Abstract
Biological N2 fixation (BNF) in the rhizosphere of Podocarpaceae is currently attributed to unspecific diazotrophs with negligible impact on N acquisition. Here, we report specific and high associative BNF in dead cells of root nodules of Lepidothamnus fonkii distributed in ombrotrophic peatlands of Patagonia. BNF of nodulated roots, intact plants of L. fonkii and rhizospheric peat was assessed by 15N2 and acetylene reduction. Diazotrophs were identified by electron microscopy, analysis of nitrogenase encoding genes (nifH) and transcripts, and 16S rRNA. Nitrogenase encoding nifH transcripts from root nodules point to Beijerinckiaceae (Rhizobiales), known as free-living diazotrophs. Electron microscopy and 16S rRNA analysis likewise identified active Beijerinckiaceae in outer dead cells of root nodules. NifH transcripts from the rhizopshere peat revealed diverse active diazotrophs including Beijerinckiaceae. Both methods revealed high activity of nitrogenase rates in cut roots of L. fonkii (2.5 μmol N g−1 d.w. d−1 based on 15N2 assay; 2.4 μmol C2H4 g−1 d.w. d−1 based on acetylene reduction assay). The data suggest that (i) nodules recruit diazotrophic Beijerinckiaceae from peat, (ii) dead nodule cells provide an exclusive habitat for Beijerinckiaceae, and (iii) BNF in L. fonkii is one potent pathway to overcome N deficiency in ombrotrophic peatlands of Patagonia.
Biological dinitrogen (N2) fixation (BNF) by plant-associated prokaryotes is a widespread and effective process of N acquisition1. However, the capability of plants to host N2 fixing endosymbiotic prokaryotes is restricted to few plant species and bacteria2. Especially in peatlands, the existence of such mutualistic associations is restricted to very few plant species. One example is the shrub Myrica gale (L.) that grows in some peatlands of the Northern hemisphere and fixes considerably amounts of atmospheric N2 in root nodules3. Mutualistic BNF in peatlands of the southern hemisphere is not known, but other strategies of BNF can also occur in peatlands.
Conifers of the family Podocarpaceae form nodules and host arbuscular mycorrhizal fungi therein as shown for few species4,5. BNF in root nodules of Podocarpaceae has been postulated in several studies for more than a century6,7,8,9,10,11. Low N2 fixation activities in root nodules were confirmed for Podocarpus rospigliosii6 and P. macrophyllus10 (Table 1). In contrast, an absence of any N2 fixation was reported for P. totara12. The current view is that the observed N2 fixation activities arise from methodological artifacts and free-living diazotrophs in the rhizosphere, possibly due to incomplete removal of rhizosphere soil11,13.
Table 1. Nitrogen fixation in nodules and nodulated roots of Podocarpus species.
| Species | (nmol N g−1 d−1 nodules) | (nmol N g−1 d−1 nodulated roots) |
|---|---|---|
| P. lawrencei7 | 1128† | 429*,† |
| P. rospigliosii6 | 3143† | 1194*,† |
| P. totara11 | 3–7‡ | |
| P. macrophyllus10 | 720†,‡ | |
| P. latifolius9 | 36†,‡ | |
| L. fonkii§ | 2470 |
Literature data were recalculated to nodulated roots and for dry weight to allow for comparison.
*assuming 38% nodule mass per root mass7; †assuming 10% dry matter per fresh weight; ‡assuming a conversion factor of acetylene reduction to nitrogen fixation of 2; §This study, root samples only.
Podocarpaceae are restricted to nutrient poor environments of the Southern Hemisphere14. Many nutrient poor Sphagnum bogs of Patagonia host the podocarp Lepidothamnus fonkii (Phil), a small coniferous shrub of up to 30–60 cm height with hitherto unknown associated N2 fixation (Fig. 1A). Pristine ombrotrophic bogs receive inorganic N from two sources: (i) atmospheric deposition and (ii) N2 fixation by non-symbiotic diazotrophic microorganisms15,16,17,18,19. Atmospheric N deposition is secondary relative to BNF as indicated by exceptionally low atmospheric deposition rates of less than 0.1 g N m−2 yr−1 20 and BNF associated with Sphagnum or other mosses ranging from 0.5–6.4 g N m−2 yr−1 21.
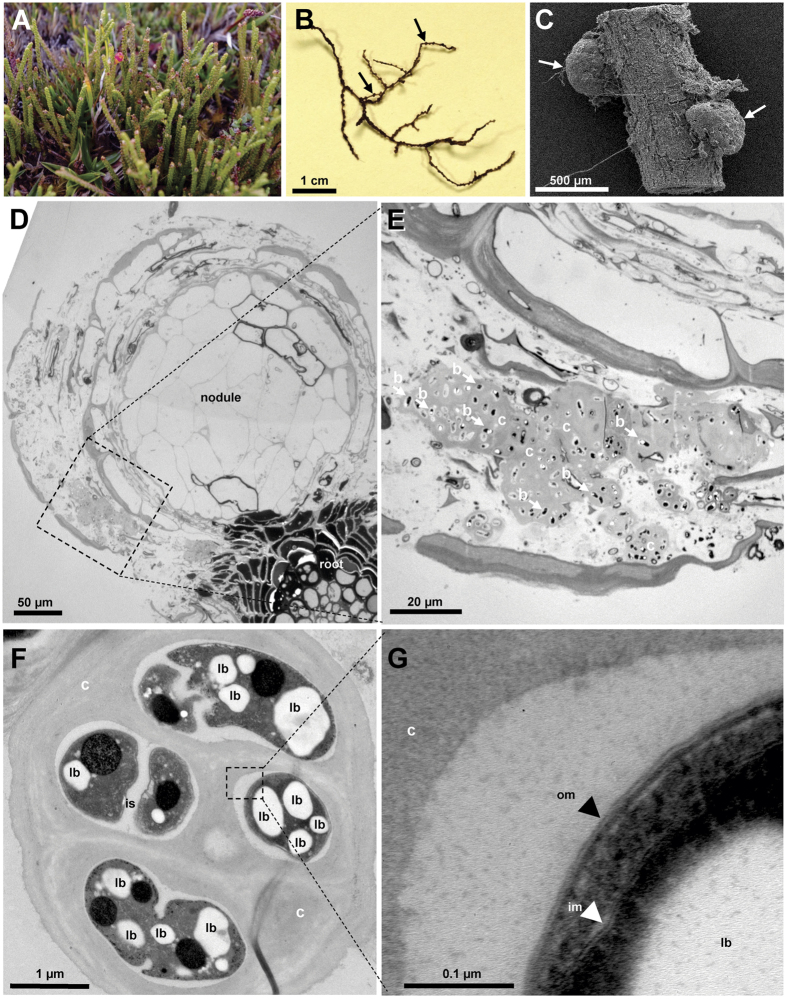
Figure 1.
