Abstract
IMPORTANCE
As many as 60% of patients with Parkinson disease (PD) experience psychosis, 80% develop dementia, and the use of antipsychotics (APs) in the population with PD is common. The use of APs by patients with dementia in the general population is associated with increased mortality, but whether this risk extends to patients with PD remains unknown.
OBJECTIVE
To determine whether AP use in patients with PD is associated with increased mortality.
DESIGN, SETTING, AND PARTICIPANTS
This retrospective matched-cohort study used data from a Veterans Health Administration database from fiscal years 1999 to 2010 to examine the risk associated with AP use in a cohort of patients with idiopathic PD and recent stable physical health. The rates of 180-day mortality were compared in 7877 patients initiating AP therapy and 7877 patients who did not initiate AP therapy (matched for age ±2.5 years, sex, race, index year, presence and duration of dementia, PD duration, delirium, hospitalization, Charlson Comorbidity Index, and new nonpsychiatric medications). Data were analyzed from October 19, 2012, to September 21, 2015.
MAIN OUTCOMES AND MEASURES
Mortality rates at 180 days in those patients who initiated AP therapy compared with matched patients who did not use APs. Cox proportional hazards regression models were used with intent-to-treat (ITT) and exposure-only analyses.
RESULTS
The study population included 7877 matched pairs of patients with PD (65 women [0.8%] and 7812 men [99.2%] in each cohort; mean [SD] age, 76.3 [7.7] years for those who initiated AP therapy and 76.4 [7.6] years for those who did not). Antipsychotic use was associated with more than twice the hazard ratio (HR) of death compared with nonuse (ITT HR, 2.35; 95% CI, 2.08–2.66; P < .001). The HR was significantly higher for patients who used typical vs atypical APs (ITT HR, 1.54; 95% CI, 1.24–1.91; P < .001). Among the atypical APs used, HRs relative to nonuse of APs in descending order were 2.79 (95% CI, 1.97–3.96) for olanzapine, 2.46 (95% CI, 1.94–3.12) for risperidone, and 2.16 (95% CI, 1.88–2.48) for quetiapine fumarate.
CONCLUSIONS AND RELEVANCE
Use of APs is associated with a significantly increased mortality risk in patients with PD, after adjusting for measurable confounders. This finding highlights the need for cautious use of APs in patients with PD. Future studies should examine the role of nonpharmacologic strategies in managing psychosis in PD. In addition, new pharmacologic treatments that do not increase mortality in patients with neurodegenerative diseases need to be developed.
A range of nonmotor symptoms, including psychosis and dementia, are common in Parkinson disease (PD).1 Psychosis occurs in as many as 60%2,3 and dementia in as many as 80% of patients with long-term PD.4,5 Further, dementia is a known correlate of psychosis in PD.6
Use of antipsychotics (APs) in PD is common. One study reported that approximately one-third of patients with newly diagnosed PD were prescribed an AP within 7 years,7 and another reported a 6-year cumulative probability of initiating AP treatment at 50%.8 In an examination of approximately 2600 patients with PD and psychosis (using Veterans Affairs [VA] data from fiscal year 2008),9 50% were prescribed an AP during a 1-year period, with dementia significantly associated with increased AP use.
In 2005, the US Food and Drug Administration issued a public health advisory that the treatment of behavioral disorders in elderly patients with dementia with atypical AP medications is associated with increased mortality.10 A similar US Food and Drug Administration alert for typical APs followed in 2008,11 with evidence that typical (or conventional) APs may be more harmful than atypical APs for morbidity12 and mortality.13 Since then, Kales et al14 have demonstrated a differential risk among AP classes and specific APs.
Specific features of PD (eg, rigidity, gait and balance impairment, orthostatic hypotension, dysphagia, and autonomic system cardiac changes) may place these patients at even higher risk of morbidity and mortality than patients with general dementia. For example, patients with PD are already at a nearly 3-fold increased risk for hip fracture owing to gait and balance impairment.15
Little research has examined the risks associated with AP use in PD. A study using Medicaid data16 found that the use of quetiapine fumarate, risperidone, or olanzapine was associated with a higher rate of fracture in patients with a diagnosis of parkinsonism. Another study using Canadian health care administrative data17 compared deceased with matched living patients with PD; the investigators found that individuals exposed to APs had higher odds of death and that typical APs were associated with an increased odds of death compared with atypical APs. However, the investigators could not control for confounding by indication, and only 242 AP-treated patients with PD were included.
