Abstract
Classically, white adipose tissue (WAT) was considered an inert component of connective tissue but is now appreciated as a major regulator of metabolic physiology and endocrine homeostasis. Recent work defining how WAT develops and expands in vivo emphasizes the importance of specific locations of WAT or depots in metabolic regulation. Interestingly, mature white adipocytes are integrated into several tissues. A new perspective regarding the in vivo regulation and function of WAT in these tissues has highlighted an essential role of adipocytes in tissue homeostasis and regeneration. Finally, there has been significant progress in understanding how mature adipocytes regulate the pathology of several diseases. In this review, we discuss these novel roles of WAT in the homeostasis and regeneration of epithelial, muscle, and immune tissues and how they contribute to the pathology of several disorders.
Keywords: adipose tissue, regeneration, skin, adipocyte
INTRODUCTION
In vertebrates, mature white adipocytes store energy in the form of triglycerides, control organismal energy balance and metabolism through hormone or adipokine secretion, and provide mechanical cushioning for organs (Rosen & Spiegelman 2006). Pathologically, the expansion of white adipose tissue (WAT) during obesity is associated with metabolic and cardiovascular disease, with defects in wound healing, and with cancer. Recent studies have highlighted novel roles of WAT in tissue regeneration and stem cell regulation, highlighting the central role of adipose tissue in multiple aspects of organismal biology. In this review, we focus on the roles of adipocytes in skin, mammary gland (MG), and skeletal muscle homeostasis and repair.
TYPES OF ADIPOSE TISSUE
Mammals have two major types of adipose tissue with distinct functions: brown adipose tissue (BAT) and WAT. Mature adipocytes in BAT contain multiple small lipid droplets and a high number of mitochondria, whereas mature adipocytes in WAT contain few mitochondria and a unilocular lipid droplet for triglyceride storage (Berry & Rodeheffer 2013). BAT generates heat from a lipolytic process that involves uncoupling of the mitochondrial proton gradient from ATP generation (Nicholls & Locke 1984). In contrast, the triglycerides in WAT are released and transported to tissue for use as energy through oxidation (Frayn et al. 2006).
Both BAT and WAT develop in discrete locations within the body as large collections of adipocytes that are identified as specific depots. Large depots of WAT found in the subcutaneous and visceral regions of mammalian organisms have been studied due to the association of visceral fat with metabolic disease (Lee et al. 2013). The subcutaneous WAT (sWAT) and visceral WAT (vWAT) depots display distinct adipokine secretions and rates of lipolysis and triglyceride synthesis (Tchkonia et al. 2013). Whereas several recent reviews have discussed the biology, function, and regulation of traditional WAT depots (Rosen & Spiegelman 2006), here we focus on the role of WAT in additional depots associated with the skin, MG, bone marrow, and skeletal muscle.
BIRTH AND DYNAMICS OF DISTINCT ADIPOSE DEPOTS
The development and maintenance of WAT occur through distinct mesenchymal precursor cells that differentiate into mature adipocytes. Genetic lineage-tracing tools in mice have revealed that the various depots develop at distinct times and from specific mesenchymally derived progenitor populations (reviewed in Sanchez-Gurmaches & Guertin 2014). For instance, dorsal-anterior adipocyte depots arise from Myf5- or Pax3-expressing precursor cells, and cephalic adipocytes arise from Sox10-expressing neural crest precursors (Billon et al. 2007). In the skin, delta-like homologue 1 (Dlk1)-expressing embryonic mesenchymal precursors generate both dermal fibroblasts and adipocytes until E16.5, when they become restricted in their contribution to the dermal WAT (dWAT) (Driskell et al. 2013, 2014; Wojciechowicz et al. 2008, 2013), and lipid-filled mature adipocytes are present in the dermis of mice following the formation of hair follicles (Hausman et al. 1981) (Figure 1). In mice, the three anterior MG pairs form in thoracic WAT depots, which are partially associated with BAT, whereas two posterior MG pairs develop in the inguinal WAT pad (Gouon-Evans & Pollard 2002, Hovey et al. 1999).
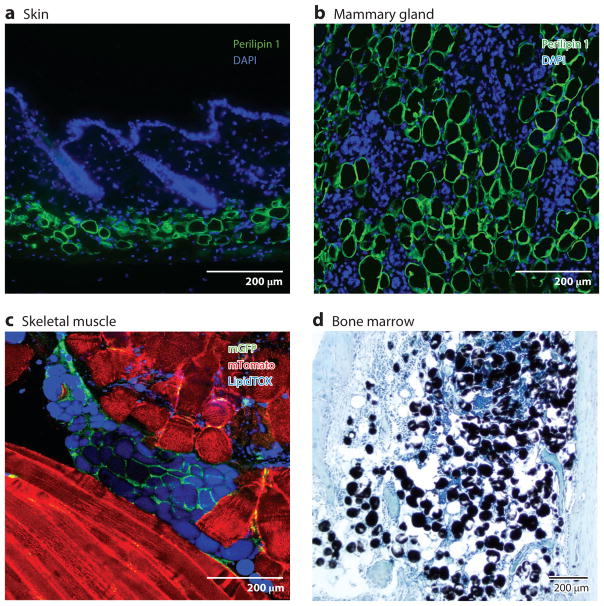
Figure 1.
White adipocytes reside in multiple tissues. (a) In skin, small perilipin 1–positive adipocytes are located in the deepest layer of the dermis. (b) Perilipin 1–positive adipocytes are found within the mammary gland stroma. The images show mouse mammary glands after 7 days after involution. (c) Lipid-filled mature adipocytes (LipidTOX positive) are present in skeletal muscle following injury. These cells are lineage traced from adipocyte precursor cells by crossing PdgfraCre with an mTomato/mGFP (membrane tomato/membrane GFP) reporter mouse. mGFP indicates lineage tracing. (d) Bone marrow adipose tissue expands in response to many diseases. Adipocytes in bone marrow are stained black by using osmium, and tissue is stained with toluidine blue. Images courtesy of Dr. Matthew Rodeheffer (panel c) and Dr. Mark Horowitz (panel d).
Studies of human adipocytes labeled with stable isotopes during nuclear weapons testing revealed that turnover of mature adipocytes is approximately 10% per year (Spalding et al. 2008). However, WAT depots can expand or reduce their mass dramatically in response to nutritional states (Krotkiewski & Björntorp 1975, Krotkiewski et al. 1983). The expansion of WAT can occur through hypertrophy (an increase in mature adipocyte size) or hyperplasia (an increase in mature adipocyte number). During states of overnutrition, the accumulation of lipid rapidly promotes hypertrophy of mature adipocytes (Krotkiewski et al. 1983). A major advance in our understanding of the regulation of WAT hyperplasia came with in vivo identification of adipocyte precursor cells (APs) within WAT that are identified by several cell surface proteins, including Sca1 and CD24, and that express Pdgfrα (Berry & Rodeheffer 2013, Joe et al. 2010, Rodeheffer et al. 2008).
In response to overfeeding, individual WAT depots display distinct mechanisms of growth. In humans, upper-body sWAT depots display hypertrophic growth, whereas hyperplasia contributes to expansion of depots below the waist (Tchoukalova et al. 2010). Distinct responses of individual depots to dietary cues also occur in mice. Male mice fed a high-fat diet (HFD) display hyperplasia in the visceral depot, but not in the sWAT (Jeffery et al. 2015). These hyperplastic responses are in part due to proliferation and differentiation of APs in response to HFD in an Akt-dependent manner (Jeffery et al. 2015).
Dramatic changes in WAT mass can also occur in dWAT in response to multiple stimuli. As hair follicles regress and grow cyclically during the hair cycle, adipocytes regress and expand in parallel with the hair follicle to double the size of the dermis (Chase et al. 1953). During hair follicle growth, dWAT displays a dynamic and rapid expansion of mass due to both hypertrophy and hyperplasia via Sca1+ AP proliferation (Festa et al. 2011). Recent work has also revealed that dWAT expands in mice in response to skin bacterial infection (Zhang et al. 2015). Interestingly, whereas cold stress can induce alterations in white adipocytes toward a brown adipose phenotype in other depots (Jankovic et al. 2015), dWAT expands in response to cold stress (Kasza et al. 2014).
Adipose tissue is also highly dynamic during the pregnancy, lactation, and involution cycle in the MG (Figure 1). MG adipocytes expand by almost 20% during pregnancy (Elias et al. 1973, Pujol et al. 2006). During lactation, the MG epithelium of rodents differentiates to produce milk, and stromal adipocytes are depleted (Elias et al. 1973). Cessation of lactation results in a robust regression of MG epithelium (Watson 2006), and the stromal adipose tissue in mice expands rapidly to repopulate the stroma within a few days (Elias et al. 1973). These dynamic changes in MG WAT have been reported to occur through transdifferentiation from epithelial cells in the gland (Morroni et al. 2004). Future studies exploring whether resident APs or other cell types contribute to WAT dynamics in the MG will be interesting avenues of investigation.
In addition to developing in depots within the skin and MG, WAT develops in skeletal muscle during muscle atrophy (Figure 1). Tissue-resident APs exist in skeletal muscle and, as in other depots, express Pdgfrα, Sca1, and CD34 (Joe et al. 2010, Uezumi et al. 2010), similar to APs found in WAT depots (Festa et al. 2011, Rodeheffer et al. 2008). These cells have the dual capacity to generate fibroblasts and adipocytes and are thus thought to represent a fibro-adipogenic progenitor (FAP). Not surprisingly, other muscle locations, such as cardiac muscle, also show the presence of adipocytes that are generated by stromal cells (Sommariva et al. 2016). Although skeletal muscle adipose tissue emerges in different injury and disease states, whether WAT expands in skeletal muscle in other conditions in rodents is unclear.
An additional depot of WAT exists in the marrow space of bone. Although bone marrow WAT (mWAT) stores triglycerides, as does WAT in other depots (Tavassoli et al. 1977) (Figure 1), the size of mature adipocytes in mWAT is smaller. Surprisingly, in contrast to the reduction of most WAT depots with caloric restriction, mWAT expands in the anorexic mouse and human (Bredella et al. 2009, Devlin et al. 2010, Fazeli et al. 2012). Sca1+, CD24+ adipogenic precursors present in other WAT depots are not found in mWAT (Fazeli et al. 2013). Yet, mature adipocytes within mWAT do form from precursor cells that express Pdgfra, Osterix1, Leptin receptor, and Nestin (Mizoguchi et al. 2014, Morikawa et al. 2009, Pinho et al. 2013, Zhou et al. 2014), suggesting that the cellular progenitors that maintain mWAT may be distinct from those of other WATs. Thus, there may be a mesenchymal multipotent precursor cell in bone marrow capable of giving rise to multiple lineages, including bone, cartilage, and mWAT.
FUNCTIONS OF ADIPOSE DEPOTS IN TISSUE DEVELOPMENT, HOMEOSTASIS, AND REGENERATION
Skin
Mature adipocytes are one of the most abundant cell types within the skin’s dermis, which supports the epithelial keratinocytes of the epidermis. Although dWAT was previously believed to merely provide insulation and protective cushioning, tremendous strides have been made in our understanding of how dWAT contributes to skin biology, supporting the current view that dWAT is central to cutaneous function.
Hair follicle regeneration
One role of dWAT is the intimate regulation of hair follicle growth by adipocyte lineage cells (Figure 2). The growth of hair follicles in skin is tightly regulated and can be divided into specific stages (Schneider et al. 2009, Stenn & Paus 2001). The activation of hair follicle growth (anagen) involves the proliferation of epithelial stem cells located in the bulge region of the hair follicle (Blanpain et al. 2004, Cotsarelis et al. 1990, Zhang et al. 2009) and their communication with the mesenchymal component of the hair follicle, the dermal papillae (Jahoda et al. 1984, Rompolas et al. 2012). Following anagen, the hair follicle enters into a regression state (catagen) followed by a phase of relative quiescence termed telogen.