Photograph (A), stereo microscope image (B), scanning electron (SEM; C) and transmission electron microscope (TEM; D–G) images of Lepidothamnus fonkii. Photograph of L. fonkii at the SKY field site at Seno Skyring (Southern Patagonia, Chile; A) and roots densely covered by nodules (B). Root nodules (arrows) were smaller than 500 μm in diameter (SEM; C). Ultrastructure of root with nodule (TEM; D) revealed capsules with multiple bacteria located primarily at the vicinity of the nodules (arrows indicate some of the bacterial cells; E) Enlarged capsules indicate ultrastructure of bacteria containing lipoid bodies (F). Intact outer and inner membranes (black and white arrowheads, respectively; G) of bacteria were indicative of living gram negatives, which is in agreement with active diazotrophic Beijerinckiaceae-related bacteria detected at the roots. Bar represents a scale bar (B–G). Squares and dashed lines indicate areas that were enlarged in the following panel. Abbreviations: b, bacteria; c, capsule; im, inner membrane; is, intercellular space; lb, lipoid bodies; om, outer membrane.
Net N retention rates and thus N storage in the peat of 2.0 g m−2 yr−1 reported for northern Alberta bogs were mainly attributed to BNF17. Patagonian bogs could have net N retention of 0.4–1.0 g N m−2 yr−1 based on published carbon (C) storage rates of 15–40 g C m−2 yr−1 in Yu et al.22 and average C/N ratios of about 40 in the top 1 m19,23. BNF thus likely represents the predominant N source (0.4–1.2 g N m−2 yr−1) in Patagonian bogs given the low N deposition. The widespread occurrence of L. fonkii in such habitats and the limited knowledge on BNF in Podocarpaceae nodules necessitates studies on the potential of L. fonkii for BNF. Thus, our objectives were to (i) assess associative N2 fixation in root nodules of L. fonkii, (ii) to identify the genetic potential for diazotrophy in root nodules as well as active diazotrophs, and to (iii) estimate the importance of L. fonkii for N acquisition in two pristine Patagonian bogs.
Materials and Methods
Intact plants of L. fonkii (Fig. 1A) and peat cores of 100 cm2, 20 cm depth, with L. fonkii were sampled at two pristine bogs in Southern Patagonia, Chile, in March 2014: a 2–4 m deep peat deposit at the Seno Skyring (site ‘SKY’, −52.508667°S, 72.127278°W)23 and a 2 m deep peat deposit at the Seno Obstruccion (site ‘OBS’, 52.135907°S, 72.446037°W). Additional peat cores were obtained at each site using a Russian type peat corer (5 cm diameter), in which we determined peat C and N concentrations, C/N ratios, and δ15N signatures of the upper 80 cm (provided in the supporting information; Supplementary Table S1). L. fonkii grew in communities with Astelia pumila (Asteliaceae), Donatia fascicularis (Stylidiaceae), Sphagnum magellanicum (Sphagnaceae), Empetrum rubrum (Ericaceae), Gaultheria pumila (Ericaceae), Drosera uniflora (Droseraceae), Marsippospermum grandiflorum (Juncaceae), and Caltha appendiculata (Ranunculaceae). For estimates of plant biomass (n = 6) additional intact peat blocks were extruded (March 2013 and 2014). All samples were packed in plastic bags and transported to the lab. Leaf biomass, stems, and roots were manually picked, washed, dried, and weighed.
BNF was determined using both the 15N2 (98 atom%, Sigma Aldrich, batch No. MBBB0968V, St. Louis, USA) assay and the acetylene (Riessner-Gase, Lichtenfels, Germany) reduction assay (ARA)24. According to Dabundo et al.25 we cannot exclude that 15N2 was contaminated with reactive N gas compounds such as 15NH3 and 15NOx. A mean contamination as reported in Dabundo et al.25 (e.g. 1 mmol 15N mol−1 15N2) would overestimate the N2 fixation rates by about 13%, assuming an uptake of 50% of the total contamination. We assume that moist paper tissues in the jars (see below) trapped a large fraction of potential impurities during the incubation.
