Abstract
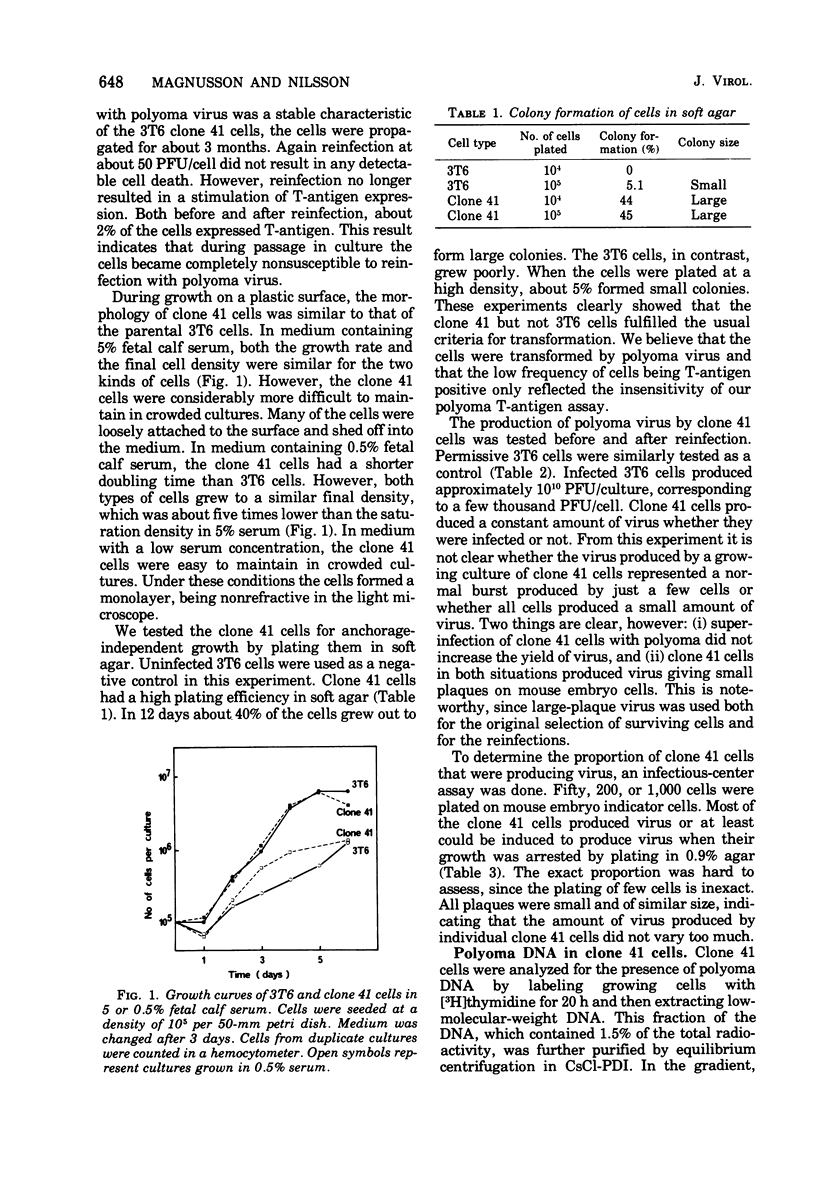
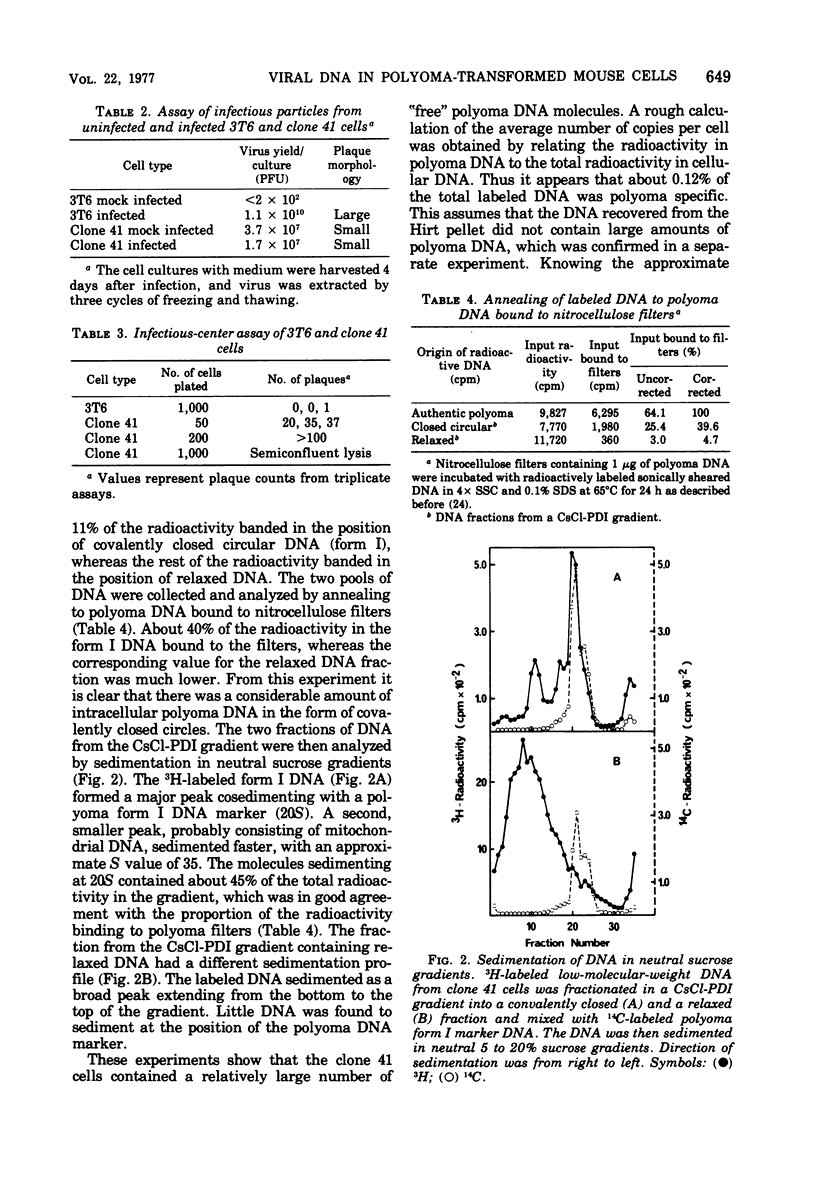
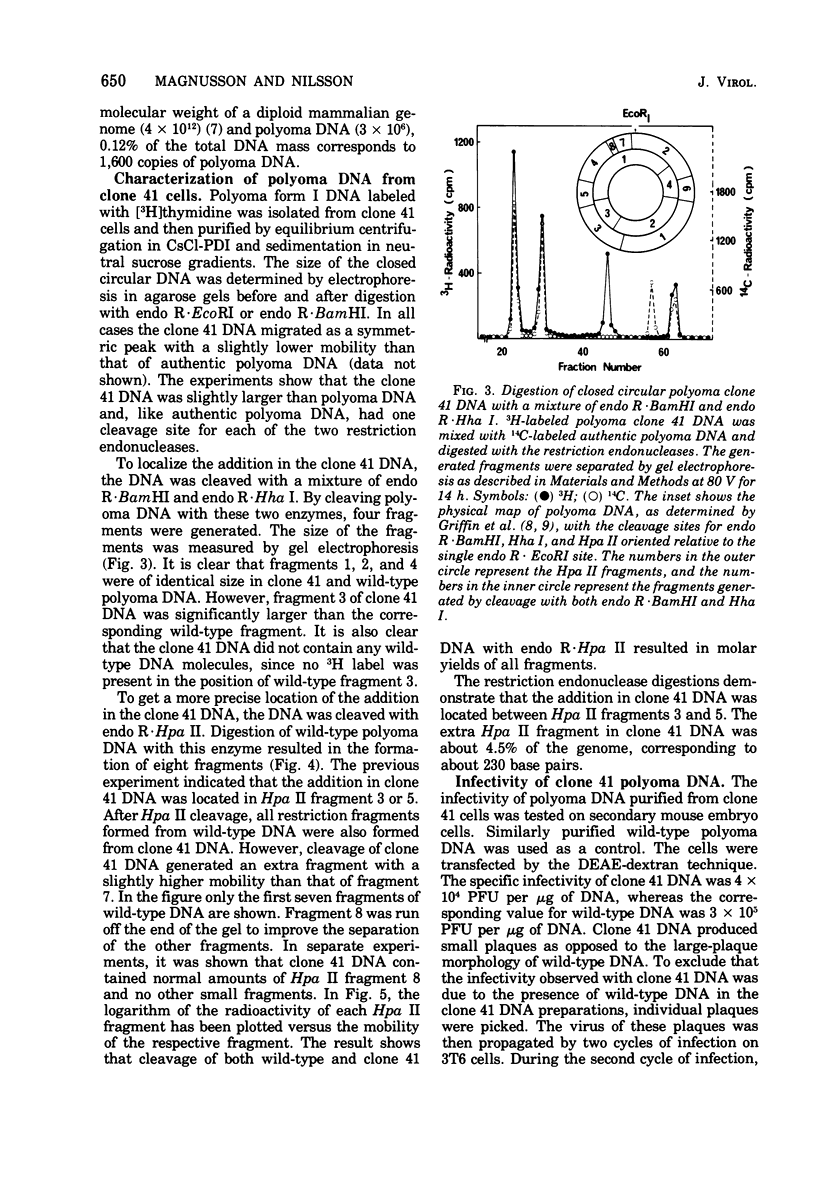
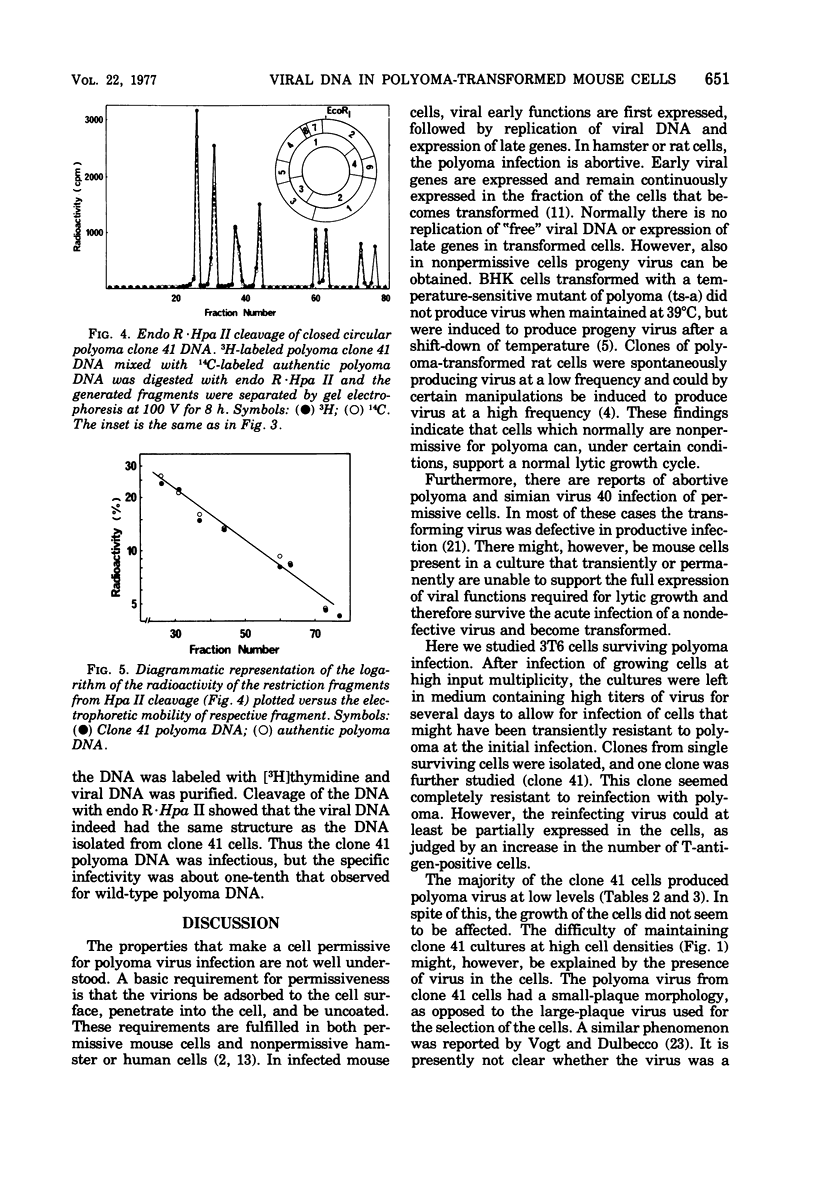
Mouse 3T6 cells were infected with polyoma virus at high multiplicity, and survivors were isolated. Clones from single cells were then established and were found to be resistant to a second infection. However, in some clones viral functions could at least be partially expressed during reinfection, as judged from a stimulation of nuclear tumor antigen expression. One such clone was studied in detail. These cells were transformed and produced low amounts of virus (less than 1 PFU per cell per generation). The persistent infection did not seem to be a carrier-state phenomenon, since infectious-center assays showed that most cells produced virus. The resistance of the cells to reinfection can be explained by interference from viral DNA present in the cells, averaging about 1,500 “free” copies per cell. This DNA had the normal physical characteristics of polyoma DNA. However, it had a slightly larger size than authentic polyoma DNA. Mapping with restriction endonucleases showed that the addition to the DNA was about 5% of the wild-type genome and was located close