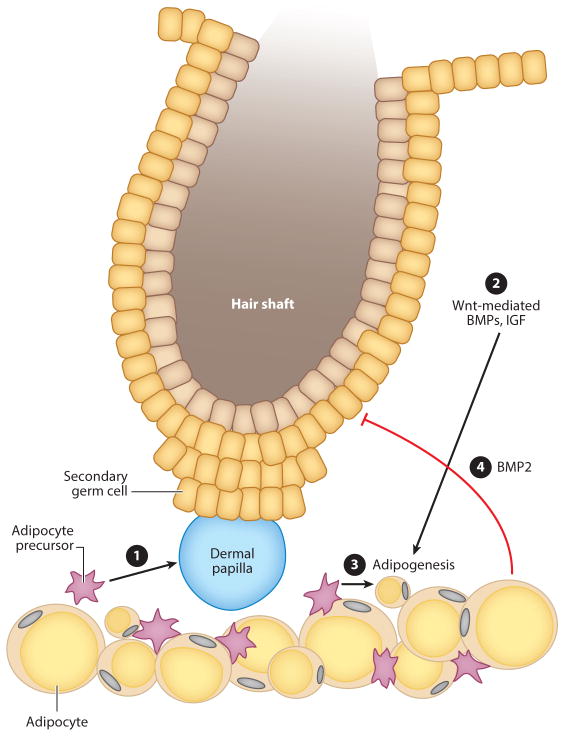
Figure 2.
Interaction between dermal white adipose tissue (dWAT) and hair follicle cycling. Hair follicles undergo cyclic growth (anagen), regression (catagen), and rest (telogen). ➊ During the telogen stage, signaling from adipocyte precursor cells (APs) activates hair follicle growth. ➋, ➌ Adipogenic factors are released by keratinocytes downstream of Wnt/β-catenin signaling, resulting in expansion of dWAT. Adipocyte size continues to increase during anagen. ➍ Adipocytes express high levels of BMP2 during late catagen/early telogen; BMP2 maintains the hair follicle in a resting state. Proliferation leads to AP pool expansion during this stage of the hair follicle cycle.
Mature adipocytes in dWAT may be key regulators of the rest stage of the hair cycle through the expression of Bmp2 mRNA (Festa et al. 2011, Plikus et al. 2008) (Figure 2). BMP signaling in hair follicle stem cells is required for their quiescence (Kandyba et al. 2013, Kobielak et al. 2007), and the increased formation of mature adipocytes during hair growth leads to enhanced levels of Bmp ligand expression in the dermis (Plikus et al. 2008). The combination of BMP expression by adipocytes and that by dermal papillae cells (Sennett & Rendl 2012) may suppress stem cell activity. Later in hair growth, adipocyte-derived BMPs may also support hair lineage specification and/or differentiation (Kobielak et al. 2003, Kwan et al. 2004) because hair follicle lineage cells detect BMP ligands at specific stages of differentiation (Genander et al. 2014).
In addition, immature APs can stimulate hair follicle stem cell activity (Festa et al. 2011). Genetic and pharmacological loss of APs in the skin delays hair growth initiation. Furthermore, intradermal transplantation of APs activates hair follicle growth, indicating that APs are a key component of the hair follicle stem cell niche. Mature intradermal adipocytes express the adipokine leptin and may also stimulate hair growth, as treatment of human hair follicle organ cultures with leptin can enhance hair shaft length (Yang et al. 2015), and injection of leptin into mouse skin can induce activation of hair follicle stem cells and hair growth (Sumikawa et al. 2014, Watabe et al. 2014). Whether adipocytes signal directly to epithelial cells or act through the dermal papilla remains to be determined.
The interaction between dWAT and hair follicles seems to be bidirectional, as activation of hair follicle growth through epidermal activation of Wnt/β-catenin stimulates expansion of dWAT (Donati et al. 2014) (Figure 2). Although the precise mechanism is not known, in vitro experiments suggest that keratinocytes secrete proadipogenic molecules such as Bmp2, Bmp6, and Igf2 (Donati et al. 2014). Unanswered questions remain regarding the interaction between the hair follicle and intradermal adipocytes. Are signaling pathways activated during skin adipogenesis, as are pathways activated during adipogenesis in other depots? Do alterations in dWAT contribute to pathologies with defective hair follicle growth? Addressing these questions will provide a better understanding of the relationship between adipose tissue, epidermis, and hair growth and could lead to new treatments to remedy hair loss.
Wound healing
Although changes in dWAT were observed in association with wound healing decades ago (Montagna et al. 1988), understanding of how adipocyte lineage cells aid skin repair following injury is in its infancy. Although various cells with mesenchymal stem cell markers are important in dermal wound healing, adipose-derived stem cells are an attractive substitute for mesenchymal stem cells from bone marrow because the former cells are more easily accessible, are more available, and are often discarded as medical waste (Condé-Green et al. 2016). Multiple groups have investigated the role of adipocyte lineage cells during skin repair in an attempt to harness their therapeutic potential.
Intradermal injection of adipose-derived stem cells following injury accelerates dermal wound healing in mice (Kim et al. 2007). These cells survive for up to one year after dermal injection, without detection in distant immune organs or tumor formation at the site of injection (Koellensperger et al. 2014). A common observation across these models is an increase in angiogenesis; this increase is mediated by vascular endothelial growth factor (VEGF). Consequently, using adipose-derived stromal cells as delivery agents for VEGF to dorsal wounds accelerated healing (Nauta et al. 2012, Song et al. 2012). Even though adipose-derived stem cells can accelerate wound healing, the heterogeneity of cells described as adipose-derived (mesenchymal) stem or stromal cells do not have a universal definition or preparation protocol (Baer & Geiger 2012). Adherent cells isolated from the stromal vascular fraction of enzymatically digested adipose tissue are frequently used for transplantation. This adherent fraction consists of APs, fibroblasts, and various immune cells. Additionally, confusion persists over the boundaries, relationships, and classification of closely associated WAT depots in humans, as the terms dermal, intradermal, subcutaneous, hypodermis, or simply skin are inaccurately used interchangeably (Driskell et al. 2014).
In addition to immature adipocyte lineage cells, mature adipocytes have been implicated in regulating skin wound healing. Multiple adipokines can alter the rate of wound repair. The two primary adipokines that have been investigated during wound healing are adiponectin and leptin. The most striking wound healing phenotype in adiponectin-deficient mice is severely delayed reepithelialization. Cultured keratinocytes treated with adiponectin show increased proliferation and migration through ERK signaling and in vivo administration of adiponectin to mouse skin wounds, accelerated wound healing, and reepithelialization (Jin et al. 2015, Salathia et al. 2012, Shibata et al. 2012). Administration of adiponectin in an ear punch model accelerated reepithelialization and wound closure so effectively that wound contraction and complete dermal repair were delayed (Salathia et al. 2012). Interestingly, another group demonstrated that adiponectin can enhance apoptosis, suggesting that decreased levels of adiponectin in diabetic ulcers may contribute to peripheral hyperkeratosis through persistence of the wound-associated hyperthickened epithelium (Kawai et al. 2008). These data demonstrate that adiponectin is an important factor for driving reepithelialization of skin wounds and a promising therapeutic target for delayed wound healing.
In addition to adiponectin, leptin promotes wound healing. Leptin signaling can enhance reepithelialization and angiogenesis following injury (Frank et al. 2000, Ring et al. 2000, Sierra-Honigmann et al. 1998). The systemic or local application of leptin to leptin-deficient (ob/ob) mice accelerates reepithelialization and contraction of full-thickness skin wounds independently of revascularization (Frank et al. 2000, Ring et al. 2000).
Although the majority of wound healing studies have focused on revascularization and reepithelialization of wound beds, deep dermal fat is present within hypertrophic scars (Matsumura et al. 2001), suggesting a relationship between dWAT and extracellular matrix (ECM) deposition. Interestingly, stromal cells from skin-associated adipose tissue have increased levels of collagen 1, alpha smooth muscle actin (αSMA), and lysyl hydroxylase-2b (a critical enzyme in collagen cross-linking) compared with levels in total dermis (van den Bogaerdt et al. 2009). Additionally, purified human adipose-derived stem cells can increase human dermal fibroblast proliferation, migration, and production of collagen 1 (Kim et al. 2007). These data suggest that the local WAT environment may be promoting a more fibrotic phenotype of resident fibroblasts. We have shown that adipocytes are required for fibroblast repopulation of dorsal skin wound beds by triggering fibroblast migration (Schmidt & Horsley 2013). A-ZIP mice, which lack mature adipocytes, initially have normal reepithelialization of wound beds but subsequently show impaired fibroblast repopulation of wound beds. An altered fibroblast response was not seen in diabetic ob/ob mice, suggesting that this result is not the sequela of systemic metabolic derangement but is rather due to the lack of adipocytes. This study was confirmed using pharmacological antagonists of PPARγ, which inhibits adipogenesis and results in reduced wound bed fibroblast repopulation (Schmidt & Horsley 2013). Impaired fibroblast reconstitution and ECM deposition resulted in recurring wounds in these models.
These findings demonstrate the tremendous translational value of adipocyte lineage cells in cutaneous wound healing. In the future, it is important for the field to have more unified definitions of adipose-derived cells and for future work to parse out the differences in function between adipose-derived stem cells, adipocytes, and other cells present in adipose tissue during wound healing beyond angiogenesis and fibroblast deployment.
Thermoregulation
The role of the skin in thermoregulation is not fully understood (Romanovsky 2014). Recent evidence suggests that dWAT responds to and may regulate thermoregulation in response to cold stress (Kasza et al. 2014). Kasza et al. (2014) showed that cold stress in mice can expand dWAT thickness in addition to its additional physiological changes in activating uncoupling protein-1 (UCP-1) in BAT and a cold stress response in liver glycogen and oxygen consumption. Interestingly, in mice lacking the heparin sulfate proteoglycan syndecan-1 (Sdc−/− mice), dWAT thickness was significantly decreased, whereas other WAT depots were not dramatically altered. In response to overnight fasting at room temperature, Sdc−/− mice exhibited lethargy, systemic metabolic defects, and activation of UCP-1 and stress-induced p38α in BAT, similar to what is observed in response to cold stress. This phenotype was prevented by housing mice at thermoneutral conditions (31°C), indicating that loss of syndecan-1 leads to systemic cold stress in mice housed at 21°C. The authors demonstrated that loss of syndecan-1 resulted in decreased adipogenesis in culture and that administration of the PPARγ agonist rosiglitazone was able to rescue dWAT thickness and prevent the cold stress–induced response of Sdc−/− mice housed at 21°C (Kasza et al. 2014). Interestingly, the diminished dWAT thickness in Sdc−/− mice was rectified during hair growth, indicating that adipogenic and hypertrophic signals during hair cycling do not rely on syndecan-1. How thermal changes control dWAT and the role of dWAT in thermal protection will be interesting areas of future investigation.
Mammary Gland
Although WAT composes approximately 80% of the MG stroma, its function has only recently been explored. Several factors secreted by adipocytes can promote MG tumorigenesis (Iyengar et al. 2003, 2005) and differentiation of mammary epithelial organoids (Howlett & Bissell 1993, Zangani et al. 1999). The development of a functional mammary ductal tree structure requires mature adipocytes because lipodystrophic A-ZIP mice (Moitra et al. 1998) and a mouse model with inducible loss of adipocytes (Pajvani et al. 2005) display rudimentary mammary anlagen and severely distended mammary ducts (Couldrey et al. 2002, Landskroner-Eiger et al. 2010). Furthermore, the transplantation of embryonic mammary epithelium, single MG stem cells, or sweat gland epithelial progenitors into WAT is sufficient to induce morphogenesis of the mammary ductal tree (Lu et al. 2012, Sakakura et al. 1982, Stingl et al. 2006). Interestingly, leptin may be involved in mammary gland development because ob/ob mice and db/db mice (mice lacking the leptin receptor) lack postnatal ductal epithelium (Hu et al. 2002).