Using methods similar to what Kales et al14,18 used to document the increased risk for mortality associated with AP use in patients with general dementia, we used national VA health system administrative data to examine 180-day mortality risks associated with AP use in a large cohort of patients with PD. We compared patients who initiated AP treatment (exposed) with those who did not (unexposed) while controlling for a wide array of potential confounding factors. We hypothesized that AP-exposed patients with PD would have increased mortality compared with matched non–AP-exposed patients with PD.
Methods
Study Design
This retrospective, matched-cohort study used VA data from fiscal years 1999–2010. For every unique patient with PD who filled a new AP prescription, we randomly selected 1 individual from the risk set with replacement of matched non–AP-exposed patients. Matching (as outlined below) was performed to identify the day the exposed patient with PD filled the AP prescription as a phantom start date for the non–AP-exposed patients with PD so that the observation periods were matched by calendar time and comparisons would be unaffected by secular trends and other selection biases. We only included the first new AP prescription for each unique patient, which was defined as no AP exposure in the prior 6 months, which was a matching factor. We used National Death Index data19 to assess mortality. Typical APs included haloperidol, chlorpromazine hydrochloride, fluphenazine, loxapine, mesoridazine besylate, molindone hydrochloride, perphenazine, thioridazine hydrochloride, thiothixene hydrochloride, and trifluoperazine hydrochloride; atypical APs included olanzapine, quetiapine, risperidone, aripiprazole, clozapine, and ziprasidone. This study was approved by the VA Central institutional review board, which waived the need for informed consent.
Participants
As in the VA research study of patients with PD, psychosis, and dementia by Weintraub et al,9 patients with idiopathic PD were identified using code 332.0 from the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). Psychosis diagnoses included ICD-9-CM codes 293.81, 293.82, 297.0, 297.1, 297.2, 297.3, 297.8, 297.9, 298.0, 298.1, 298.2, 298.3, 298.4, 298.8, 298.9, 368.16, and 780.1; patients with schizophrenia and bipolar disorder were excluded. Because patients with PD constituted the population of interest, patients with a diagnosis of dementia with Lewy bodies ICD-9-CM code 331.82) were excluded. A patient was counted as having PD with dementia and included in the study if the dementia diagnosis followed the PD diagnosis by at least 1 year, to match the exclusion criteria for dementia with Lewy bodies.20 Dementia diagnoses included ICD-9-CM codes 290.0, 290.1, 290.11, 290.12, 290.13, 290.2, 290.21, 290.3, 290.4, 290.41, 290.42, 290.43, 291.2, 294.1, 294.11, 331.0, 331.1, 331.11, 331.19, and 331.2.
Inclusion and Exclusion Criteria, Matching Variables, and Covariates
Inclusion and Exclusion Criteria
Patients with an ICD-9-CM diagnosis of idiopathic PD were included. We excluded patients with (1) a diagnosis of dementia with Lewy bodies; (2) a dementia diagnosis preceding or within 1 year of the PD diagnosis; (3) a hospitalization or an emergency department visit within 14 days of the index new AP prescription (to ensure medical stability); (4) AP exposure in the preceding 180 days; (5) initiation of AP therapy before the PD diagnosis; (6) younger than 50 years at initiation of AP therapy; (7) more than 1 type of AP included on the same fill date; (8) a diagnosis of bipolar disorder, schizophrenia, schizoaffective disorder, or Huntington disease; and (9) an AP index prescription that occurred during a nursing home or inpatient stay. For every unique patient with PD who filled a new AP prescription, the AP fill date was defined as the index date, and we constructed a risk set of matched patients not treated with APs on the index date.
