Abstract
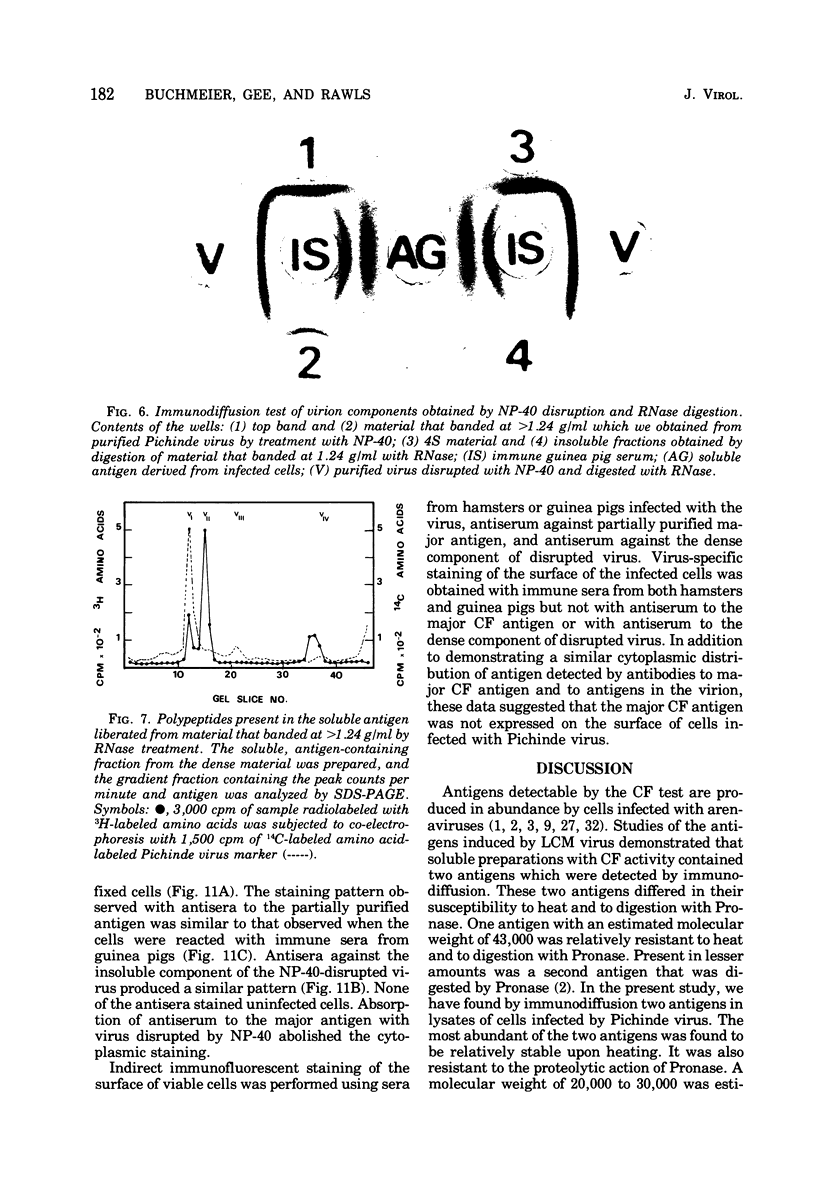
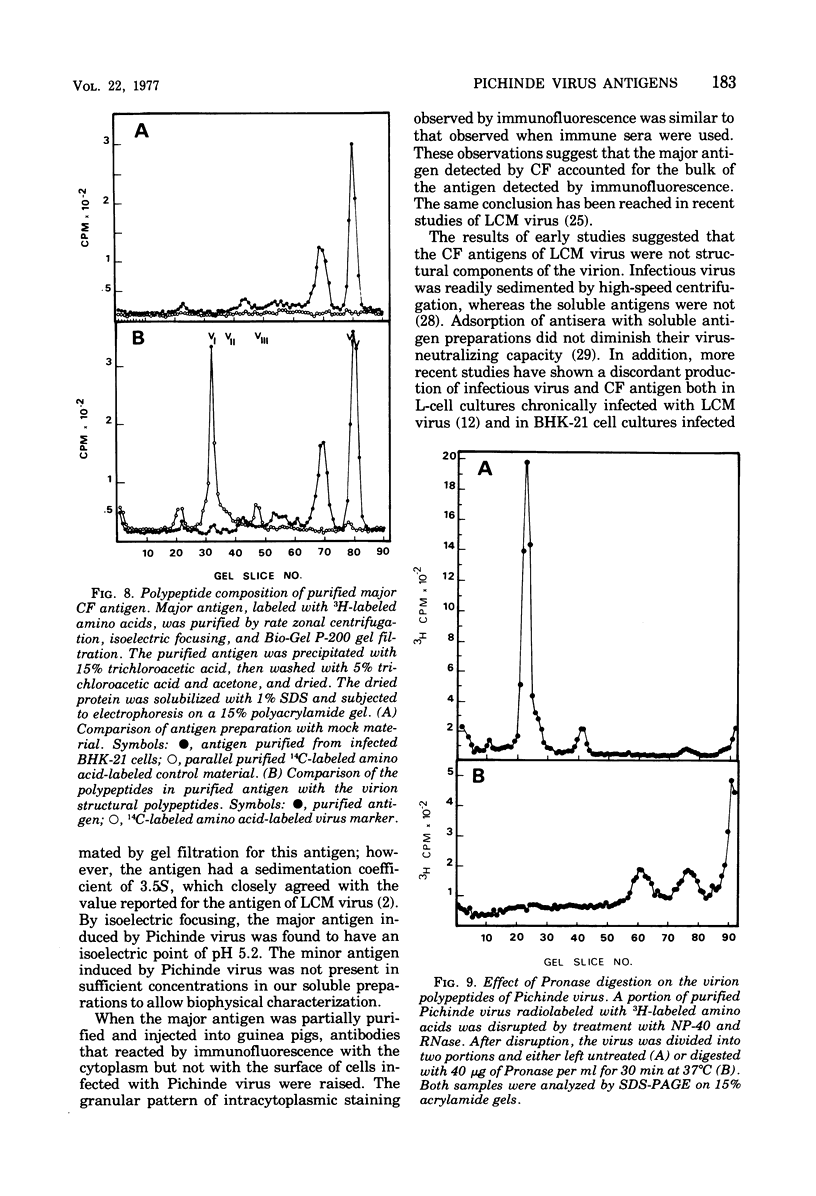
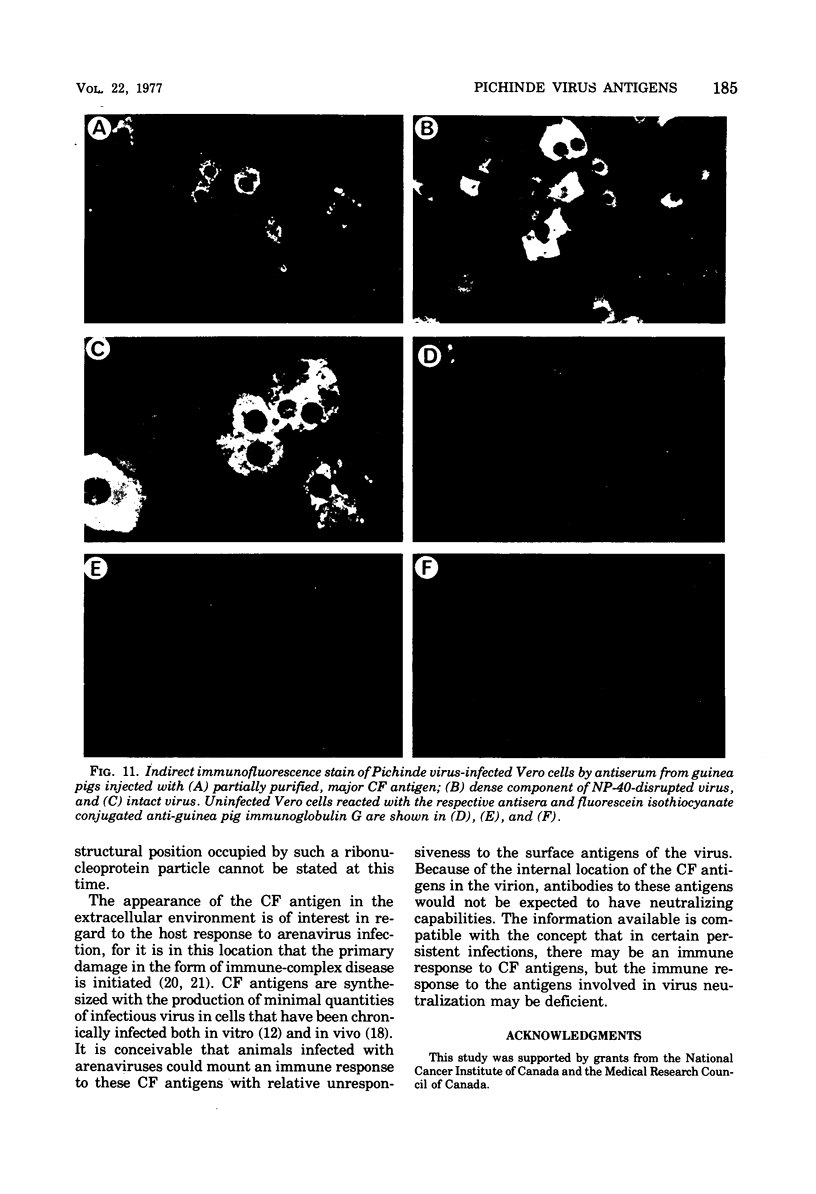
Antigens detected by the complement-fixation (CF) test were prepared from BHK-21 cells infected with Pichinde virus.The preparations contained two antigens demonstrable by immunodiffusion. The antigen present in abundance was heat stable, Pronase resistant, and had a molecular weight of 20,000 to 30,000 as estimated by gel filtration. Polyacrylamide gel electrophoresis of purified antigen demonstrated two low-molecular-weight polypeptides. An identical antigenic determinant was found by disrupting purified virus with Nonidet P-40; however, none of the viral polypeptides co-migrated with the polypeptides derived from purified CF antigen. Pronase digestion of disrupted virus did not alter antigenicity but degraded the viral peptides to sizes similar to those associated with the major CF antigen. These observations suggest that the major CF antigen of Pichnide virus is a cleavage product of the structural proteins of the virus.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abelson H. T., Smith G. H., Hoffman H. A., Rowe W. P. Use of enzyme-labeled antibody for electron microscope localization of lymphocytic choriomeningitis virus antigens in infected cell cultures. J Natl Cancer Inst. 1969 Mar;42(3):497–515. [PubMed] [Google Scholar]
- Bro-Jorgensen K. Characterization of virus-specific antigen in cell culture infected with lymphocytic choriomeningitis virus. Acta Pathol Microbiol Scand B Microbiol Immunol. 1971;79(4):466–474. doi: 10.1111/j.1699-0463.1971.tb03796.x. [DOI] [PubMed] [Google Scholar]