Skeletal Muscle
Although adipose tissue in other depots can positively affect tissue function, the presence of skeletal muscle–associated adipose tissue is associated mostly with muscular disorders (Wallace & McNally 2009) and is correlated with insulin resistance and increased body mass index (Albu et al. 2005, Yim et al. 2007). Whereas defective wound repair occurs in skeletal muscle of ob/ob and db/db mutant mice (Nguyen et al. 2011), mature adipocytes are sparse in young, healthy skeletal muscle, and an abundant pool of FAPs resides in skeletal muscle (Joe et al. 2010; Uezumi et al. 2010, 2011). Whereas mature adipocytes may impair muscle physiology, FAPs contribute to successful wound healing. Three to four days after acute muscle injury, FAPs are more abundant at wound edges and dramatically increase proliferation (Joe et al. 2010, Uezumi et al. 2010). Coculturing myogenic precursors with FAPs enhances muscle differentiation, possibly through expression of antiadipogenic, promyogenic Igf-1, IL-6, Wnt1, Wnt3A, and Wnt5A (Joe et al. 2010). Interestingly, a similar population of cells exists in human skeletal muscle (Arrighi et al. 2015, Uezumi et al. 2014). Given that degenerative muscle fibers are able to induce adipogenesis (Hosoyama et al. 2009), accumulation of mature adipocytes may be secondary to, and not the cause of, deterioration of muscle tissue. It will be exciting for future studies to determine whether mature adipocytes have a functional contribution to the dysfunction of muscle tissue seen in disease states.
Immunity
Communication between adipocytes and immune cells is an emerging area with tremendous implications for tissue regeneration and stem cell biology. Mesenchymal precursors within the bone marrow that can form adipocytes are important for organizing hematopoiesis by regulating hematopoietic stem cells through BMP (Zhang et al. 2003) and Tie2 (Arai et al. 2004) signaling. Adipocytes within mWAT may also regulate hematopoiesis (Naveiras et al. 2009). Outside of mWAT, several mouse models of obesity display an increase in diverse immune cell subsets in vWAT (Kanneganti & Dixit 2012). Indeed, salicylate anti-inflammatory agents were shown in the early 1900s to ameliorate diabetic symptoms in humans. However, only recently has the importance of WAT depots outside of the bone marrow in regulating immunity come to light.
In a lean state, adipose tissue macrophages (ATMs) are skewed toward an anti-inflammatory phenotype (described as M2, or alternatively activated) (DiSpirito & Mathis 2015, Kanneganti & Dixit 2012). This skewing of ATMs is necessary to support tissue expansion, and WAT signaling to the immune system is required to activate adipogenesis in vWAT during short-term HFD (Asterholm et al. 2014). As adipose tissue expands, ATMs stimulate angiogenesis through increased production of PDGFβ (Pang et al. 2008). Although the initial inflammatory process is beneficial for tissue growth, prolonged adipose tissue inflammation can lead to fibrosis, hindering adipocyte expansion and preventing further lipid storage (Khan et al. 2009, Pasarica et al. 2009). Continued expansion of WAT during obesity results in excess lipids and increased levels of macrophage chemoattractant-1 (MCP-1), which rapidly induces infiltration of a large number of ATMs into vWAT (Schipper et al. 2012, Xu et al. 2003) that can induce insulin resistance (reviewed in Osborn & Olefsky 2012). These ATMs are similar to inflammatory macrophages described in other tissues (DiSpirito & Mathis 2015, Lumeng et al. 2007, Nguyen et al. 2007). Ablation of inflammatory macrophages (Patsouris et al. 2008, Weisberg et al. 2006) or deletion of IKK-β (Arkan et al. 2005), JNK1 (Solinas et al. 2007), the insulin receptor (Mauer et al. 2010), or fatty acid–binding protein 4 (FABP4) (Furuhashi et al. 2008) in macrophages protects mice from obesity-induced insulin resistance.
Other local immune cells, such as regulatory T cells (Tregs), influence macrophage phenotype. Treg secretions elicit a Th2 immune response that promotes anti-inflammatory ATM gene expression and suppresses inflammatory immune responses that promote inflammatory macrophage migration in WAT during obesity (Feuerer et al. 2009) and aging (Cipolletta et al. 2015, Kolodin et al. 2015, Vasanthakumar et al. 2015). Increasing the number of Treg cells can improve insulin sensitivity during obesity (Feuerer et al. 2009). Furthermore, deletion of PPARG in Tregs resulted in reduced age-related weight gain and improved metabolic parameters, including insulin resistance, glucose tolerance, and calorie expenditure (Bapat et al. 2015).
Given the importance of inflammatory cytokines and macrophage phenotype in regenerative processes in many tissues after injury (Goren et al. 2010, Lucas et al. 2010, Sciorati et al. 2016), the impact of adipocytes on macrophage phenotype may influence tissue regeneration more broadly. Not only do skin macrophages influence hair follicle activation (Castellana et al. 2014), but unique macrophage phenotypes are associated with various phases of skin regeneration after injury (Daley et al. 2010, Mirza & Koh 2014). Macrophages are also required for the involution of the MG epithelium that is associated with MG WAT expansion (O’Brien et al. 2010). Given that obesity induces inflammatory pathways beyond vWAT, such as in the liver (Cai et al. 2005) and muscle (Bandyopadhyay et al. 2005, Itani et al. 2002), the strong correlation between obesity, inflammation, and comorbidity with many diseases underscores the importance of uncovering the complex interactions between immune cells and adipocytes. It is promising that future studies could uncover adipocyte and immune cell interactions that regulate reparative processes and disease states in different tissues.
ADIPOCYTES IN CUTANEOUS DISEASE
Just as adipose tissue contributes to proper function in various tissues, dysfunction of adipose tissue contributes to impaired physiology in multiple tissues. Several dermatological conditions in humans are associated with altered dermal adiposity. Recent studies have demonstrated a central role of adipocytes in multiple disease states. Below, we discuss how dWAT in a lean state has a protective role in skin homeostasis, how loss of adipocytes corresponds with the progression of certain cutaneous diseases, and how altered adipocyte biology associated with obesity promotes disease progression.
Skin Disorders
Various lipodystrophic conditions are characterized by hair loss or alopecia concomitantly with a loss of adipose tissue; however, a causative role of human dWAT in hair follicle maintenance has not been described (Fukumoto et al. 2009, Hegele 2005, Jeninga et al. 2009). Additionally, as many as 61% of patients suffering from anorexia have hair loss or fragile hair (Strumia 2009), along with lower levels of leptin (Janas-Kozik et al. 2011, Uzum et al. 2009). Hyperkeratosis, immune cell infiltration, and alopecia can also be observed in the scalps of patients experiencing lipoedema (Bridges et al. 2000, Fukumoto et al. 2009, Piraccini et al. 2006), and these symptoms can worsen as adiposity increases. Moreover, lipodystrophic individuals with mutations in PPARG frequently exhibit hyperpigmentation, hirsutism, and cutaneous eruptive xanthomata (Hegele 2005, Herrmann et al. 2003). Future studies are required to determine whether changes in skin biology are a direct result of altered dWAT or whether changes in adiposity are a symptom of the disease.
Infection
A recent study demonstrated that dermal adipocytes contribute directly to the innate immune response following skin infection (Zhang et al. 2015). Shortly after Staphylococcus aureus infection of mouse back skin, local AP proliferation and hypertrophy of mature adipocytes lead to a dramatic expansion of dWAT (Figure 3). As adipose tissue expands, mature adipocytes produce the antimicrobial peptide cathelicidin. Pharmacological inhibition of adipogenesis during infection reduced host ability to combat infection and resulted in greater numbers of S. aureus colony-forming units. Additionally, cultured human adipocytes increased expression of cathelicidin in response to S. aureus, indicating that human adipocytes may also contribute to innate immunity following infection (Zhang et al. 2015). Interestingly, common inflammatory skin diseases such as atopic dermatitis, psoriasis, and rosacea are characterized by defects in cutaneous cathelicidin. Future studies will be required to determine whether changes in adipocyte-derived cathelicidin contribute to the pathology of these diseases.
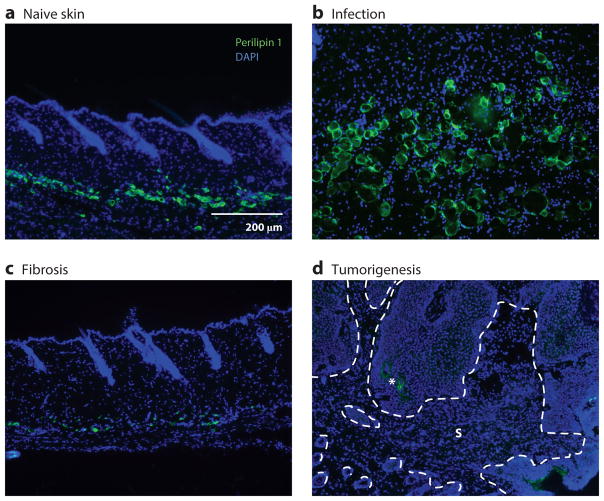
Figure 3.
Dermal white adipose tissue (dWAT) mature adipocytes are altered during cutaneous disease. (a) In naive skin in the rest stage of the hair cycle, small perilipin 1–positive mature adipocytes reside deep in the dermis. (b) Three days after infection of mouse skin with subcutaneous injections of Staphylococcus aureus, dWAT undergoes a dramatic expansion. (c) During bleomycin-induced skin fibrosis in mice, perilipin 1–positive cells become depleted. (d) Perilipin 1–positive adipocytes are absent from the stromal compartment of TPA/DMBA-induced skin tumors. The dotted lines denote the epithelial-stroma (S) border. The asterisk indicates autofluorescence. Image in panel b courtesy of Dr. Richard Gallo and Dr. Ling-juan Zhang.
Fibrosis
Just as adipocytes and their precursor cells play important roles in scar deposition during wound healing, it is becoming increasingly appreciated that adipocytes may play key roles in the development of fibrosis. Fibrosis is a chronic pathology characterized by excessive and chronic ECM deposition in the interstitial spaces of affected organs, ultimately leading to increased organ stiffness and loss of function (Friedman et al. 2013, Wynn 2008). In dermal fibrosis, deposition of fibrous ECM often occurs in conjunction with subcutaneous adipose tissue atrophy (Figure 3), a phenomenon observed in both human scleroderma patients and animal models (Fleischmajer et al. 1971, Smith & Chan 2010). Fibrosis also manifests in many diverse pathologies, including obesity, epithelial tumors, and cancer cachexia (Bing et al. 2006, Bochet et al. 2013, Sun et al. 2013).
The primary producer of ECM in fibrosis is an activated cell type known as the myofibroblast, a contractile, fibroblast-like cell that produces large amounts of collagens and SMA (Gabbiani et al. 1971, Hinz et al. 2012). The origins of these cells are largely debated due to the diversity of lineage-tracing schemes in different fibrosis models, as we recently reviewed (Ebmeier & Horsley 2015). Recent evidence has pointed to a possible adipocyte origin of some myofibroblasts in dermal fibrosis. Through the use of a lineage-tracing strategy in which adiponectin-driven Cre recombinase was used to label mature adipocytes with tdTomato, adiponectin-traced cells lost labeling with the adipocyte lipid vesicle marker perilipin and colocalized with αSMA in bleomycin-induced dermal fibrosis (Marangoni et al. 2014). This development was accompanied by a morphological change of the cells from a round adipocyte-like morphology to a spindle-shaped fibroblast-like morphology. The authors also reported that a loss of adipogenic gene expression preceded increased expression of fibrogenic genes and reported increased dermal thickness associated with dermal fibrosis. In a subsequent study, Martins et al. (2015) observed that a profibrotic protein termed FIZZ1 induced cultured adipocytes to acquire myofibroblast characteristics, including lipolysis and increased expression of αSMA and collagen 1. FIZZ1 was upregulated in mice with bleomycin-induced fibrosis, and deletion of FIZZ1 from the genome reduced the severity of bleomycin-induced fibrosis (Martins et al. 2015).
