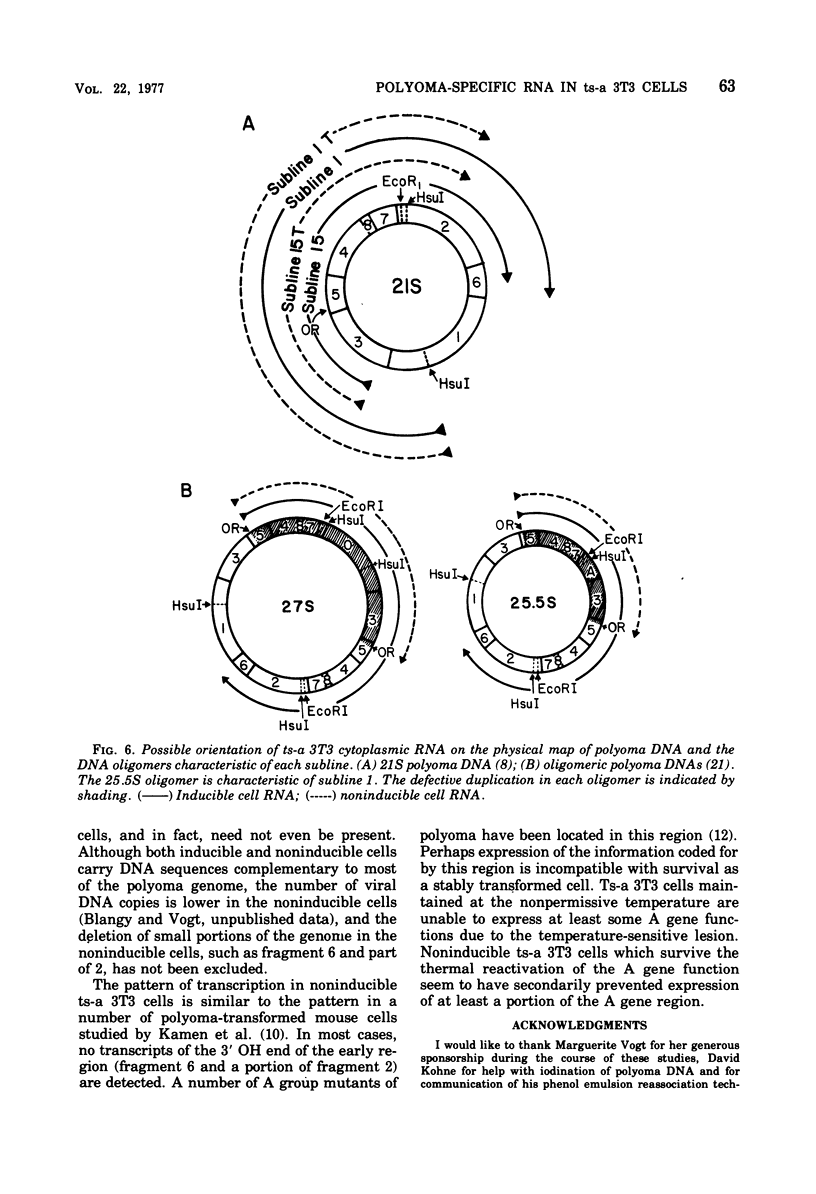
Virus-specific RNA transcription has been measured in 3T3 cells transformed by the ts-a mutant of polyoma virus by RNA-excess hybridization to the separated strands of polyoma DNA. In two cloned sublines maintained at 39 degrees C, the nonpermissive temperature for the A gene function, RNA transcripts of a large fraction of the "early" strand are detected in both nuclear and cytoplasmic RNA fractions, but no "late" strand transcription is detected. Temperature shift to 31.5 degrees C, the permissive temperature, induces viral DNA replication and virus production accompanied by late strand transcription. In two independently derived noninducible cell lines, L strand transcription is never observed, even after cultivation at the permissive temperature. A smaller fraction of the E strand is transcribed in each noninducible cell than in its inducible parent, and this difference is further characterized as a lack of transcripts of portions of HpaII restriction endonuclease fragments 2 and 6.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beard P., Acheson N. H., Maxwell I. H. Strand-specific transcription of polyoma virus DNA-early in productive infection and in transformed cells. J Virol. 1975 Jan;17(1):20–26. doi: 10.1128/jvi.17.1.20-26.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commerford S. L. Iodination of nucleic acids in vitro. Biochemistry. 1971 May 25;10(11):1993–2000. doi: 10.1021/bi00787a005. [DOI] [PubMed] [Google Scholar]
- Cuzin F., Vogt M., Dieckmann M., Berg P. Induction of virus multiplication in 3T3 cells transformed by a thermosensitive mutant of polyoma virus. II. Formation of oligometric polyoma DNA molecules. J Mol Biol. 1970 Feb 14;47(3):317–333. doi: 10.1016/0022-2836(70)90305-0. [DOI] [PubMed] [Google Scholar]
- FRIED M. CELL-TRANSFORMING ABILITY OF A TEMPERATURE-SENSITIVE MUTANT OF POLYOMA VIRUS. Proc Natl Acad Sci U S A. 1965 Mar;53:486–491. doi: 10.1073/pnas.53.3.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin R. M. Purification and properties of the replicative intermediate of the RNA bacteriophage R17. Proc Natl Acad Sci U S A. 1966 Jun;55(6):1504–1511. doi: 10.1073/pnas.55.6.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried M. Characterization of a temperature-sensitive mutant of polyoma virus. Virology. 1970 Mar;40(3):605–617. doi: 10.1016/0042-6822(70)90205-9. [DOI] [PubMed] [Google Scholar]
- Griffin B. E., Fried M., Cowie A. Polyoma DNA: a physical map. Proc Natl Acad Sci U S A. 1974 May;71(5):2077–2081. doi: 10.1073/pnas.71.5.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Kamen R., Lindstrom D. M., Shure H., Old R. W. Virus-specific RNA in cells productively infected or transformed by polyoma virus. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):187–198. doi: 10.1101/sqb.1974.039.01.025. [DOI] [PubMed] [Google Scholar]