After washing and removal of peat particles, intact, non-sterilized plants were incubated in 325 ml glass jars. Roots were wrapped in moist paper tissue to prevent desiccation. For 15N2 assays, the jars (800 ml) were closed, evacuated to ~350 mbar and refilled with 15N2 to achieve about 70 atom% 15N2 in the head space (verified by mass spectrometry). In addition, freshly cut roots (0.1–0.2 g d.w.) and peat without live roots (about 0.1 g d.w.) were separately incubated in 22 ml vials with similar 15N2 enrichment in the headspace as for intact plant samples. In parallel batches all samples were incubated with 10 vol. % of acetylene in the headspace for the ARA. To mimic oxygen concentrations in the rhizosphere, we adjusted O2 concentrations to about 2%. Three replicates per site, each with 2–3 intact plants per jar were incubated for 65 hours at 15 °C in a climate chamber with 12 hours light per day (about 500 μmol s−1 photon flux). In the ARA, ethylene (C2H4) concentrations were analyzed after 0, 6, 12 and 24 hours (L. fonkii samples) or after 0, 1.5 and 5 h (T. repens controls), C2H4 production rates were obtained from linear increase of concentration over time and expressed in μmol C2H4 g−1 d.w. d−1. All ethylene time series were highly linear, with an r2 > 0.95. From 15N2 incubations, jars were opened and the plants were separated into roots, stems, and leaf biomass. After oven drying at 40 °C, the dry weight was determined and the material was milled for subsequent isotope analysis. In addition to intact plant incubations, three replicates per site of cut roots of L. fonkii were incubated separately in 20 ml flasks at 15 °C in the dark for 72 hours. As a control, parallel incubations of fresh, nodulated roots of Trifolium repens were carried out in triplicate. N2 fixation in the incubation was expressed in μmol N per gram dry biomass and day (μmol N g−1 d.w. d−1) and was calculated based on 15N natural abundance of control plants (Table S2) and 15N enrichment in atom % after incubation for each respective plant component. We are aware that some studies suggested to include only data from short term incubations for ARA and 15N2 techniques26,27, but recent studies in wetlands have confirmed linearity also over long incubation time (>48 h)16,17,28. Moreover, longer incubation times would also overcome at least in part methodological issues of an underestimation of rates due to incomplete equilibration of the added gas (acetylene or 15N2) and the water phase29 surrounding the samples of wetland plants.
Concentrations of 15N2 in the headspace were analyzed using a Delta Plus XL isotope ratio mass spectrometer (Thermo Finnigan, Bremen, Germany), after equilibration of the masses 28, 29, and 30 in a microwave (GMW 24–201, AHF Analysentechnik, Tübingen, Germany)30. Ethylene concentration was analyzed by gas chromatography (Model 8610C, SRI Instruments Inc., Las Vegas, NV, USA). Peat and plant analysis for 15N was done using a Eurovector/HEKAtech Elemental CNS analyzer (HEKAtech, Wegberg, Germany) coupled to a Nu Horizon isotope ratio mass spectrometer (Nu Instruments, Wrexham, UK).
Sections of L. fonkii roots were fixed in glutaraldehyde (2%) and OsO4 (2%) prior to positive staining with uranylaceate (2%) for ultrastructural analyses. For taking images with scanning electron microscopy (SEM, Philips ESEM XL 30), fixed sections were dehydrated in acetone, followed by critical point drying and sputtering with a gold layer. For transmission electron microscopy (TEM) (Zeiss CEM 902 or a JEOL JEM-2110) sections were dehydrated in ethanol/propylenoxid, embedded in epon, and thin sections (50–70 μM thickness) were produced in a Leica Ultracut UCT microtome.
Nitrogenase encoding genes (nifH) and transcripts, as well as 16S rRNA were analyzed from L. fonkii roots and peat to identify microbes driving N2 fixation and those colonizing roots. One mixed, representative sample of young densely nodulated roots from multiple individuals (600 mg) of L. fonkii were washed 2x with 70% ethanol and 3x with sterile phosphate buffered saline to remove root surface attached microbes prior to pestling and DNA/RNA extraction with the RNA PowerSoil and DNA Elution Accessory Kit (MoBio, Carlsbad, CA, USA). A representative, mixed sample of peat was extracted in a similar way. Reverse transcription was done with random hexamer priming and SuperScriptIII reverse transcriptase (Invitrogen, Karlsruhe, Germany) according to the manufacturer’s protocol31. nifH was PCR amplified from DNA and cDNA of roots and peat with primers IGK3 (GCI WTH TAY GGI AAR GGI GGI ATH GGI AA) and DVV (ATI GCR AAI CCI CCR CAI ACI ACR TC) currently covering the broadest range of nifH diversity (including nifH of Cyanobacteria) utilizing the Kappa2G Robust HotStart PCR Kit (Peqlab, Nuremberg, Germany) as described32. In brief, reaction mixtures contained 1x buffer A, 0.2 mM of each dNTP, 1.0 μM of each primer, 1x KAPPA Enhancer 1, 2.5 mM MgCl2, and 1 unit of KAPPA2G Robust HotStart DNA polymerase. Thermal protocol was: Initial denaturation at 95 °C (5 min); 40 cycles of 95 °C (1 min), 58 °C (0.5 min), and 72 °C (1 min); final elongation at 72 °C (5 min). PCR products were gel purified, ligated into pGEM-T (PROMEGA, Madison, WI, USA) and TOP10 competent cells were transformed. Four gene libraries were constructed (i.e., one each for root DNA, root cDNA; peat DNA, peat cDNA). Per gene library, plasmids were extracted from 96 clones, and inserts were Sanger sequenced (4 gene libraries x 96) at LGC genomics (Berlin, Germany). nifH sequences were clustered with JAGUC2 and OTUs were called at 97% sequence similarity33,34. Cluster representatives were phylogenetically affiliated with BLASTX35. 264 nifH genes and transcripts grouping into 2αTUs were recovered and are presented in the supporting information (Tables S3, S4). Diversity measures were calculated as described in Palmer et al.34. Coverages for all gene libraries were always >85%.
16S rRNA amplicons originating from RNA, were generated from roots and peat with primers 341F-785R as described36. Sequencing of the two amplicon libraries was done on the Illumina MiSeq V3 platform at LGC Genomics (Berlin, Germany) and approximately 13,000 quality-filtered reads were obtained per amplicon library. 16S rRNA derived sequences were analyzed with the QIIME pipeline37. In particular, OTUs were called at 97% sequence similarity and OTU representatives were aligned using PyNast. Chimeras were excluded uding ChimeraSlayer, and taxonomy was assigned to OTU representatives using RDP classifier.