Not only are mature adipocytes implicated in the development of fibrosis, but APs also have a functional relationship with fibroblasts. In multiple models of fibrosis and acute injury, cells that share characteristics with APs become activated and lead to myofibroblast accumulation (reviewed in Ebmeier & Horsley 2015). APs in culture can be stimulated to acquire an activated fibroblast phenotype with decreased adipogenic potential when stimulated with cytokines or fibrotic factors such as TGFβ1 or bleomycin (Ohgo et al. 2013). APs also require the ability to remodel collagen to differentiate, suggesting that increased ECM produced during fibrosis may mechanically constrain adipogenesis (Chavey et al. 2003, Chun et al. 2006, Mariman & Wang 2010). In vivo, treatment of mice with factors that facilitate adipogenesis, such as rosiglitazone, or factors that suppress the inhibition of adipogenesis, such as TGFβ-neutralizing antibody, has shown success in reversing fibrosis in the skin (Du et al. 2013, Shi-wen et al. 2009). It will be important to better understand how adipocytes might acquire a myofibroblast phenotype in fibrosis and how their interactions with surrounding cell types might alter cell behavior to promote fibrosis.
Aging
Changes in adipose tissue occur during the aging process. vWAT increases whereas other depots, such as dWAT, shrink (Kuk et al. 2009, Tchkonia et al. 2010, Treiber et al. 2011). This decrease in the adiposity of the skin during aging may be related to the decreased capacity of APs to differentiate into mature adipocytes. APs isolated from human subjects can enter into a senescent state that impairs their ability to differentiate (Mitterberger et al. 2014). Recent research suggests that senescence of APs in WAT increases their expression of activin A, which is capable of inhibiting adipogenesis in neighboring APs (Xu et al. 2015). Determining whether senescence is a mechanism that regulates loss of adipose tissue in skin and elucidating whether there are changes in the numbers of APs that contribute to the diminished dWAT will be interesting future areas of investigation.
Cancer
Changes in the stromal macroenvironment can influence tumorigenesis and metastasis, yet exactly how these alterations contribute to tumor formation and progression is not well defined (Sleeman et al. 2012). Although adipocyte lineage cells are one of the most abundant lineages in skin, their involvement in skin cancer has not been examined. In other tissues, adipocytes contribute to tumorigenesis and protect tumor cells from treatments. Bone marrow–derived adipokine secretions have been implicated in the stimulation of metastatic prostate tumor growth and invasiveness (Herroon et al. 2013). When murine and human breast cancer cell lines were cocultured with mature adipocytes on transwells, the tumor cells showed greater invasion into matrigel and homing into mouse lungs after tail vein injection. Interestingly, coculture with APs had no effect on tumor cell invasion. Subsequently, mature adipocytes decreased lipid storage and gene expression of adipocyte-specific genes while upregulating genes encoding inflammation-associated IL-6, IL-1β, CCL-2, CCL-5, and TNFα in response to coculture with cancer cell lines (Dirat et al. 2011, Picon-Ruiz et al. 2016). These findings highlight the bidirectional communication between adipocytes and cancer cells. The contribution of specific adipokines to cancer cell biology is just beginning to be examined (Giordano et al. 2015, Lee et al. 2016) and should provide significant insight into the molecular signaling between adipocytes and cancer cells. Recently, it was shown that preincubation of breast cancer cells with adipocytes increases cancer cell migration and resistance to trastuzumab treatment (Duong et al. 2015). A similar effect has been attributed to bone marrow adipocytes in myeloma chemotherapy resistance (Liu et al. 2015). Due to the decreased presence of dermal adipocytes in the stromal fraction of cutaneous tumors (Figure 3), many questions remain. Examining the fate of adipocytes, adipocyte lipid stores, and potential contributions of adipokines during tumor formation and metastasis will be an interesting area of investigation for future studies.
Obesity and Diabetes
Accumulation of WAT throughout the body during obesity is associated with many alterations in adipocyte physiology. In obese mice and humans, the balance of adipokine secretions is shifted toward an inflammatory state, and innate and adaptive immune cells become activated. Beneficial adipokines, such as adiponectin, are reduced, while cytokines that promote inflammation and insulin resistance, such as leptin and IL-6, increase (Frank et al. 2000, Guilherme et al. 2008, Ouchi et al. 2011, Wetzler et al. 2000). As systemic levels of beneficial adipokines decrease, the elevation of inflammatory cytokines creates low-grade inflammation in multiple organs, including the skin. Examination of serum adipokine levels in lean patients suffering from inflammatory diseases suggests that adipose tissue increases anti-inflammatory adipokine release in a putative attempt to dampen the immune response; however, in the obese state, anti-inflammatory adipokine levels are frequently decreased compared with lean, healthy baseline levels (Fantuzzi 2008). This observation demonstrates the systemic complexity associated with obesity and diabetes and helps to explain the high comorbidity of obesity and diabetes with inflammatory diseases.
Although the direct contribution of dWAT is unknown, obese individuals have a greater risk of acquiring many different cutaneous diseases, including ulceration, infection, and a diminished capacity to heal wounds (Frank et al. 2000, Guilherme et al. 2008, Shipman & Millington 2011, Yosipovitch et al. 2007). Relative to lean controls, obese women have a greater risk of S. aureus colonization (Olsen et al. 2013), and obese patients have a greater risk of infection after surgical procedures. Obese psoriatic patients are more likely to develop severe psoriatic outbreaks that are difficult to treat. As in other inflammatory diseases, the elevated inflammatory baseline associated with obesity is believed to contribute to activation of dendritic cells and T cells in psoriatic skin (Lowes et al. 2014). However, although the serum levels of many adipokines, such as adiponectin, leptin, and IL-6, are altered in psoriasis (Gerdes et al. 2011), there is little evidence to support that dWAT directly contributes to these altered serum levels.
Progression of obesity to type II diabetes is associated with insulin resistance and additional defects in skin biology (Guilherme et al. 2008). Altered insulin signaling leads to defects in human and mouse keratinocyte differentiation and migration in vitro (Benoliel et al. 1997, Wertheimer et al. 2000). Cell-intrinsic defects in keratinocytes may contribute to the inability of many diabetics to close skin wounds. Wound healing is significantly impaired in both diabetic patients and many animal models. Diabetic ob/ob and db/db mice have slower healing rates and persisting inflammation. In db/db mice, the numbers of neutrophils and inflammatory macrophages remain elevated for up to 10 days after injury and γδT cells are dysfunctional (Frank et al. 2000, Goren et al. 2003, Mirza & Koh 2011, Taylor et al. 2011, Wetzler et al. 2000). Wound beds of diabetic mice also have diminished levels of keratinocyte growth factor and acidic and basic FGF during the tissue formation phase of wound healing (Werner et al. 1994).
In addition to showing defects in keratinocyte function during diabetic murine wound healing, fibroblasts generate less collagen, resulting in both decreased tensile strength and decreased wound closure (Enser & Avery 1984, Goodson & Hunt 1979). Interestingly, human dermal fibroblasts cultured with high levels of leptin increased secretion of inflammatory cytokines (IL-6, CXCL-1, IL-8, and MCP-1), demonstrating that dermal fibroblasts may receive signals from adipose tissue and may contribute to a cutaneous inflammatory state (Ommen et al. 2015).
CONCLUDING REMARKS
Until recently, it was believed that WAT participated primarily in systemic metabolism and endocrine function. WAT is integrated into many mammalian tissues, and its expansion is associated with protective, regenerative, or pathological conditions. The diverse beneficial and deleterious contributions of WAT to tissue physiology make WAT an exciting and powerful target for translational biology. Over the past few years, many new mouse models have been developed to study mature adipocytes and APs in vivo (Jiang et al. 2014, Sassmann et al. 2010, Wang et al. 2013). It will be exciting to see these models implemented to selectively examine the function of WAT in skin, muscle, MG, and bone marrow and to determine how fat contributes to maintenance of these tissues and to the development of human diseases.
Acknowledgments
We thank Dr. Matthew Rodeheffer, Dr. Mark Horowitz, Dr. Richard Gallo, and Dr. Ling-juan Zhang for providing the images in the figures. V.H. is funded by the NIH (AR060295) and the Connecticut Department of Public Health (12SCBYALE01). B.S. is a New York Stem Cell Foundation Druckenmiller Fellow. This research was supported by the New York Stem Cell Foundation.
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- Albu JB, Kovera AJ, Allen L, Wainwright M, Berk E, et al. Independent association of insulin resistance with larger amounts of intermuscular adipose tissue and a greater acute insulin response to glucose in African American than in white nondiabetic women. Am J Clin Nutr. 2005;82(6):1210–17. doi: 10.1093/ajcn/82.6.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai F, Hirao A, Ohmura M, Sato H, Matsuoka S, et al. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell. 2004;118(2):149–61. doi: 10.1016/j.cell.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Arkan MC, Hevener AL, Greten FR, Maeda S, Li Z-W, et al. IKK-β links inflammation to obesity-induced insulin resistance. Nat Med. 2005;11(2):191–98. doi: 10.1038/nm1185. [DOI] [PubMed] [Google Scholar]
- Arrighi N, Moratal C, Ment NCE, Giorgetti-Peraldi S, Peraldi P, et al. Characterization of adipocytes derived from fibro/adipogenic progenitors resident in human skeletal muscle. Cell Death Dis. 2015;6(4):e1733–10. doi: 10.1038/cddis.2015.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asterholm IW, Tao C, Morley TS, Wang QA, Delgado-Lopez F, et al. Adipocyte inflammation is essential for healthy adipose tissue expansion and remodeling. Cell Metab. 2014;20(1):103–18. doi: 10.1016/j.cmet.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baer PC, Geiger H. Adipose-derived mesenchymal stromal/stem cells: tissue localization, characterization, and heterogeneity. Stem Cells Int. 2012;2012:812693. doi: 10.1155/2012/812693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandyopadhyay GK, Yu JG, Ofrecio J, Olefsky JM. Increased p85/55/50 expression and decreased phosphotidylinositol 3-kinase activity in insulin-resistant human skeletal muscle. Diabetes. 2005;54(8):2351–59. doi: 10.2337/diabetes.54.8.2351. [DOI] [PubMed] [Google Scholar]