- Kamen R., Shure H. Topography of polyoma virus messenger RNA molecules. Cell. 1976 Mar;7(3):361–371. doi: 10.1016/0092-8674(76)90165-3. [DOI] [PubMed] [Google Scholar]
- Miller L. K., Fried M. Construction of the genetic map of the polyoma genome. J Virol. 1976 Jun;18(3):824–832. doi: 10.1128/jvi.18.3.824-832.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder C., Vogt M. Production of non-defective and defective oligomers of viral DNA in mouse 3T3 cells transformed by a thermosensitive mutant of polyoma virus. J Mol Biol. 1973 Apr 25;75(4):601–608. doi: 10.1016/0022-2836(73)90294-5. [DOI] [PubMed] [Google Scholar]
- Oxman M. N., Takemoto K. K., Eckhart W. Polyoma T antigen synthesis by temperature-sensitive mutants of polyoma virus. Virology. 1972 Sep;49(3):675–682. doi: 10.1016/0042-6822(72)90524-7. [DOI] [PubMed] [Google Scholar]
- Paulin D., Cuzin F. Polyoma virus T antigen. I. Synthesis of modified heat-labile T angiten in cells transformed with the ts-a mutant. J Virol. 1975 Feb;15(2):393–397. doi: 10.1128/jvi.15.2.393-397.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penman S. RNA metabolism in the HeLa cell nucleus. J Mol Biol. 1966 May;17(1):117–130. doi: 10.1016/s0022-2836(66)80098-0. [DOI] [PubMed] [Google Scholar]
- Sambrook J., Sharp P. A., Keller W. Transcription of Simian virus 40. I. Separation of the strands of SV40 DNA and hybridization of the separated strands to RNA extracted from lytically infected and transformed cells. J Mol Biol. 1972 Sep 14;70(1):57–71. doi: 10.1016/0022-2836(72)90163-5. [DOI] [PubMed] [Google Scholar]
- Schachat F. H., Hogness D. S. Repetitive sequences in isolated Thomas circles from Drosophila melanogaster. Cold Spring Harb Symp Quant Biol. 1974;38:371–381. doi: 10.1101/sqb.1974.038.01.040. [DOI] [PubMed] [Google Scholar]
- Sharp P. A., Sugden B., Sambrook J. Detection of two restriction endonuclease activities in Haemophilus parainfluenzae using analytical agarose--ethidium bromide electrophoresis. Biochemistry. 1973 Jul 31;12(16):3055–3063. doi: 10.1021/bi00740a018. [DOI] [PubMed] [Google Scholar]
- Vogt M., Bacheler L. T., Boice L. Proposed structure of two defective viral DNA oligomers produced in 3T3 cells transformed by the ts-a mutant of polyoma virus. J Virol. 1976 Mar;17(3):1009–1026. doi: 10.1128/jvi.17.3.1009-1026.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt M. Induction of virus multiplication in 3T3 cells transformed by a thermosensitive mutant of polyoma virus. I. Isolation and characterization of Ts-a-3T3 cells. J Mol Biol. 1970 Feb 14;47(3):307–316. doi: 10.1016/0022-2836(70)90304-9. [DOI] [PubMed] [Google Scholar]