Results
Root, stem and leaf biomass of L. fonkii (n = 6) studied in Patagonia at both sites SKY and OBS amounted to means of 221 (range 79–367), 135 (range 49–225) and 119 (range 10–255) g d.w. m−2, respectively. Roots of L. fonkii had diameters of <1 mm and were densely covered by 15–20 nodules cm−1 (Fig. 1B,C). After incubation of L. fonkii from both sites with 15N2, fixed 15N was recovered in nodulated roots, stems, and leaf biomass, resulting in δ15N values of 4470 ± 1730, 3340 ± 1270, and 905 ± 245‰, respectively. Estimated BNF from 15N2 uptake into roots of intact plants were higher compared to rates obtained from incubation of cut roots (Table 2). Latter yielded about 70% of BNF in nodulated roots of control white clover plants (Trifolium repens) (Table 2). Ethylene production rates in cut roots of L. fonkii were about 8 times lower than ethylene production [i.e. acetylene reduction assay (ARA) as a proxy for nitrogenase activity] of T. repens cut roots. On the other hand, ethylene production rates of intact L. fonkii plants were similar to rates of cut roots. Both N2 fixation and ethylene production rates were lowest for root-free peat from 20 cm depth.
Table 2. N2 fixation and ethylene (C2H4) production rates in Lepidothamnus fonkii.
| N2 fixation (μmol N g−1 d.w. d−1) | C2H4 production (μmol C2H4 g−1 d.w. d−1) | C2H4/N2 | |
|---|---|---|---|
| L. fonkii (entire plants)*, site OBS | 1.02 ± 0.27 | 2.43 ± 0.45 | 4.90 ± 0.92 |
| L. fonkii (entire plants)*, site SKY | 1.25 ± 0.21 | 1.68 ± 0.25 | 2.69 ± 0.04 |
| L. fonkii, intact roots† | 3.09 ± 1.24 | n.d. | n.d. |
| L. fonkii, cut roots‡ | 2.47 ± 0.28 | 2.35 ± 0.63 | 1.85 ± 0.79 |
| T. repens, cut roots‡ | 3.94 ± 0.63 | 18.01 ± 5.27 | 9.07 ± 1.22 |
| Root-free peat (0–10 cm) | 1.69 ± 0.68 | 1.25 ± 0.32 | 1.67 ± 0.69 |
Mean ± s.d. in-vitro N2 fixation rates (determined by the 15N2 assay), unconverted C2H4 production rates (determined by the acetylene reduction assay), and ratios of C2H4 production to N2 fixation in entire plants of L. fonkii from OBS and SKY (n = 3), in nodulated roots of L. fonkii and Trifolium repens (white clover) and peat of 0–10 cm depth (all n = 6), n.d. = not detected.
*Incubation of intact plants and weighted average of N2 fixation in nodulated roots, stems and leaf biomass.
†N2 fixation of live roots, obtained from incubations of intact plants.
‡incubation of freshly cut, nodulated roots.
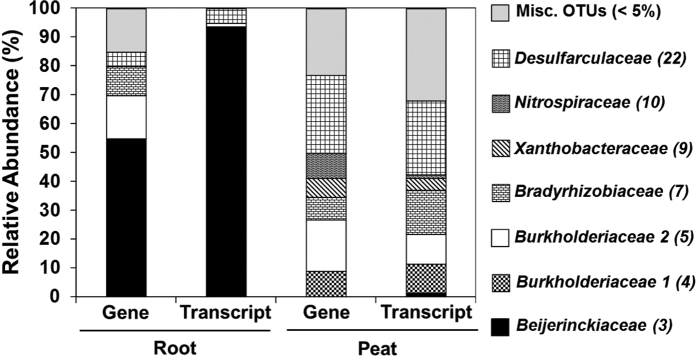
Scanning and transmission electron microscopy of roots or root thin sections indicated a high abundance of encapsulated, gram-negative bacteria with lipoid bodies and intact outer and inner membranes in presumably dead, peripheral cells of the nodules (Fig. 1C–F) of L. fonkii roots. Capsules and encapsulated cells resembled morphologies of Beijerinckia sp. grown in nitrogen free medium38. Nitrogenase encoding nifH genes and transcripts were diverse in peat and included operational taxonomic units (OTUs) related to Rhizobiales, Burkholderiales, and Desulfarculales (Fig. 2, Supplementary Tables S3, S4). Gene and transcript libraries of roots rather than those of peat were predominated by OTU3 that is closely related to nifH from Beijerinckiaceae (Supplementary Tables S3, S4). The relative abundance of OTU3 transcripts in root libraries was >93%, which indicates active N2 fixation by essentially one dominating group of root nodule-associated microbes. 16S rRNA analysis likewise revealed Beijerinckiaceae associated with roots rather than peat (Supplementary Figure S1).
Figure 2. Relative abundances of nifH genes and transcripts in gene libraries retrieved from live Lepidothamnus fonkii roots and peat material.
264 nifH sequences grouping into 22 operational taxonomic units (OTUs) were recovered. OTUs with a relative abundance of >5% in one of the libraries are given. OUT numbers are shown in parentheses and related to nifH from the family-level taxa as indicated. Misc. OTUs (<5%): miscellaneous OTUs, relative abundance <5%. OTU 3 is associated with roots rather than with peat. (See also Tables S3, S4).