- Bapat SP, Suh JM, Fang S, Liu S, Zhang Y, et al. Depletion of fat-resident Treg cells prevents age-associated insulin resistance. Nature. 2015;528(7580):137–41. doi: 10.1038/nature16151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoliel AM, Kahn-Perles B, Imbert J, Verrando P. Insulin stimulates haptotactic migration of human epidermal keratinocytes through activation of NF-κB transcription factor. J Cell Sci. 1997;110(Pt. 17):2089–97. doi: 10.1242/jcs.110.17.2089. [DOI] [PubMed] [Google Scholar]
- Berry R, Rodeheffer MS. Characterization of the adipocyte cellular lineage in vivo. Nat Cell Biol. 2013;15(3):302–8. doi: 10.1038/ncb2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billon N, Iannarelli P, Monteiro MC, Glavieux-Pardanaud C, Richardson WD, et al. The generation of adipocytes by the neural crest. Development. 2007;134(12):2283–92. doi: 10.1242/dev.002642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bing C, Russell S, Becket E, Pope M, Tisdale MJ. Adipose atrophy in cancer cachexia: morphologic and molecular analysis of adipose tissue in tumour-bearing mice. Br J Cancer. 2006;95(8):1028–37. doi: 10.1038/sj.bjc.6603360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanpain C, Lowry WE, Geoghegan A, Polak L, Fuchs E. Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell. 2004;118(5):635–48. doi: 10.1016/j.cell.2004.08.012. [DOI] [PubMed] [Google Scholar]
- Bochet L, Lehuédé C, Dauvillier S, Wang YY, Dirat B. Adipocyte-derived fibroblasts promote tumor progression and contribute to the desmoplastic reaction in breast cancer. Cancer Res. 2013;73(18):5657–68. doi: 10.1158/0008-5472.CAN-13-0530. [DOI] [PubMed] [Google Scholar]
- Bredella MA, Fazeli PK, Miller KK, Misra M, Torriani M, et al. Increased bone marrow fat in anorexia nervosa. J Clin Endocrinol Metab. 2009;94(6):2129–36. doi: 10.1210/jc.2008-2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges AG, von Kuster LC, Estes SA. Lipedematous alopecia. Cutis. 2000;65(4):199–202. [PubMed] [Google Scholar]
- Cai D, Yuan M, Frantz DF, Melendez PA, Hansen L, et al. Local and systemic insulin resistance resulting from hepatic activation of IKK-β and NF-κB. Nat Med. 2005;11(2):183–90. doi: 10.1038/nm1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellana D, Paus R, Perez-Moreno M. Macrophages contribute to the cyclic activation of adult hair follicle stem cells. PLOS Biol. 2014;12(12):e1002002. doi: 10.1371/journal.pbio.1002002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase HB, Montagna W, Malone JD. Changes in the skin in relation to the hair growth cycle. Anat Rec. 1953;116(1):75–81. doi: 10.1002/ar.1091160107. [DOI] [PubMed] [Google Scholar]
- Chavey C, Mari B, Monthouel M-N, Bonnafous S, Anglard P, et al. Matrix metalloproteinases are differentially expressed in adipose tissue during obesity and modulate adipocyte differentiation. J Biol Chem. 2003;278(14):11888–96. doi: 10.1074/jbc.M209196200. [DOI] [PubMed] [Google Scholar]
- Chun T-H, Hotary KB, Sabeh F, Saltiel AR, Allen ED, Weiss SJ. A pericellular collagenase directs the 3-dimensional development of white adipose tissue. Cell. 2006;125(3):577–91. doi: 10.1016/j.cell.2006.02.050. [DOI] [PubMed] [Google Scholar]
- Cipolletta D, Cohen P, Spiegelman BM, Benoist C, Mathis D. Appearance and disappearance of the mRNA signature characteristic of Treg cells in visceral adipose tissue: age, diet, and PPARγ effects. PNAS. 2015;112(2):482–87. doi: 10.1073/pnas.1423486112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condé-Green A, Kotamarti V, Marano MA, Lee ES, Granick MS. Adipose stem cells isolated from excised burned tissue: Is there potential for clinical use? Plast Reconstr Surg. 2016;137(4):767e–68e. doi: 10.1097/PRS.0000000000002000. [DOI] [PubMed] [Google Scholar]
- Cotsarelis G, Sun TT, Lavker RM. Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell. 1990;61(7):1329–37. doi: 10.1016/0092-8674(90)90696-c. [DOI] [PubMed] [Google Scholar]
- Couldrey C, Moitra J, Vinson C, Anver M, Nagashima K, Green J. Adipose tissue: a vital in vivo role in mammary gland development but not differentiation. Dev Dyn. 2002;223(4):459–68. doi: 10.1002/dvdy.10065. [DOI] [PubMed] [Google Scholar]
- Daley JM, Brancato SK, Thomay AA, Reichner JS, Albina JE. The phenotype of murine wound macrophages. J Leukoc Biol. 2010;87(1):59–67. doi: 10.1189/jlb.0409236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin MJ, Cloutier AM, Thomas NA, Panus DA, Lotinun S, et al. Caloric restriction leads to high marrow adiposity and low bone mass in growing mice. J Bone Miner Res. 2010;25(9):2078–88. doi: 10.1002/jbmr.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirat B, Bochet L, Dabek M, Daviaud D, Dauvillier S, et al. Cancer-associated adipocytes exhibit an activated phenotype and contribute to breast cancer invasion. Cancer Res. 2011;71(7):2455–65. doi: 10.1158/0008-5472.CAN-10-3323. [DOI] [PubMed] [Google Scholar]
- DiSpirito JR, Mathis D. Immunological contributions to adipose tissue homeostasis. Semin Immunol. 2015;27(5):315–21. doi: 10.1016/j.smim.2015.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donati G, Proserpio V, Lichtenberger BM, Natsuga K, Sinclair R, et al. Epidermal Wnt/β-catenin signaling regulates adipocyte differentiation via secretion of adipogenic factors. PNAS. 2014;111(15):E1501–9. doi: 10.1073/pnas.1312880111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driskell RR, Jahoda CAB, Chuong C-M, Watt FM, Horsley V. Defining dermal adipose tissue. Exp Dermatol. 2014;23(9):629–31. doi: 10.1111/exd.12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driskell RR, Lichtenberger BM, Hoste E, Kretzschmar K, Simons BD, et al. Distinct fibroblast lineages determine dermal architecture in skin development and repair. Nature. 2013;504(7479):277–81. doi: 10.1038/nature12783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du B, Cawthorn WP, Su A, Doucette CR, Yao Y, et al. The transcription factor paired-related homeobox 1 (Prrx1) inhibits adipogenesis by activating transforming growth factor-β (TGFβ) signaling. J Biol Chem. 2013;288(5):3036–47. doi: 10.1074/jbc.M112.440370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duong MN, Cleret A, Matera E-L, Chettab K, Mathé D, et al. Adipose cells promote resistance of breast cancer cells to trastuzumab-mediated antibody-dependent cellular cytotoxicity. Breast Cancer Res. 2015;17(1):57. doi: 10.1186/s13058-015-0569-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebmeier S, Horsley V. Origin of fibrosing cells in systemic sclerosis. Curr Opin Rheumatol. 2015;27(6):555–62. doi: 10.1097/BOR.0000000000000217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias JJ, Pitelka DR, Armstrong RC. Changes in fat cell morphology during lactation in the mouse. Anat Rec. 1973;177(4):533–47. doi: 10.1002/ar.1091770407. [DOI] [PubMed] [Google Scholar]
- Enser M, Avery NC. Mechanical and chemical properties of the skin and its collagen from lean and obese-hyperglycaemic (ob/ob) mice. Diabetologia. 1984;27(1):44–49. doi: 10.1007/BF00253500. [DOI] [PubMed] [Google Scholar]
- Fantuzzi G. Adiponectin and inflammation: consensus and controversy. J Allergy Clin Immunol. 2008;121(2):326–30. doi: 10.1016/j.jaci.2007.10.018. [DOI] [PubMed] [Google Scholar]
- Fazeli PK, Bredella MA, Freedman L, Thomas BJ, Breggia A, et al. Marrow fat and preadipocyte factor-1 levels decrease with recovery in women with anorexia nervosa. J Bone Miner Res. 2012;27(9):1864–71. doi: 10.1002/jbmr.1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazeli PK, Horowitz MC, MacDougald OA, Scheller EL, Rodeheffer MS, et al. Marrow fat and bone—new perspectives. J Clin Endocrinol Metab. 2013;98(3):935–45. doi: 10.1210/jc.2012-3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Festa E, Fretz J, Berry R, Schmidt B, Rodeheffer M, et al. Adipocyte lineage cells contribute to the skin stem cell niche to drive hair cycling. Cell. 2011;146(5):761–71. doi: 10.1016/j.cell.2011.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feuerer M, Herrero L, Cipolletta D, Naaz A, Wong J, et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med. 2009;15(8):930–39. doi: 10.1038/nm.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischmajer R, Damanio V, Nedwich A. Scleroderma and the subcutaneous tissue. Science. 1971;171(3975):1019–21. doi: 10.1126/science.171.3975.1019. [DOI] [PubMed] [Google Scholar]
- Frank S, Stallmeyer B, Kämpfer H, Kolb N, Pfeilschifter J. Leptin enhances wound reepithelialization and constitutes a direct function of leptin in skin repair. J Clin Investig. 2000;106(4):501–9. doi: 10.1172/JCI9148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frayn KN, Arner P, Yki-Järvinen H. Fatty acid metabolism in adipose tissue, muscle and liver in health and disease. Essays Biochem. 2006;42:89–103. doi: 10.1042/bse0420089. [DOI] [PubMed] [Google Scholar]
- Friedman SL, Sheppard D, Duffield JS. Therapy for fibrotic diseases: nearing the starting line. Sci Transl Med. 2013;5(167):167sr1. doi: 10.1126/scitranslmed.3004700. [DOI] [PubMed] [Google Scholar]
- Fukumoto D, Kubo Y, Saito M, Arase S. Centrifugal lipodystrophy of the scalp presenting with an arch-form alopecia: a 10-year follow-up observation. J Dermatol. 2009;36(9):499–503. doi: 10.1111/j.1346-8138.2009.00685.x. [DOI] [PubMed] [Google Scholar]
- Furuhashi M, Fucho R, Görgün CZ, Tuncman G, Cao H, Hotamisligil GS. Adipocyte/macrophage fatty acid–binding proteins contribute to metabolic deterioration through actions in both macrophages and adipocytes in mice. J Clin Investig. 2008;118(7):2640–50. doi: 10.1172/JCI34750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbiani G, Ryan GB, Majno G. Presence of modified fibroblasts in granulation tissue and their possible role in wound contraction. Experientia. 1971;27(5):549–50. doi: 10.1007/BF02147594. [DOI] [PubMed] [Google Scholar]
- Genander M, Cook PJ, Ramsköld D, Keyes BE, Mertz AF, et al. BMP signaling and its pSMAD1/5 target genes differentially regulate hair follicle stem cell lineages. Cell Stem Cell. 2014;15(5):619–33. doi: 10.1016/j.stem.2014.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdes S, Rostami-Yazdi M, Mrowietz U. Adipokines and psoriasis. Exp Dermatol. 2011;20(2):81–87. doi: 10.1111/j.1600-0625.2010.01210.x. [DOI] [PubMed] [Google Scholar]
- Giordano C, Chemi F, Panza S, Barone I, Bonofiglio D, et al. Leptin as a mediator of tumor-stromal interactions promotes breast cancer stem cell activity. Oncotarget. 2015;7(2):1262–75. doi: 10.18632/oncotarget.6014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson WH, Hunt TK. Deficient collagen formation by obese mice in a standard wound model. Am J Surg. 1979;138(5):692–94. doi: 10.1016/0002-9610(79)90350-7. [DOI] [PubMed] [Google Scholar]
- Goren I, Allmann N, Yogev N, Schürmann C, Linke A, et al. A transgenic mouse model of inducible macrophage depletion. AJPA. 2010;175(1):132–47. doi: 10.2353/ajpath.2009.081002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goren I, Kämpfer H, Podda M, Pfeilschifter J, Frank S. Leptin and wound inflammation in diabetic ob/ob mice: differential regulation of neutrophil and macrophage influx and a potential role for the scab as a sink for inflammatory cells and mediators. Diabetes. 2003;52(11):2821–32. doi: 10.2337/diabetes.52.11.2821. [DOI] [PubMed] [Google Scholar]