Matching Variables and Covariates
We matched patients by (1) age (±2.5 years); (2) sex; (3) race (white, black, other, and unknown); (4) index year; (5) comorbid dementia diagnosis within the year before the index date; (6) time from PD diagnosis to index date (±180 days); (7) delirium diagnosis within the year before the index date; (8) hospitalization in the prior year; (9) 3-category Charlson Comorbidity Index21 based on prior 1-year data (0, 1, or >1, where >1 could be >1 condition or a single condition with weighted score >1; the following conditions were weighted [weight]: hepatic failure [3], diabetes mellitus with complications [2], hemiplegia [2], chronic renal disease [2], malignant neoplasm [2], leukemia [2], lymphomas [2], metastatic solid tumor [6], human immunodeficiency virus without AIDS [2], and AIDS [6]); and (10) prescription of new nonpsychiatric medications within 14 days before and including the index date, to help ensure medical stability. For patients with comorbid dementia, an additional matching variable was time from dementia diagnosis to index date (±180 days), because longer duration of dementia is associated with mortality. For patients without comorbid dementia, additional matching variables were comorbid post-traumatic stress disorder and substance abuse diagnoses due to between-group differences in the distribution of these variables for patients without dementia.
Covariates included (1) marital status; (2) use of any new psychiatric medications within 14 days before the index date; (3) number of psychiatric and nonpsychiatric outpatient visits within 180 days before the index date; (4) medication use within the previous 1 year (separately for antidepressants, opioids, benzodiazepines, and cholinesterase inhibitors); (5) psychiatric diagnoses (separately for depression, other psychoses, alcohol abuse, other drug abuse, posttraumatic stress disorder, personality disorders, and other anxiety disorders); (6) number of days in the hospital in the year before the index date; (7) number of days in a nursing home in the year before the index date; (8) having an outpatient visit with a neurologist for PD within the year before the index date; (9) individual diseases from the Charlson Comorbidity Index21 (myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular disease, chronic obstructive pulmonary disease, rheumatologic disease, peptic ulcer disease, cirrhosis, hepatic failure, diabetes mellitus with and without complications, hemiplegia, chronic renal disease, malignant neoplasm, leukemia, lymphomas, metastatic solid tumor, and human immunodeficiency virus with and without AIDS); (10) academic affiliation of the facility; (11) urban vs rural facility; and (12) size of the facility. In subanalyses that include only those patients with AP exposure, matching variables were also entered as covariates.
Statistical Analysis
Data were analyzed from October 19, 2012, to September 21, 2015. We used intention-to-treat (ITT) analysis and exposure-only analysis approaches to measure the exposure period. For the ITT analysis, exposure period for the initial exposure status continued until 180 days after the index date (or the phantom fill date for unexposed participants) or death, whichever occurred first, regardless of any changes in exposure status (ie, stopping or switching AP treatments for the AP-exposed group or starting an AP treatment for the non–AP-exposed group). In the exposure-only analysis, the exposure period continued until the end of 180 days or to the time of switch, stop, or death, whichever occurred first.
Time to death since the index date was used to compare mortality risks during the 180-day period by baseline AP treatment status (exposed vs nonexposed), across baseline AP classes, and across different APs. Cox proportional hazards regression models stratified by the matched pairs using the robust sandwich estimate of Lin and Wei22 were used to adjust for covariates and to obtain adjusted hazard ratios (HRs). Comparisons across the different APs used only those patients exposed to an AP on the index date. Covariate-adjusted survival function for an average patient in the cohort by baseline AP exposure group was generated based on the predicted survival functions from the Cox proportional hazards regression without accounting for matching.
Results
Characteristics of Study Cohort
The demographic characteristics of the 7877 matched pairs (65 women [0.8%] and 7812 men [99.2%] in each cohort; mean [SD] age, 76.3 [7.7] years for those who initiated AP therapy and 76.4 [7.6] years for those who did not) are given in eTable 1 in the Supplement. A total of 905 AP-exposed patients were not included in the matched analyses because a matched non–AP-exposed participant could not be found. For the analyses comparing mortality rates in patients with typical vs atypical AP exposure, we included 8782 AP-exposed patients with or without a matched unexposed participant. Despite matching, AP-exposed and non–AP-exposed patients differed in a number of characteristics measuring mental health, including psychoses other than bipolar disorder or schizophrenia, alcohol abuse, other drug abuse, other anxiety disorders, personality disorders, and use of other psychiatric medications, so these variables were included as covariates in the models.