Discussion
Our finding of BNF, indicated by both 15N2 uptake and ARA, refute the common view that the BNF activity of Podocarpaceae is related to unspecific diazotrophs in the rhizosphere11,13. Although there is no endophytic symbiosis, electron microscopy and nifH transcripts indicated abundant living gram-negative bacteria in peripheral dead cells of root nodules of L. fonkii. Interestingly, nodulated roots of L. fonkii displayed similar rates of BNF as roots of a model plant with typical symbiotic N2 fixation by diazotrophic Rhizobia in nodules (Table 2; ref. 39). N2 fixation and ethylene production rates of other Podocarpaceae species, such as P. lawrencei7 were substantially lower than, but for P. rospigliosii6 of similar magnitude as those of L. fonkii roots when calculated on an entire root basis (Table 1). C2H4/15N2 ratios of 1.9–4.9 for L. fonkii fell in a range typically observed for most important nitrogenases40. We cannot exclude that a contamination of the applied 15N2 gas with 15NH3 or 15NOx resulted in an overestimation of the BNF rate25. This bias, however, is likely small in our case, considering comparably high 15N enrichment, the acetylene reduction rates and respective C2H4/15N2 ratios observed for nodulated roots of T. repens, which compared well to values reported for T. pratense41. A significant contamination of the applied 15N2 gas and absorption of 15NH3 or 15NOx could have led to lower C2H4/15N2 ratios and to an overestimation of N2 fixation rates of about 13%.
Nitrogenase genes (nifH) affiliating with Bradyrhizobiaceae and Burkholderiaceae were associated with peat and to some extent in L. fonkii roots (Fig. 2). Such diazotrophs were also detected in other studies on root-associated BNF of trees, but the diversity of active diazotrophs was much higher in these studies, indicating a non-specific association of diazotrophs with tree roots42,43. In our study, however, the expression of Bradyrhizobiaceae and Burkholderiaceae related nifH was essentially only detected in peat, indicating that free-living Bradyrhizobiaceae and Burkholderiaceae contribute to BNF in peat rather than to BNF in root nodules of L. fonkii. Accordingly, we assume that these taxa make a substantial contribution to BNF in the peat at our study sites. As Bradyrhizobiaceae and Burkholderiaceae are abundant taxa in peatlands or in other acidic and organic matter rich soils44,45,39, they could be key organisms for BNF in N limited ecosystems (see discussion below).
Despite the relatively small importance for BNF in root nodules of L. fonkii, active Bradyrhizobiaceae colonized roots of L. fonkii rather than peat as indicated by 16 rRNA amplicon sequencing (Fig. S1). Rhizobial Nod factors were reported to suppress plant innate immune response in nonlegumes46. Thus, it is tempting to speculate that initial colonization of roots by Bradyrhizobiaceae might enable subsequent colonization of other microbes.
The predominant and active N2 fixing, gram-negative bacteria of the Beijerinckiaceae identified in root nodules of L. fonkii (Figs 1 and 2) are well known as free-living diazotrophic bacteria that occur in water and soil including the rhizosphere of acidic peat soils38,47. Beijerinckiaceae-like nifH genes were recently also detected in association with Sphagnum mosses in an alpine bog48. Microbial diazotrophy accounted for most of the new N input associated with Sphagnum mosses17. Thus, plant associated diazotrophy was until recently underrated in bogs and in particular the role of diazotrophic Beijerinckiaceae may merit further attention.
Our study provides evidence that L. fonkii root associated, active diazotrophs fix substantial amounts of atmospheric N2. 15N2 enrichment in stems and leaf biomass supports significant and rapid translocation of fixed N excreted or leaking from diazotrophs in the root nodules to aboveground tissues (Table 2). Our results strongly support that this occurs in a specific association with Beijerinckiaceae in nodulated roots and refutes that reported N2 fixation by Podocarpaceae may only result from the activity of free-living bacteria in the rhizosphere13. Observed ranges of natural abundance of 15N in other Podocarpaceae, e.g. δ15N of −8 to −3‰ for P. hallii and P. urbanii49,50, suggest that such associated nitrogen fixation is certainly not a general feature of Podocarpaceae, however, or at least its contribution may not always be significant. For L. fonkii, specifically the following findings support an effective and specific association: (i) high N2 fixation rates, confirmed by both 15N2 uptake and active acetylene reduction (ii) molecular evidence of nitrogenase gene expression predominated by Beijerinckiaceae compared to a diverse diazotroph community in the surrounding rhizopheric peat soil (Fig. 2), and (iii) electron-microscopic images showing encapsulated bacteria with Beijerinckiaceae-like morphology densely colonizing the peripheral dead cell tissue of nodules (Fig. 1). Intact outer and inner cell membranes and lipoid bodies possibly consisting of poly-β-hydroxybutyrate (PHB) are typical for the gram-negative Beijerinckiaceae, further consolidating the conclusion that active Beijerinckiaceae reside inside nodules51. Obviously, peripheral dead cells of root nodules represent a favorable habitat that allows specific colonization and growth by Beijerinckiaceae. The high energy demand for N2 fixation and nutrients for growth may arise from enzymatic decay of plant cell compounds and dead bacterial cells. The layered ultrastructure of L. fonkii nodules (Fig. 1) suggests that Beijerinckiaceae are supported by continuous segregation of plant cells. High nitrogenase and BNF activities are in agreement with the formation of capsules (Fig. 1) that protects the oxygen sensitive nitrogenase38,52. The widespread occurrence of Beijerinckiaceae in a wide range of soils including those of low pH and high C-to-N ratio, their occurrence in the rhizoplane, and their well-recognized role as plant growth promoting bacteria might suggest a broad relevance for N-input of N-limited systems53,54. Thus, the proposed mechanism of plant-microbe interaction via necrosis of root cells and N-transfer from living bacteria to the host might represent an early variant of symbiotic diazotrophy and deserves more attention in future studies.