- Gouon-Evans V, Pollard JW. Unexpected deposition of brown fat in mammary gland during postnatal development. Mol Endocrinol. 2002;16(11):2618–27. doi: 10.1210/me.2001-0337. [DOI] [PubMed] [Google Scholar]
- Guilherme A, Virbasius JV, Puri V, Czech MP. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat Rev Mol Cell Biol. 2008;9(5):367–77. doi: 10.1038/nrm2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausman GJ, Campion DR, Richardson RL, Martin RJ. Adipocyte development in the rat hypodermis. Am J Anat. 1981;161(1):85–100. doi: 10.1002/aja.1001610107. [DOI] [PubMed] [Google Scholar]
- Hegele RA. Lessons from human mutations in PPARγ. Int J Obes Relat Metab Disord. 2005;29:S31–35. doi: 10.1038/sj.ijo.0802911. [DOI] [PubMed] [Google Scholar]
- Herrmann T, van der Hoeven F, Grone H-J, Stewart AF, Langbein L, et al. Mice with targeted disruption of the fatty acid transport protein 4 (Fatp 4, Slc27a4) gene show features of lethal restrictive dermopathy. J Cell Biol. 2003;161(6):1105–15. doi: 10.1083/jcb.200207080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herroon MK, Rajagurubandara E, Hardaway AL, Powell K, Turchick A, et al. Bone marrow adipocytes promote tumor growth in bone via FABP4-dependent mechanisms. Oncotarget. 2013;4(11):2108–23. doi: 10.18632/oncotarget.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinz B, Phan SH, Thannickal VJ, Prunotto M, Desmouliere A, et al. Recent developments in myofibroblast biology. Am J Pathol. 2012;180(4):1340–55. doi: 10.1016/j.ajpath.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosoyama T, Ishiguro N, Yamanouchi K, Nishihara M. Degenerative muscle fiber accelerates adipogenesis of intramuscular cells via RhoA signaling pathway. Differentiation. 2009;77(4):350–59. doi: 10.1016/j.diff.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Hovey RC, McFadden TB, Akers RM. Regulation of mammary gland growth and morphogenesis by the mammary fat pad: a species comparison. J Mammary Gland Biol Neoplasia. 1999;4(1):53–68. doi: 10.1023/a:1018704603426. [DOI] [PubMed] [Google Scholar]
- Howlett AR, Bissell MJ. The influence of tissue microenvironment (stroma and extracellular matrix) on the development and function of mammary epithelium. Epithel Cell Biol. 1993;2(2):79–89. [PubMed] [Google Scholar]
- Hu X, Juneja SC, Maihle NJ, Cleary MP. Leptin—a growth factor in normal and malignant breast cells and for normal mammary gland development. J Natl Cancer Inst. 2002;94(22):1704–11. doi: 10.1093/jnci/94.22.1704. [DOI] [PubMed] [Google Scholar]
- Itani SI, Ruderman NB, Schmieder F, Boden G. Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C, and IκB-α. Diabetes. 2002;51(7):2005–11. doi: 10.2337/diabetes.51.7.2005. [DOI] [PubMed] [Google Scholar]
- Iyengar P, Combs TP, Shah SJ, Gouon-Evans V, Pollard JW, et al. Adipocyte-secreted factors synergistically promote mammary tumorigenesis through induction of anti-apoptotic transcriptional programs and proto-oncogene stabilization. Oncogene. 2003;22(41):6408–23. doi: 10.1038/sj.onc.1206737. [DOI] [PubMed] [Google Scholar]
- Iyengar P, Espina V, Williams TW, Lin Y, Berry D, et al. Adipocyte-derived collagen VI affects early mammary tumor progression in vivo, demonstrating a critical interaction in the tumor/stroma microenvironment. J Clin Investig. 2005;115(5):1163–76. doi: 10.1172/JCI23424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahoda CA, Horne KA, Oliver RF. Induction of hair growth by implantation of cultured dermal papilla cells. Nature. 1984;311(5986):560–62. doi: 10.1038/311560a0. [DOI] [PubMed] [Google Scholar]
- Janas-Kozik M, Stachowicz M, Krupka-Matuszczyk I, Szymszal J, Krysta K, et al. Plasma levels of leptin and orexin A in the restrictive type of anorexia nervosa. Regul Pept. 2011;168(1–3):5–9. doi: 10.1016/j.regpep.2011.02.005. [DOI] [PubMed] [Google Scholar]
- Jankovic A, Golic I, Markelic M, Stancic A, Otasevic V, et al. Two key temporally distinguishable molecular and cellular components of white adipose tissue browning during cold acclimation. J Physiol. 2015;593(15):3267–80. doi: 10.1113/JP270805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery E, Church CD, Holtrup B, Colman L, Rodeheffer MS. Rapid depot-specific activation of adipocyte precursor cells at the onset of obesity. Nat Cell Biol. 2015;17(4):376–85. doi: 10.1038/ncb3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeninga EH, Gurnell M, Kalkhoven E. Functional implications of genetic variation in human PPARγ. Trends Endocrinol Metab. 2009;20(8):380–87. doi: 10.1016/j.tem.2009.04.005. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Berry DC, Tang W, Graff JM. Independent stem cell lineages regulate adipose organogenesis and adipose homeostasis. Cell Rep. 2014;9(3):1007–22. doi: 10.1016/j.celrep.2014.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin CE, Xiao L, Ge ZH, Zhan XB, Zhou HX. Role of adiponectin in adipose tissue wound healing. Genet Mol Res. 2015;14(3):8883–91. doi: 10.4238/2015.August.3.11. [DOI] [PubMed] [Google Scholar]
- Joe AWB, Yi L, Natarajan A, Le Grand F, So L, et al. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat Cell Biol. 2010;12(2):153–63. doi: 10.1038/ncb2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandyba E, Leung Y, Chen Y-B, Widelitz R, Chuong C-M, Kobielak K. Competitive balance of intrabulge BMP/Wnt signaling reveals a robust gene network ruling stem cell homeostasis and cyclic activation. PNAS. 2013;110(4):1351–56. doi: 10.1073/pnas.1121312110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanneganti T-D, Dixit VD. Immunological complications of obesity. Nat Immunol. 2012;13(8):707–12. doi: 10.1038/ni.2343. [DOI] [PubMed] [Google Scholar]
- Kasza I, Suh Y, Wollny D, Clark RJ, Roopra A, et al. Syndecan-1 is required to maintain intradermal fat and prevent cold stress. PLOS Genet. 2014;10(8):e1004514. doi: 10.1371/journal.pgen.1004514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai K, Kageyama A, Tsumano T, Nishimoto S, Fukuda K, et al. Effects of adiponectin on growth and differentiation of human keratinocytes—implication of impaired wound healing in diabetes. Biochem Biophys Res Commun. 2008;374(2):269–73. doi: 10.1016/j.bbrc.2008.07.045. [DOI] [PubMed] [Google Scholar]
- Khan T, Muise ES, Iyengar P, Wang ZV, Chandalia M, et al. Metabolic dysregulation and adipose tissue fibrosis: role of collagen VI. Mol Cell Biol. 2009;29(6):1575–91. doi: 10.1128/MCB.01300-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim W-S, Park B-S, Sung J-H, Yang J-M, Park S-B, et al. Wound healing effect of adipose-derived stem cells: a critical role of secretory factors on human dermal fibroblasts. J Dermatol Sci. 2007;48(1):15–24. doi: 10.1016/j.jdermsci.2007.05.018. [DOI] [PubMed] [Google Scholar]
- Kobielak K, Pasolli HA, Alonso L, Polak L, Fuchs E. Defining BMP functions in the hair follicle by conditional ablation of BMP receptor IA. J Cell Biol. 2003;163(3):609–23. doi: 10.1083/jcb.200309042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobielak K, Stokes N, de la Cruz J, Polak L, Fuchs E. Loss of a quiescent niche but not follicle stem cells in the absence of bone morphogenetic protein signaling. PNAS. 2007;104(24):10063–68. doi: 10.1073/pnas.0703004104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koellensperger E, Lampe K, Beierfuss A, Gramley F, Germann G, Leimer U. Intracutaneously injected human adipose tissue–derived stem cells in a mouse model stay at the site of injection. J Plast Reconstr Aesthet Surg. 2014;67(6):844–50. doi: 10.1016/j.bjps.2014.02.021. [DOI] [PubMed] [Google Scholar]
- Kolodin D, van Panhuys N, Li C, Magnuson AM, Cipolletta D, et al. Antigen- and cytokine-driven accumulation of regulatory T cells in visceral adipose tissue of lean mice. Cell Metab. 2015;21(4):543–57. doi: 10.1016/j.cmet.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krotkiewski M, Björntorp P. The effects of dexamethasone and starvation on body composition and regional adipose tissue cellularity in the rat. Acta Endocrinol. 1975;80(4):667–75. doi: 10.1530/acta.0.0800667. [DOI] [PubMed] [Google Scholar]
- Krotkiewski M, Mandroukas K, Morgan L, William-Olsson T, Feurle GE, et al. Effects of physical training on adrenergic sensitivity in obesity. J Appl Physiol Respir Environ Exerc Physiol. 1983;55(6):1811–17. doi: 10.1152/jappl.1983.55.6.1811. [DOI] [PubMed] [Google Scholar]
- Kuk JL, Saunders TJ, Davidson LE, Ross R. Age-related changes in total and regional fat distribution. Ageing Res Rev. 2009;8(4):339–48. doi: 10.1016/j.arr.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Kwan KM, Li AG, Wang X-J, Wurst W, Behringer RR. Essential roles of BMPR-IA signaling in differentiation and growth of hair follicles and in skin tumorigenesis. Genesis. 2004;39(1):10–25. doi: 10.1002/gene.20021. [DOI] [PubMed] [Google Scholar]
- Landskroner-Eiger S, Park J, Israel D, Pollard JW, Scherer PE. Morphogenesis of the developing mammary gland: stage-dependent impact of adipocytes. Dev Biol. 2010;344(2):968–78. doi: 10.1016/j.ydbio.2010.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JO, Kim N, Lee HJ, Lee YW, Kim SJ, et al. Resistin, a fat-derived secretory factor, promotes metastasis of MDA-MB-231 human breast cancer cells through ERM activation. Sci Rep. 2016;6:18923. doi: 10.1038/srep18923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M-J, Wu Y, Fried SK. Adipose tissue heterogeneity: implication of depot differences in adipose tissue for obesity complications. Mol Aspects Med. 2013;34(1):1–11. doi: 10.1016/j.mam.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Xu J, He J, Liu H, Lin P, et al. Mature adipocytes in bone marrow protect myeloma cells against chemotherapy through autophagy activation. Oncotarget. 2015;6(33):34329–41. doi: 10.18632/oncotarget.6020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowes MA, Suárez-Fariñas M, Krueger JG. Immunology of psoriasis. Annu Rev Immunol. 2014;32:227–55. doi: 10.1146/annurev-immunol-032713-120225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu CP, Polak L, Rocha AS, Pasolli HA, Chen S-C, et al. Identification of stem cell populations in sweat glands and ducts reveals roles in homeostasis and wound repair. Cell. 2012;150(1):136–50. doi: 10.1016/j.cell.2012.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas T, Waisman A, Ranjan R, Roes J, Krieg T, et al. Differential roles of macrophages in diverse phases of skin repair. J Immunol. 2010;184(7):3964–77. doi: 10.4049/jimmunol.0903356. [DOI] [PubMed] [Google Scholar]
- Lumeng CN, Deyoung SM, Bodzin JL, Saltiel AR. Increased inflammatory properties of adipose tissue macrophages recruited during diet-induced obesity. Diabetes. 2007;56(1):16–23. doi: 10.2337/db06-1076. [DOI] [PubMed] [Google Scholar]