- Brown W. J., Kirk B. E. Complement-fixing antigen from BHK-21 cell cultures infected with lymphocytic choriomeningitis virus. Appl Microbiol. 1969 Sep;18(3):496–499. doi: 10.1128/am.18.3.496-499.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchmeier M., Adam E., Rawls W. E. Serological evidence of infection by Pichinde virus among laboratory workers. Infect Immun. 1974 May;9(5):821–823. doi: 10.1128/iai.9.5.821-823.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter M. F., Biswal N., Rawls W. E. Characterization of nucleic acid of pichinde virus. J Virol. 1973 Jan;11(1):61–68. doi: 10.1128/jvi.11.1.61-68.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton A. J., Rowe W. P., Smith G. H., Wilsnack R. E., Pugh W. E. Morphological and cytochemical studies on lymphocytic choriomeningitis virus. J Virol. 1968 Dec;2(12):1465–1478. doi: 10.1128/jvi.2.12.1465-1478.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farber F. E., Rawls W. E. Isolation of ribosome-like sturctures from Pichinde virus. J Gen Virol. 1975 Jan;26(1):21–31. doi: 10.1099/0022-1317-26-1-21. [DOI] [PubMed] [Google Scholar]
- Kingsbury D. W., Webster R. G. Some properties of influenza virus nucleocapsids. J Virol. 1969 Sep;4(3):219–225. doi: 10.1128/jvi.4.3.219-225.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lehmann-Grube F., Slenczka W., Tees R. A persistent and inapparent infection of L cells with the virus of lymphocytic choriomeningitis. J Gen Virol. 1969 Jul;5(1):63–81. doi: 10.1099/0022-1317-5-1-63. [DOI] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- Mifune K., Carter M., Rawls W. Characterization studies of the Pichinde virus-a member of the arenavirus group. Proc Soc Exp Biol Med. 1971 Feb;136(2):637–644. doi: 10.3181/00379727-136-35330. [DOI] [PubMed] [Google Scholar]