Indeed, evidence from growth experiments with the model diazotroph Azotobacter vinelandii suggests that ammonia could be excreted by or leak out of actively nitrogen fixing cells and can thus be easily transferred to the plant55. In this latter study, A. vinelandii accumulated up to 50 μM of ammonium and was capable of supporting algal growth in N-free medium. Although 15N recovered in leaf biomass might partly arise from a contribution of foliar endophytic nitrogen fixation, as reported for Pinus flexilis56, a translocation of N from nodules to leaves seems more likely due to higher 15N enrichment in the stem compared to leaf biomass.
A dense root biomass further indicates that L. fonkii can play a prominent role in the N cycle of south Patagonian bogs. Keeping in mind the limitation of our laboratory approach and inherent uncertainties in an extrapolation to field conditions, the potential N2 fixation is 13 mg N m−2 d−1 for the two study sites based on live root biomass, stems, leaves, and an incubation temperature of 15 °C. We cannot exclude overall smaller and seasonal different N2 fixation rates under in-situ conditions. Greater photosynthetically active radiation may improve the growth of root nodules and thus provides more niches for diazotrophs during the growing season. Lower in-situ temperatures would particularly limit the activity of diazotrophs in the early growing season. Further, we cannot exclude that preparation of L. fonkii altered the efficiency of N2 fixation during the incubation. Despite the methodological limitations, it seems that N2 fixation in root nodules of L. fonkii is one potent strategy of N acquisition in Patagonian bogs.
Considering recent studies on N2 fixation, plants and diazotrophs evolved different strategies to overcome N deficiency in ombrotrophic peatlands. Other pathways of N2 fixation include cyanobacteria, free-living diazotrophs or bryophyte-associated diazotrophs17,19,28,48,57, highlighting the diversity and niches of diazotrophs in ombrotrophic bogs. Further pathways of N acquisition, e.g. insect prey of Drosera sp.58, have also not yet been fully evaluated.
Comparing the BNF rates in other microhabitats, it seems that L. fonkii root nodules specifically colonized by diazotrophic Beijerinckiaceae represent ‘hot spots’ of BNF and thus of N acquisition. Existence of such specific associations and other reported strategies of N fixation challenge the current view on BNF in peatlands and in Podocarpaceae.
Additional Information
Accession codes: Sequences of nifH were deposited at the European Molecular Biology Laboratory (EMBL; www.ebi.ac.uk) under accession numbers LT221262-LT221526. Illumina 16S rRNA amplicon sequences were deposited at GenBank’s short reads archive under the following accession numbers: SRA accession, SRP073705; BioProject ID, PRJNA319299; BioSamples SAMN04884733, SAMN04884734, SAMN04884735, and SAMN04884736.
How to cite this article: Borken, W. et al. Associative nitrogen fixation in nodules of the conifer Lepidothamnus fonkii (Podocarpaceae) inhabiting ombrotrophic bogs in southern Patagonia. Sci. Rep. 6, 39072; doi: 10.1038/srep39072 (2016).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
We thank Andrés Fernández for providing access to the SKY site and we thank INIA Kampenaike in Punta Arenas and Carlos Rios and Osvaldo Vidal from the Instituto de la Patagonia for infrastructural support. We thank Rita Grotjahn for SEM. This study was co-funded by the Bavarian Research Alliance (BayFOR).
Footnotes
Author Contributions W.B., M.A.H., and K.-H.K. planned and designed the study. W.B., K.-H.K., and N.A.B.A. conducted field work, W.B. and K.-H.K. performed incubations, plant and peat analysis. M.A.H. performed molecular analyses, S.G. contributed the electron microscopy images. W.B., K.-H.K., and M.A.H wrote the manuscript with equal contributions of all other authors.
References
- Cleveland C. C. et al. Global patterns of terrestrial biological nitrogen (N2) fixation in natural ecosystems. Glob. Biogeochem. Cycles 13, 623–645, doi: 10.1029/1999gb900014 (1999). [DOI] [Google Scholar]
- Burns R. C. & Hardy R. W. Nitrogen fixation in bacteria and higher plants Vol. 21 (Springer, 1975). [DOI] [PubMed] [Google Scholar]
- Schwintzer C. R. Nitrogen-fixation by Myrica gale root nodules Massachusetts wetland. Oecologia 43, 283–294, doi: 10.1007/bf00344955 (1979). [DOI] [PubMed] [Google Scholar]
- Khan A. G. Podocarpus Root Nodules in Sterile Culture. Nature 215, 1170–1170 (1967). [Google Scholar]
- Russell A. J., Bidartondo M. I. & Butterfield B. G. The root nodules of the Podocarpaceae harbour arbuscular mycorrhizal fungi. New Phytol. 156, 283–295, doi: 10.1046/j.1469-8137.2002.00504.x (2002). [DOI] [PubMed] [Google Scholar]
- Becking J. H. Nitrogen fixation and mycorrhiza in podocarpus root nodules. Plant Soil 23, 213–226, doi: 10.1007/bf01358347 (1965). [DOI] [Google Scholar]
- Bergersen F. & Costin A. Root Nodules on Podocarpus Lawrencei and Their Ecological Significance. Aust. J. Biol. Sci. 17, 44–48, doi: 10.1071/BI9640044 (1964). [DOI] [Google Scholar]
- Spratt E. R. The Formation and Physiological Significance of Root Nodules in the Podocarpineae. Ann. Bot. os –26, 801–814 (1912). [Google Scholar]
- Grobbelaar N., Strauss J. & Groenewald E. Non-leguminous seed plants in southern Africa which fix nitrogen symbiotically. Plant Soil 35, 325–334 (1971). [Google Scholar]
- Huang B., Lü C., Wu B. & Fan L. A rhizobia strain isolated from root nodule of gymnosperm Podocarpus macrophyllus. Sci. China Ser. C 50, 228–233, doi: 10.1007/s11427-007-0034-0 (2007). [DOI] [PubMed] [Google Scholar]
- Silvester W. B. & Bennett K. J. Acetylene reduction by roots and associated soil of New Zealand conifers. Soil Biol. Biochem. 5, 171–179, doi: 10.1016/0038-0717(73)90107-7 (1973). [DOI] [Google Scholar]
- Baylis G. T. S. Mycorrhizal Nodules and Growth of Podocarpus in Nitrogen-poor Soil. Nature 223, 1385–1386 (1969). [Google Scholar]
- Dickie I. A. & Holdaway R. J. Podocarp Roots, Mycorrhizas, and Nodules. Smithsonian Contributions to Botany, 175–187 (2011). [Google Scholar]
- Turner B. L. & Cernusak L. Ecology of the Podocarpaceae in tropical forests. (Smithsonian Institution Scholarly Press, 2011).