- Marangoni RG, Korman B, Wei J. Myofibroblasts in cutaneous fibrosis originate from adiponectin-positive intradermal progenitors. Arthritis Rheumatol. 2014;67(4):1062–73. doi: 10.1002/art.38990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariman ECM, Wang P. Adipocyte extracellular matrix composition, dynamics and role in obesity. Cell Mol Life Sci. 2010;67(8):1277–92. doi: 10.1007/s00018-010-0263-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins V, Gonzalez De Los Santos F, Wu Z, Capelozzi V, Phan SH, Liu T. FIZZ1-induced myofibroblast transdifferentiation from adipocytes and its potential role in dermal fibrosis and lipoatrophy. Am J Pathol. 2015;185(10):2768–76. doi: 10.1016/j.ajpath.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura H, Engrav LH, Gibran NS, Yang TM, Grant JH, et al. Cones of skin occur where hypertrophic scar occurs. Wound Repair Regen. 2001;9(4):269–77. doi: 10.1046/j.1524-475x.2001.00269.x. [DOI] [PubMed] [Google Scholar]
- Mauer J, Chaurasia B, Plum L, Quast T, Hampel B, et al. Myeloid cell–restricted insulin receptor deficiency protects against obesity-induced inflammation and systemic insulin resistance. PLOS Genet. 2010;6(5):e1000938. doi: 10.1371/journal.pgen.1000938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirza R, Koh TJ. Dysregulation of monocyte/macrophage phenotype in wounds of diabetic mice. Cytokine. 2011;56(2):256–64. doi: 10.1016/j.cyto.2011.06.016. [DOI] [PubMed] [Google Scholar]
- Mirza RE, Koh TJ. Contributions of cell subsets to cytokine production during normal and impaired wound healing. Cytokine. 2014;71(2):409–12. doi: 10.1016/j.cyto.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitterberger MC, Lechner S, Mattesich M, Zwerschke W. Adipogenic differentiation is impaired in replicative senescent human subcutaneous adipose–derived stromal/progenitor cells. J Gerontol A. 2014;69(1):13–24. doi: 10.1093/gerona/glt043. [DOI] [PubMed] [Google Scholar]
- Mizoguchi T, Pinho S, Ahmed J, Kunisaki Y, Hanoun M, et al. Osterix marks distinct waves of primitive and definitive stromal progenitors during bone marrow development. Dev Cell. 2014;29(3):340–49. doi: 10.1016/j.devcel.2014.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moitra J, Mason MM, Olive M, Krylov D, Gavrilova O, et al. Life without white fat: a transgenic mouse. Genes Dev. 1998;12(20):3168–81. doi: 10.1101/gad.12.20.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagna W, Carlisle K, Brenner RM. Wound healing in the sex skin of pig-tailed macaques. Arch Dermatol Res. 1988;280(Suppl):68–84. [PubMed] [Google Scholar]
- Morikawa S, Mabuchi Y, Kubota Y, Nagai Y, Niibe K, et al. Prospective identification, isolation, and systemic transplantation of multipotent mesenchymal stem cells in murine bone marrow. J Exp Med. 2009;206(11):2483–96. doi: 10.1084/jem.20091046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morroni M, Giordano A, Zingaretti MC, Boiani R, De Matteis R, et al. Reversible transdifferentiation of secretory epithelial cells into adipocytes in the mammary gland. PNAS. 2004;101(48):16801–6. doi: 10.1073/pnas.0407647101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauta A, Seidel C, Deveza L, Montoro D, Grova M, et al. Adipose-derived stromal cells overexpressing vascular endothelial growth factor accelerate mouse excisional wound healing. Mol Ther. 2012;21(2):445–55. doi: 10.1038/mt.2012.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naveiras O, Nardi V, Wenzel PL, Hauschka PV, Fahey F, Daley GQ. Bone-marrow adipocytes as negative regulators of the haematopoietic microenvironment. Nature. 2009;460(7252):259–63. doi: 10.1038/nature08099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen M-H, Cheng M, Koh TJ. Impaired muscle regeneration in ob/ob and db/db mice. Sci World J. 2011;11:1525–35. doi: 10.1100/tsw.2011.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen MTA, Favelyukis S, Nguyen A-K, Reichart D, Scott PA, et al. A subpopulation of macrophages infiltrates hypertrophic adipose tissue and is activated by free fatty acids via Toll-like receptors 2 and 4 and JNK-dependent pathways. J Biol Chem. 2007;282(48):35279–92. doi: 10.1074/jbc.M706762200. [DOI] [PubMed] [Google Scholar]
- Nicholls DG, Locke RM. Thermogenic mechanisms in brown fat. Physiol Rev. 1984;64(1):1–64. doi: 10.1152/physrev.1984.64.1.1. [DOI] [PubMed] [Google Scholar]
- O’Brien J, Lyons T, Monks J, Lucia MS, Wilson RS, et al. Alternatively activated macrophages and collagen remodeling characterize the postpartum involuting mammary gland across species. AJPA. 2010;176(3):1241–55. doi: 10.2353/ajpath.2010.090735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohgo S, Hasegawa S, Hasebe Y, Mizutani H, Nakata S, Akamatsu H. Bleomycin inhibits adipogenesis and accelerates fibrosis in the subcutaneous adipose layer through TGF-β 1. Exp Dermatol. 2013;22(11):769–71. doi: 10.1111/exd.12256. [DOI] [PubMed] [Google Scholar]
- Olsen K, Danielsen K, Wilsgaard T, Sangvik M, Sollid JUE, et al. Obesity and Staphylococcus aureus nasal colonization among women and men in a general population. PLOS ONE. 2013;8(5):e63716. doi: 10.1371/journal.pone.0063716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ommen P, Stjernholm T, Kragstrup T, Raaby L, Johansen C, et al. The role of leptin in psoriasis comprises a proinflammatory response by the dermal fibroblast. Br J Dermatol. 2015;174(1):187–90. doi: 10.1111/bjd.13969. [DOI] [PubMed] [Google Scholar]
- Osborn O, Olefsky JM. The cellular and signaling networks linking the immune system and metabolism in disease. Nat Med. 2012;18(3):363–74. doi: 10.1038/nm.2627. [DOI] [PubMed] [Google Scholar]
- Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11(2):85–97. doi: 10.1038/nri2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pajvani UB, Trujillo ME, Combs TP, Iyengar P, Jelicks L, et al. Fat apoptosis through targeted activation of caspase 8: a new mouse model of inducible and reversible lipoatrophy. Nat Med. 2005;11(7):797–803. doi: 10.1038/nm1262. [DOI] [PubMed] [Google Scholar]
- Pang C, Gao Z, Yin J, Zhang J, Jia W, Ye J. Macrophage infiltration into adipose tissue may promote angiogenesis for adipose tissue remodeling in obesity. Am J Physiol Endocrinol Metab. 2008;295(2):E313–22. doi: 10.1152/ajpendo.90296.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasarica M, Gowronska-Kozak B, Burk D, Remedios I, Hymel D, et al. Adipose tissue collagen VI in obesity. J Clin Endocrinol Metab. 2009;94(12):5155–62. doi: 10.1210/jc.2009-0947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patsouris D, Li P-P, Thapar D, Chapman J, Olefsky JM, Neels JG. Ablation of CD11c-positive cells normalizes insulin sensitivity in obese insulin resistant animals. Cell Metab. 2008;8(4):301–9. doi: 10.1016/j.cmet.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picon-Ruiz M, Pan C, Drews-Elger K, Jang K, Besser AH, et al. Interactions between adipocytes and breast cancer cells stimulate cytokine production and drive Src/Sox2/miR-302b-mediated malignant progression. Cancer Res. 2016;76(2):491–504. doi: 10.1158/0008-5472.CAN-15-0927. [DOI] [PubMed] [Google Scholar]
- Pinho S, Lacombe J, Hanoun M, Mizoguchi T, Bruns I, et al. PDGFRα and CD51 mark human Nestin+ sphere-forming mesenchymal stem cells capable of hematopoietic progenitor cell expansion. J Exp Med. 2013;210(7):1351–67. doi: 10.1084/jem.20122252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piraccini BM, Voudouris S, Pazzaglia M, Rech G, Vicenzi C, Tosti A. Lipedematous alopecia of the scalp. Dermatol Online J. 2006;12(2):6. [PubMed] [Google Scholar]
- Plikus MV, Mayer JA, de la Cruz D, Baker RE, Maini PK, et al. Cyclic dermal BMP signalling regulates stem cell activation during hair regeneration. Nature. 2008;451(7176):340–44. doi: 10.1038/nature06457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujol E, Proenza AM, Roca P, Lladó I. Changes in mammary fat pad composition and lipolytic capacity throughout pregnancy. Cell Tissue Res. 2006;323(3):505–11. doi: 10.1007/s00441-005-0085-0. [DOI] [PubMed] [Google Scholar]
- Ring BD, Scully S, Davis CR, Baker MB, Cullen MJ, et al. Systemically and topically administered leptin both accelerate wound healing in diabetic ob/ob mice. Endocrinology. 2000;141(1):446–49. doi: 10.1210/endo.141.1.7373. [DOI] [PubMed] [Google Scholar]
- Rodeheffer MS, Birsoy K, Friedman JM. Identification of white adipocyte progenitor cells in vivo. Cell. 2008;135(2):240–49. doi: 10.1016/j.cell.2008.09.036. [DOI] [PubMed] [Google Scholar]
- Romanovsky AA. Skin temperature: its role in thermoregulation. Acta Physiol. 2014;210(3):498–507. doi: 10.1111/apha.12231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rompolas P, Deschene ER, Zito G, Gonzalez DG, Saotome I, et al. Live imaging of stem cell and progeny behaviour in physiological hair-follicle regeneration. Nature. 2012;487:496–99. doi: 10.1038/nature11218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen ED, Spiegelman BM. Adipocytes as regulators of energy balance and glucose homeostasis. Nature. 2006;444(7121):847–53. doi: 10.1038/nature05483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakakura T, Sakagami Y, Nishizuka Y. Dual origin of mesenchymal tissues participating in mouse mammary gland embryogenesis. Dev Biol. 1982;91(1):202–7. doi: 10.1016/0012-1606(82)90024-0. [DOI] [PubMed] [Google Scholar]
- Salathia NS, Shi J, Zhang J, Glynne RJ. An in vivo screen of secreted proteins identifies adiponectin as a regulator of murine cutaneous wound healing. J Investig Dermatol. 2012;133(3):812–21. doi: 10.1038/jid.2012.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Gurmaches J, Guertin DA. Adipocytes arise from multiple lineages that are heterogeneously and dynamically distributed. Nat Commun. 2014;5:1–13. doi: 10.1038/ncomms5099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassmann A, Offermanns S, Wettschureck N. Tamoxifen-inducible Cre-mediated recombination in adipocytes. Genesis. 2010;48(10):618–25. doi: 10.1002/dvg.20665. [DOI] [PubMed] [Google Scholar]
- Schipper HS, Prakken B, Kalkhoven E, Boes M. Adipose tissue-resident immune cells: key players in immunometabolism. Trends Endocrinol Metab. 2012;23(8):407–15. doi: 10.1016/j.tem.2012.05.011. [DOI] [PubMed] [Google Scholar]
- Schmidt BA, Horsley V. Intradermal adipocytes mediate fibroblast recruitment during skin wound healing. Development. 2013;140(7):1517–27. doi: 10.1242/dev.087593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider MR, Schmidt-Ullrich R, Paus R. The hair follicle as a dynamic miniorgan. Curr Biol. 2009;19(3):R132–42. doi: 10.1016/j.cub.2008.12.005. [DOI] [PubMed] [Google Scholar]
- Sciorati C, Rigamonti E, Manfredi AA, Rovere-Querini P. Cell death, clearance and immunity in the skeletal muscle. Cell Death Differ. 2016;23:927–37. doi: 10.1038/cdd.2015.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sennett R, Rendl M. Mesenchymal-epithelial interactions during hair follicle morphogenesis and cycling. Semin Cell Dev Biol. 2012;23(8):917–27. doi: 10.1016/j.semcdb.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata S, Tada Y, Asano Y, Hau CS, Kato T, et al. Adiponectin regulates cutaneous wound healing by promoting keratinocyte proliferation and migration via the ERK signaling pathway. J Immunol. 2012;189(6):3231–41. doi: 10.4049/jimmunol.1101739. [DOI] [PubMed] [Google Scholar]
- Shipman AR, Millington GWM. Obesity and the skin. Br J Dermatol. 2011;165(4):743–50. doi: 10.1111/j.1365-2133.2011.10393.x. [DOI] [PubMed] [Google Scholar]