to the origin of DNA replication. This DNA was infectious, although it had a 10-fold lower infectivity than wild-type polyoma DNA. Both virus and DNA from the polyoma-resistant cells had a small-plaque morphology, as opposed to the large-plaque morphology of the virus used for the initial selection of cells.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOURGAUX P. MULTIPLICATION OF POLYOMA VIRUS IN CELLS OF A CONTINUOUS HAMSTER LINE SUSCEPTIBLE TO TRANSFORMATION. Virology. 1964 Sep;24:120–122. doi: 10.1016/0042-6822(64)90158-8. [DOI] [PubMed] [Google Scholar]
- BOURGAUX P. THE FATE OF POLYOMA VIRUS IN HAMSTER, MOUSE, AND HUMAN CELLS. Virology. 1964 May;23:46–55. doi: 10.1016/s0042-6822(64)80006-4. [DOI] [PubMed] [Google Scholar]
- Benjamin T. L. Host range mutants of polyoma virus. Proc Natl Acad Sci U S A. 1970 Sep;67(1):394–399. doi: 10.1073/pnas.67.1.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogel M. Induction of virus synthesis in polyoma-transformed cells by DNA antimetabolites and by irradiation after pretreatment with 5-bromodeoxyuridine. Virology. 1972 Jul;49(1):12–22. doi: 10.1016/s0042-6822(72)80003-5. [DOI] [PubMed] [Google Scholar]