- Hemond H. F. The Nitrogen Budget of Thoreau Bog. Ecology 64, 99–109 (1983). [Google Scholar]
- Larmola T. et al. Methanotrophy induces nitrogen fixation during peatland development. Proc. Natl. Acad. Sci. USA 111, 734–739, doi: 10.1073/pnas.1314284111 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vile M. et al. N2-fixation by methanotrophs sustains carbon and nitrogen accumulation in pristine peatlands. Biogeochemistry, 1–12, doi: 10.1007/s10533-014-0019-6 (2014). [DOI] [Google Scholar]
- Waughman G. & Bellamy D. Nitrogen fixation and the nitrogen balance in peatland ecosystems. Ecology, 1185–1198 (1980). [Google Scholar]
- Knorr K.-H., Horn M. A. & Borken W. Significant nonsymbiotic nitrogen fixation in Patagonian ombrotrophic bogs. Global Change Biol. 21, 2357–2365, doi: 10.1111/gcb.12849 (2015). [DOI] [PubMed] [Google Scholar]
- Godoy R., Paulino L., Oyarzún C. & Boeckx P. Atmospheric N deposition in central and southern Chile. An overview. Gayana Bot 60, 47–53 (2003). [Google Scholar]
- Basilier K., Granhall U. & Stenström T.-A. Nitrogen fixation in wet minerotrophic moss communities of a subarctic mire. Oikos, 236–246 (1978). [Google Scholar]
- Yu Z., Loisel J., Brosseau D. P., Beilman D. W. & Hunt S. J. Global peatland dynamics since the Last Glacial Maximum. Geophys. Res. Lett. 37, doi: L1340210.1029/2010gl043584 (2010). [Google Scholar]
- Broder T., Blodau C., Biester H. & Knorr K. H. Peat decomposition records in three pristine ombrotrophic bogs in southern Patagonia. Biogeosciences 9, 1479–1491, doi: 10.5194/bg-9-1479-2012 (2012). [DOI] [Google Scholar]
- Weaver R. W. & Danso S. K. A. Methods of Soil Analysis Part 2 (eds Weaver R. W., Angle J. S. & Bottomley P. S.) 1019–1045 (American Society of Agronomy, 1994). [Google Scholar]
- Dabundo R. et al. The contamination of commercial 15N2 gas stocks with 15N-labeled nitrate and ammonium and consequences for nitrogen fixation measurements. PLoS ONE 9, e110335, doi: 10.1371/journal.pone.0110335 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giller K. E. Use and abuse of the acetylene reduction assay for measurement of “associative” nitrogen fixation. Soil Biol. Biochem. 19, 783–784, doi: 10.1016/0038-0717(87)90066-6 (1987). [DOI] [Google Scholar]
- Minchin F. R., Witty J. F., Sheehy J. E. & Müller M. A major error in the acetylene reduction assay: decreases in nodular nitrogenase activity under assay conditions. J. Exp. Bot. 34, 641–649, doi: 10.1093/jxb/34.5.641 (1983). [DOI] [Google Scholar]
- Leppanen S., Rissanen A. & Tiirola M. Nitrogen fixation in Sphagnum mosses is affected by moss species and water table level. Plant Soil 389, 185–196, doi: 10.1007/s11104-014-2356-6 (2015). [DOI] [Google Scholar]
- Mohr W., Grosskopf T., Wallace D. W. & LaRoche J. Methodological underestimation of oceanic nitrogen fixation rates. PLoS ONE 5, e12583 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Well R., Becker K. W., Langel R., Meyer B. & Reineking A. Continuous flow equilibration for mass spectrometric analysis of dinitrogen emissions. Soil Sci. Soc. Amer. J. 62, 906–910 (1998). [Google Scholar]
- Dallinger A. & Horn M. A. Agricultural soil and drilosphere as reservoirs of new and unusual assimilators of 2,4‐dichlorophenol carbon. Environ. Microbiol. 16, 84–100 (2014). [DOI] [PubMed] [Google Scholar]
- Gaby J. C. & Buckley D. H. A comprehensive evaluation of PCR primers to amplify the nifH gene of nitrogenase. PLoS ONE 7 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebel M. E. et al. JAguc - a software package for environmental diversity analyses. J. Bioinform. Comput. Biol. 9, 749–773 (2011). [DOI] [PubMed] [Google Scholar]
- Palmer K., Biasi C. & Horn M. A. Contrasting denitrifier communities relate to contrasting N2O emission patterns from acidic peat soils in arctic tundra. ISME J 6, 1058–1077, doi: 10.1038/ismej.2011.172 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschul S. F., Gish W., Miller W., Myers E. W. & Lipman D. J. Basic local alignment search tool. J. Mol. Biol. 215, 403–410 (1990). [DOI] [PubMed] [Google Scholar]
- Klindworth A. et al. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. gks808 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso J. G. et al. QIIME allows analysis of high-throughput community sequencing data. Nature Methods 7, 335–336 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrity G. M., Bell J. A. & Lilburn T. G. Taxonomic outline of the prokaryotes. Bergey’s manual of systematic bacteriology (Springer, 2004).