- Shi-wen X, Eastwood M, Stratton RJ, Denton CP, Leask A, Abraham DJ. Rosiglitazone alleviates the persistent fibrotic phenotype of lesional skin scleroderma fibroblasts. Rheumatology. 2009;49(2):259–63. doi: 10.1093/rheumatology/kep371. [DOI] [PubMed] [Google Scholar]
- Sierra-Honigmann MR, Nath AK, Murakami C, García-Cardeña G, Papapetropoulos A, et al. Biological action of leptin as an angiogenic factor. Science. 1998;281(5383):1683–86. doi: 10.1126/science.281.5383.1683. [DOI] [PubMed] [Google Scholar]
- Sleeman JP, Christofori G, Fodde R, Collard JG, Berx G, et al. Concepts of metastasis in flux: the stromal progression model. Semin Cancer Biol. 2012;22(3):174–86. doi: 10.1016/j.semcancer.2012.02.007. [DOI] [PubMed] [Google Scholar]
- Smith GP, Chan ESL. Molecular pathogenesis of skin fibrosis: insight from animal models. Curr Rheumatol Rep. 2010;12(1):26–33. doi: 10.1007/s11926-009-0080-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solinas G, Vilcu C, Neels JG, Bandyopadhyay GK, Luo J-L, et al. JNK1 in hematopoietically derived cells contributes to diet-induced inflammation and insulin resistance without affecting obesity. Cell Metab. 2007;6(5):386–97. doi: 10.1016/j.cmet.2007.09.011. [DOI] [PubMed] [Google Scholar]
- Sommariva E, Brambilla S, Carbucicchio C, Gambini E, Meraviglia V, et al. Cardiac mesenchymal stromal cells are a source of adipocytes in arrhythmogenic cardiomyopathy. Eur Heart J. 2016 doi: 10.1093/eurheartj/ehv579. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S-H, Lee M-O, Lee J-S, Jeong H-C, Kim H-G, et al. Genetic modification of human adipose-derived stem cells for promoting wound healing. J Dermatol Sci. 2012;66(2):98–107. doi: 10.1016/j.jdermsci.2012.02.010. [DOI] [PubMed] [Google Scholar]
- Spalding KL, Arner E, Westermark PO, Bernard S, Buchholz BA, et al. Dynamics of fat cell turnover in humans. Nature. 2008;453(7196):783–87. doi: 10.1038/nature06902. [DOI] [PubMed] [Google Scholar]
- Stenn KS, Paus R. Controls of hair follicle cycling. Physiol Rev. 2001;81(1):449–94. doi: 10.1152/physrev.2001.81.1.449. [DOI] [PubMed] [Google Scholar]
- Stingl J, Eirew P, Ricketson I, Shackleton M, Vaillant F, et al. Purification and unique properties of mammary epithelial stem cells. Nature. 2006;439(7079):993–97. doi: 10.1038/nature04496. [DOI] [PubMed] [Google Scholar]
- Strumia R. Skin signs in anorexia nervosa. Dermato-Endocrinology. 2009;1(5):268–70. doi: 10.4161/derm.1.5.10193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumikawa Y, Inui S, Nakajima T, Itami S. Hair cycle control by leptin as a new anagen inducer. Exp Dermatol. 2014;23(1):27–32. doi: 10.1111/exd.12286. [DOI] [PubMed] [Google Scholar]
- Sun K, Tordjman J, Clément K, Scherer PE. Fibrosis and adipose tissue dysfunction. Cell Metab. 2013;18(4):470–77. doi: 10.1016/j.cmet.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavassoli M, Houchin DN, Jacobs P. Fatty acid composition of adipose cells in red and yellow marrow: a possible determinant of haematopoietic potential. Scand J Haematol. 1977;18(1):47–53. doi: 10.1111/j.1600-0609.1977.tb01476.x. [DOI] [PubMed] [Google Scholar]
- Taylor KR, Costanzo AE, Jameson JM. Dysfunctional γΔT cells contribute to impaired keratinocyte homeostasis in mouse models of obesity. J Investig Dermatol. 2011;131(12):2409–18. doi: 10.1038/jid.2011.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchkonia T, Morbeck DE, Von Zglinicki T, Van Deursen J, Lustgarten J, et al. Fat tissue, aging, and cellular senescence. Aging Cell. 2010;9(5):667–84. doi: 10.1111/j.1474-9726.2010.00608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchkonia T, Thomou T, Zhu Y, Karagiannides I, Pothoulakis C, et al. Mechanisms and metabolic implications of regional differences among fat depots. Cell Metab. 2013;17(5):644–56. doi: 10.1016/j.cmet.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchoukalova YD, Votruba SB, Tchkonia T, Giorgadze N, Kirkland JL, Jensen MD. Regional differences in cellular mechanisms of adipose tissue gain with overfeeding. PNAS. 2010;107(42):18226–31. doi: 10.1073/pnas.1005259107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treiber N, Maity P, Singh K, Kohn M, Keist AF, et al. Accelerated aging phenotype in mice with conditional deficiency for mitochondrial superoxide dismutase in the connective tissue. Aging Cell. 2011;10(2):239–54. doi: 10.1111/j.1474-9726.2010.00658.x. [DOI] [PubMed] [Google Scholar]
- Uezumi A, Fukada S, Yamamoto N, Ikemoto-Uezumi M, Nakatani M, et al. Identification and characterization of PDGFRα+ mesenchymal progenitors in human skeletal muscle. Cell Death Dis. 2014;5:e1186. doi: 10.1038/cddis.2014.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uezumi A, Fukada S-I, Yamamoto N, Takeda S, Tsuchida K. Mesenchymal progenitors distinct from satellite cells contribute to ectopic fat cell formation in skeletal muscle. Nat Cell Biol. 2010;12(2):143–52. doi: 10.1038/ncb2014. [DOI] [PubMed] [Google Scholar]
- Uezumi A, Ito T, Morikawa D, Shimizu N. Fibrosis and adipogenesis originate from a common mesenchymal progenitor in skeletal muscle. J Cell Sci. 2011;124(Pt. 21):3654–64. doi: 10.1242/jcs.086629. [DOI] [PubMed] [Google Scholar]
- Uzum AK, Yucel B, Omer B, Issever H, Ozbey NC. Leptin concentration indexed to fat mass is increased in untreated anorexia nervosa (AN) patients. Clin Endocrinol. 2009;71(1):33–39. doi: 10.1111/j.1365-2265.2008.03423.x. [DOI] [PubMed] [Google Scholar]
- van den Bogaerdt AJ, van der Veen VC, van Zuijlen PPM, Reijnen L, Verkerk M, et al. Collagen cross-linking by adipose-derived mesenchymal stromal cells and scar-derived mesenchymal cells: Are mesenchymal stromal cells involved in scar formation? Wound Repair Regen. 2009;17(4):548–58. doi: 10.1111/j.1524-475X.2009.00501.x. [DOI] [PubMed] [Google Scholar]
- Vasanthakumar A, Moro K, Xin A, Liao Y, Gloury R, et al. The transcriptional regulators IRF4, BATF and IL-33 orchestrate development and maintenance of adipose tissue–resident regulatory T cells. Nat Immunol. 2015;16(3):276–85. doi: 10.1038/ni.3085. [DOI] [PubMed] [Google Scholar]
- Wallace GQ, McNally EM. Mechanisms of muscle degeneration, regeneration, and repair in the muscular dystrophies. Annu Rev Physiol. 2009;71:37–57. doi: 10.1146/annurev.physiol.010908.163216. [DOI] [PubMed] [Google Scholar]
- Wang QA, Tao C, Gupta RK, Scherer PE. Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nat Med. 2013;19(10):1338–44. doi: 10.1038/nm.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watabe R, Yamaguchi T, Kabashima-Kubo R, Yoshioka M, Nishio D, Nakamura M. Leptin controls hair follicle cycling. Exp Dermatol. 2014;23(4):228–29. doi: 10.1111/exd.12335. [DOI] [PubMed] [Google Scholar]
- Watson CJ. Involution: apoptosis and tissue remodelling that convert the mammary gland from milk factory to a quiescent organ. Breast Cancer Res. 2006;8(2):203. doi: 10.1186/bcr1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisberg SP, Hunter D, Huber R, Lemieux J, Slaymaker S, et al. CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Investig. 2006;116(1):115–24. doi: 10.1172/JCI24335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner S, Breeden M, Hübner G, Greenhalgh DG, Longaker MT. Induction of keratinocyte growth factor expression is reduced and delayed during wound healing in the genetically diabetic mouse. J Investig Dermatol. 1994;103(4):469–73. doi: 10.1111/1523-1747.ep12395564. [DOI] [PubMed] [Google Scholar]
- Wertheimer E, Trebicz M, Eldar T, Gartsbein M, Nofeh-Moses S, Tennenbaum T. Differential roles of insulin receptor and insulin-like growth factor-1 receptor in differentiation of murine skin keratinocytes. J Investig Dermatol. 2000;115(1):24–29. doi: 10.1046/j.1523-1747.2000.00008.x. [DOI] [PubMed] [Google Scholar]
- Wetzler C, Kämpfer H, Stallmeyer B, Pfeilschifter J, Frank S. Large and sustained induction of chemokines during impaired wound healing in the genetically diabetic mouse: prolonged persistence of neutrophils and macrophages during the late phase of repair. J Investig Dermatol. 2000;115(2):245–53. doi: 10.1046/j.1523-1747.2000.00029.x. [DOI] [PubMed] [Google Scholar]
- Wojciechowicz K, Gledhill K, Ambler CA, Manning CB, Jahoda CAB. Development of the mouse dermal adipose layer occurs independently of subcutaneous adipose tissue and is marked by restricted early expression of FABP4. PLOS ONE. 2013;8(3):e59811. doi: 10.1371/journal.pone.0059811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojciechowicz K, Markiewicz E, Jahoda CAB. C/EBPα identifies differentiating preadipocytes around hair follicles in foetal and neonatal rat and mouse skin. Exp Dermatol. 2008;17(8):675–80. doi: 10.1111/j.1600-0625.2007.00689.x. [DOI] [PubMed] [Google Scholar]
- Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214(2):199–210. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Barnes GT, Yang Q, Tan G, Yang D, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Investig. 2003;112(12):1821–30. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Palmer AK, Ding H, Weivoda MM, Pirtskhalava T, et al. Targeting senescent cells enhances adipogenesis and metabolic function in old age. eLife. 2015;4:e12997. doi: 10.7554/eLife.12997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C-C, Sheu H-M, Chung P-L, Chang C-H, Tsai Y-S, et al. Leptin of dermal adipose tissue is differentially expressed during the hair cycle and contributes to adipocyte-mediated growth inhibition of anagen-phase vibrissa hair. Exp Dermatol. 2015;24(1):57–60. doi: 10.1111/exd.12566. [DOI] [PubMed] [Google Scholar]
- Yim J-E, Heshka S, Albu J, Heymsfield S, Kuznia P, et al. Intermuscular adipose tissue rivals visceral adipose tissue in independent associations with cardiovascular risk. Int J Obes Relat Metab Disord. 2007;31(9):1400–5. doi: 10.1038/sj.ijo.0803621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yosipovitch G, DeVore A, Dawn A. Obesity and the skin: skin physiology and skin manifestations of obesity. J Am Acad Dermatol. 2007;56(6):901–16. doi: 10.1016/j.jaad.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Zangani D, Darcy KM, Shoemaker S, Ip MM. Adipocyte-epithelial interactions regulate the in vitro development of normal mammary epithelial cells. Exp Cell Res. 1999;247(2):399–409. doi: 10.1006/excr.1998.4373. [DOI] [PubMed] [Google Scholar]
- Zhang J, Niu C, Ye L, Huang H, He X, et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425(6960):836–41. doi: 10.1038/nature02041. [DOI] [PubMed] [Google Scholar]
- Zhang L-J, Guerrero-Juarez CF, Hata T, Bapat SP, Ramos R, et al. Dermal adipocytes protect against invasive Staphylococcus aureus skin infection. Science. 2015;347(6217):67–71. doi: 10.1126/science.1260972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YV, Cheong J, Ciapurin N, McDermitt DJ, Tumbar T. Distinct self-renewal and differentiation phases in the niche of infrequently dividing hair follicle stem cells. Cell Stem Cell. 2009;5(3):267–78. doi: 10.1016/j.stem.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou BO, Yue R, Murphy MM, Peyer JG, Morrison SJ. Leptin-receptor-expressing mesenchymal stromal cells represent the main source of bone formed by adult bone marrow. Cell Stem Cell. 2014;15(2):154–68. doi: 10.1016/j.stem.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]