- Folk W. R. Induction of virus synthesis in polyoma-transformed BHK-21 cells. J Virol. 1973 Mar;11(3):424–431. doi: 10.1128/jvi.11.3.424-431.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried M. Isolation and partial characterization of different defective DNA molecules derived from polyoma virus. J Virol. 1974 May;13(5):939–946. doi: 10.1128/jvi.13.5.939-946.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelb L. D., Kohne D. E., Martin M. A. Quantitation of Simian virus 40 sequences in African green monkey, mouse and virus-transformed cell genomes. J Mol Biol. 1971 Apr 14;57(1):129–145. doi: 10.1016/0022-2836(71)90123-9. [DOI] [PubMed] [Google Scholar]
- Griffin B. E., Fried M. Amplification of a specific region of the polyoma virus genome. Nature. 1975 Jul 17;256(5514):175–179. doi: 10.1038/256175a0. [DOI] [PubMed] [Google Scholar]
- Griffin B. E., Fried M., Cowie A. Polyoma DNA: a physical map. Proc Natl Acad Sci U S A. 1974 May;71(5):2077–2081. doi: 10.1073/pnas.71.5.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Kamen R., Lindstrom D. M., Shure H., Old R. W. Virus-specific RNA in cells productively infected or transformed by polyoma virus. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):187–198. doi: 10.1101/sqb.1974.039.01.025. [DOI] [PubMed] [Google Scholar]