- Murphy F. A., Webb P. A., Johnson K. M., Whitfield S. G., Chappell W. A. Arenoviruses in Vero cells: ultrastructural studies. J Virol. 1970 Oct;6(4):507–518. doi: 10.1128/jvi.6.4.507-518.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy F. A., Winn W. C., Jr, Walker D. H., Flemister M. R., Whitfield S. G. Early lymphoreticular viral tropism and antigen persistence. Tamiami virus infection in the cotton rat. Lab Invest. 1976 Feb;34(2):125–140. [PubMed] [Google Scholar]
- Oldstone M. B., Dixon F. J. Lymphocytic choriomeningitis: production of antibody by "tolerant" infected mice. Science. 1967 Dec 1;158(3805):1193–1195. doi: 10.1126/science.158.3805.1193. [DOI] [PubMed] [Google Scholar]
- Oldstone M. B., Dixon F. J. Pathogenesis of chronic disease associated with persistent lymphocytic choriomeningitis viral infection. I. Relationship of antibody production to disease in neonatally infected mice. J Exp Med. 1969 Mar 1;129(3):483–505. doi: 10.1084/jem.129.3.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldstone M. B., Dixon F. J. Pathogenesis of chronic disease associated with persistent lymphocytic choriomeningitis viral infection. II. Relationship of the anti-lymphocytic choriomeningitis immune response to tissue injury in chronic lymphocytic choriomeningitis disease. J Exp Med. 1970 Jan 1;131(1):1–19. doi: 10.1084/jem.131.