- Phillips D. A. Efficiency of Symbiotic Nitrogen Fixation in Legumes. Annual Review of Plant Physiology 31, 29–49, doi: 10.1146/annurev.pp.31.060180.000333 (1980). [DOI] [Google Scholar]
- Bellenger J. P., Xu Y., Zhang X., Morel F. M. M. & Kraepiel A. M. L. Possible contribution of alternative nitrogenases to nitrogen fixation by asymbiotic N2-fixing bacteria in soils. Soil Biol. Biochem. 69, 413–420, doi: 10.1016/j.soilbio.2013.11.015 (2014). [DOI] [Google Scholar]
- Warembourg F. R., Lafont F. & Fernandez M. P. Economy of symbiotically fixed nitrogen in red clover (Trifolium pratense L.). Ann. Bot. 80, 515–523, doi: 10.1006/anbo.1997.0484 (1997). [DOI] [Google Scholar]
- Burbano C. S., Gronemeyer J. L., Hurek T. & Reinhold-Hurek B. Microbial community structure and functional diversity of nitrogen-fixing bacteria associated with Colophospermum mopane. FEMS Microbiol. Ecol. 91, doi: 10.1093/femsec/fiv030 (2015). [DOI] [PubMed] [Google Scholar]
- da Silva M. D. S. et al. Nitrogen-fixing bacteria in Eucalyptus globulus plantations. PLoS ONE 9, doi: 10.1371/journal.pone.0111313 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaintreuil C. et al. Photosynthetic bradyrhizobia are natural endophytes of the African wild rice Oryza breviligulata. Appl. Environ. Microbiol. 66, 5437–5447, doi: 10.1128/aem.66.12.5437-5447.2000 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halda-Alija L. Identification of indole-3-acetic acid producing freshwater wetland rhizosphere bacteria associated with Juncus effusus L. Can. J. Microbiol. 49, 781–787, doi: 10.1139/w03-103 (2003). [DOI] [PubMed] [Google Scholar]
- Liang Y. et al. Nonlegumes respond to rhizobial nod factors by suppressing the innate immune response. Science 341, 1384–1387, doi: 10.1126/science.1242736 (2013). [DOI] [PubMed] [Google Scholar]
- Vorobev A. V. et al. Methyloferula stellata gen. nov., sp nov., an acidophilic, obligately methanotrophic bacterium that possesses only a soluble methane monooxygenase. Int. J. Syst. Evol. Microbiol. 61, 2456–2463, doi: 10.1099/ijs.0.028118-0 (2011). [DOI] [PubMed] [Google Scholar]
- Bragina A., Berg C., Müller H., Moser D. & Berg G. Insights into functional bacterial diversity and its effects on Alpine bog ecosystem functioning. Sci. Rep. 3, 1955 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menge D. N. L., Troy Baisden W., Richardson S. J., Peltzer D. A. & Barbour M. M. Declining foliar and litter δ15N diverge from soil, epiphyte and input δ15N along a 120 000 yr temperate rainforest chronosequence. New Phytol. 190, 941–952, doi: 10.1111/j.1469-8137.2010.03640.x (2011). [DOI] [PubMed] [Google Scholar]
- Brearley F. Q. Nitrogen stable isotopes indicate differences in nitrogen cycling between two contrasting Jamaican montane forests. Plant Soil 367, 465–476, doi: 10.1007/s11104-012-1469-z (2013). [DOI] [Google Scholar]
- Dunfield P. F., Belova S. E., Vorob’ev A. V., Cornish S. L. & Dedysh S. N. Methylocapsa aurea sp. nov., a facultative methanotroph possessing a particulate methane monooxygenase, and emended description of the genus Methylocapsa. Int. J. Syst. Evol. Microbiol. 60, 2659–2664, doi: 10.1099/ijs.0.020149-0 (2010). [DOI] [PubMed] [Google Scholar]
- Zhao Y., Bian S. M., Zhou H. N. & Huang J. F. Diversity of nitrogenase systems in diazotrophs. J. Integr. Plant Biol. 48, 745–755 (2006). [Google Scholar]
- Marín I. & Arahal D. R. The Prokaryotes: Alphaproteobacteria and Betaproteobacteria (eds Eugene Rosenberg et al.) 115–133 (Springer, 2014). [Google Scholar]
- Xu Z., Hansen M. A., Hansen L. H., Jacquiod S. & Sørensen S. J. Bioinformatic Approaches Reveal Metagenomic Characterization of Soil Microbial Community. PLoS ONE 9, e93445, doi: 10.1371/journal.pone.0093445 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz-Marquez J. C. F., Do Nascimento M., de los Angeles Dublan M. & Curatti L. Association with an ammonium-excreting bacterium allows diazotrophic culture of oil-rich eukaryotic microalgae. Appl. Environ. Microbiol. 78, 2345–2352 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyes A. B. et al. Evidence for foliar endophytic nitrogen fixation in a widely distributed subalpine conifer. New Phytol. 210, 657–668, doi: 10.1111/nph.13850 (2016). [DOI] [PubMed] [Google Scholar]
- Berg A., Danielsson A. & Svensson B. H. Transfer of fixed-N from N2-fixing cyanobacteria associated with the moss Sphagnum riparium results in enhanced growth of the moss. Plant Soil 362, 271–278, doi: 10.1007/s11104-012-1278-4 (2013). [DOI] [Google Scholar]
- Millett J., Jones R. I. & Waldron S. The contribution of insect prey to the total nitrogen content of sundews (Drosera spp.) determined in situ by stable isotope analysis. New Phytol. 158, 527–534, doi: 10.1046/j.1469-8137.2003.00763.x (2003). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.