- Kamen R., Shure H. Topography of polyoma virus messenger RNA molecules. Cell. 1976 Mar;7(3):361–371. doi: 10.1016/0092-8674(76)90165-3. [DOI] [PubMed] [Google Scholar]
- Mackay R. L., Consigli R. A. Early events in polyoma virus infection: attachment, penetration, and nuclear entry. J Virol. 1976 Aug;19(2):620–636. doi: 10.1128/jvi.19.2.620-636.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnusson G. Hydroxyurea-induced accumulation of short fragments during polyoma DNA replication. I. Characterization of fragments. J Virol. 1973 Sep;12(3):600–608. doi: 10.1128/jvi.12.3.600-608.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller L. K., Fried M. Construction of infectious polyoma hybrid genomes in vitro. Nature. 1976 Feb 19;259(5544):598–601. doi: 10.1038/259598a0. [DOI] [PubMed] [Google Scholar]
- Miller L. K., Fried M. Construction of the genetic map of the polyoma genome. J Virol. 1976 Jun;18(3):824–832. doi: 10.1128/jvi.18.3.824-832.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peacock A. C., Dingman C. W. Resolution of multiple ribonucleic acid species by polyacrylamide gel electrophoresis. Biochemistry. 1967 Jun;6(6):1818–1827. doi: 10.1021/bi00858a033. [DOI] [PubMed] [Google Scholar]