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldstone M. B., Dixon F. J. Persistent lymphocytic choriomeningitis viral infection. 3. Virus-anti-viral antibody complexes and associated chronic disease following transplacental infection. J Immunol. 1970 Oct;105(4):829–837. [PubMed] [Google Scholar]
- Ramos B. A., Courtney R. J., Rawls W. E. Structural proteins of Pichinde virus. J Virol. 1972 Oct;10(4):661–667. doi: 10.1128/jvi.10.4.661-667.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawls W. E., Banerjee S. N., McMillan C. A., Buchmeier M. J. Inhibition of Pichinde virus replication by actinomycin D. J Gen Virol. 1976 Dec;33(3):421–434. doi: 10.1099/0022-1317-33-3-421. [DOI] [PubMed] [Google Scholar]
- Rawls W. E., Buchmeier M. Arenaviruses: purification and physicochemical nature. Bull World Health Organ. 1975;52(4-6):393–401. [PMC free article] [PubMed] [Google Scholar]
- Schild G. C., Pereira H. G. Characterization of the ribonucleoprotein and neuraminidase of influenza A viruses by immunodiffusion. J Gen Virol. 1969 Apr;4(3):355–363. doi: 10.1099/0022-1317-4-3-355. [DOI] [PubMed] [Google Scholar]
- Simon M. Multiplication of lymphocytic choriomeningitis virus in various systems. Acta Virol. 1970 Sep;14(5):369–376. [PubMed] [Google Scholar]
- Trapido H., Sanmartín C. Pichindé virus, a new virus of the Tacaribe group from Colombia. Am J Trop Med Hyg. 1971 Jul;20(4):631–641. [PubMed] [Google Scholar]
- Webb P. A., Johnson K. M., Hibbs J. B., Kuns M. L. Parana, a new Tacaribe complex virus from Paraguay. Arch Gesamte Virusforsch. 1970;32(4):379–388. doi: 10.1007/BF01250066. [DOI] [PubMed] [Google Scholar]