- Prasad I., Zouzias D., Basilico C. State of the viral DNA in rat cells transformed by polyoma virus. I. Virus rescue and the presence of nonintergrated viral DNA molecules. J Virol. 1976 May;18(2):436–444. doi: 10.1128/jvi.18.2.436-444.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp P. A., Sugden B., Sambrook J. Detection of two restriction endonuclease activities in Haemophilus parainfluenzae using analytical agarose--ethidium bromide electrophoresis. Biochemistry. 1973 Jul 31;12(16):3055–3063. doi: 10.1021/bi00740a018. [DOI] [PubMed] [Google Scholar]
- VOGT M., DULBECCO R. Studies on cells rendered neoplastic by polyoma virus: the problem of the presence of virus-related materials. Virology. 1962 Jan;16:41–51. doi: 10.1016/0042-6822(62)90200-3. [DOI] [PubMed] [Google Scholar]
- Vogt M., Dulbecco R. VIRUS-CELL INTERACTION WITH A TUMOR-PRODUCING VIRUS. Proc Natl Acad Sci U S A. 1960 Mar;46(3):365–370. doi: 10.1073/pnas.46.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winnacker E. L., Magnusson G., Reichard P. Replication of polyoma DNA in isolated nuclei. I. Characterization of the system from mouse fibroblast 3T6 cells. J Mol Biol. 1972 Dec 30;72(3):523–537. doi: 10.1016/0022-2836(72)90172-6. [DOI] [PubMed] [Google Scholar]