AP Classes and Specific Medications Prescribed
Among the 7877 AP-exposed patients, 422 (5.4%) were prescribed a typical (or conventional/traditional) AP, with haloperidol (282 [3.6%] of all AP prescriptions) being the most commonly prescribed typical AP. For atypical APs, the most commonly prescribed medication was quetiapine (5270 [66.9%]), followed by risperidone (1155 [14.7%]), olanzapine (837 [10.6%]), and other atypical APs (193 [2.5%]).
Unadjusted Mortality Rates by AP Class and Specific Medication
For AP-exposed patients, the unadjusted mortality rates (per 100 person-years) in the ITT analysis were highest for haloperidol, followed by other typical APs, risperidone, olanzapine, and quetiapine. In the exposure-only analysis, the rates were highest for haloperidol, followed by risperidone, other typical APs, olanzapine, and quetiapine (Table 1).
Table 1.
Unadjusted Mortality Rates by AP Exposure
| Antipsychotic | No. Patients Died/Total (%) | Total Person-years | Mortality Rate per 100 Person-years (95% CI) |
|---|---|---|---|
| Intention-to-treat analysis | |||
| Haloperidol | 60/282 (21.3) | 122.5 | 49.0 (37.4–63.0) |
| Other typical AP | 20/140 (14.3) | 64.0 | 31.3 (19.1–48.3) |
| Olanzapine | 113/837 (13.5) | 386.3 | 29.3 (24.1–35.2) |
| Quetiapine fumarate | 462/5270 (8.8) | 2488.8 | 18.6 (16.9–20.3) |
| Risperidone | 164/1155 (14.2) | 529.5 | 31.0 (26.4–36.1) |
| Other atypical AP | 13/193 (6.7) | 91.3 | 14.2 (7.6–24.3) |
| Exposure-only analysis | |||
| Haloperidol | 35/282 (12.4) | 68.7 | 50.9 (35.5–70.9) |
| Other typical AP | 8/140 (5.7) | 32.5 | 24.6 (10.6–48.5) |
| Olanzapine | 54/837 (6.5) | 240.1 | 22.5 (16.9–29.3) |
| Quetiapine | 268/5270 (5.1) | 1754.2 | 15.3 (13.5–17.2) |
| Risperidone | 96/1155 (8.3) | 340.8 | 28.2 (22.8–34.4) |
| Other atypical AP | 7/193 (3.6) | 59.1 | 11.8 (4.8–24.4) |
Abbreviation: AP, antipsychotic.
Mortality Rates in AP-Exposed vs Non–AP-Exposed Groups
After we adjusted for covariates, AP-exposed patients had greater than 2-fold higher hazards for mortality in the 180-day period after AP exposure in the ITT and exposure-only analyses compared with nonexposed patients (Table 2). This finding applied to patients exposed to typical and atypical APs, with an HR in the ITT analysis of 2.26 (95% CI, 1.98–2.57) in atypical AP-exposed vs non–AP-exposed patients and an HR in the exposure-only analysis of 3.65 (95% CI, 2.47–5.39) in typical AP-exposed vs non–AP-exposed patients. Survival probability graphs are presented for ITT analyses in the Figure and for exposure-only analyses in the eFigure in the Supplement. The HR was significantly higher for patients who used typical vs atypical APs (ITT HR, 1.54; CI, 1.24–1.91; P < .001).
Table 2.
Adjusted HRs for 180-Day Mortality in AP-Exposed vs Non–AP-Exposed Patients
| Group | HR (95% CI) | P Valuea |
|---|---|---|
| Intention-to-treat analysis | ||
| No AP exposure | 1 [Reference] | NA |
| AP exposure | 2.35 (2.08–2.66) | <.001 |
| AP exposure by type | ||
| No AP exposure | 1 [Reference] | NA |
| Atypical AP exposure | 2.26 (1.98–2.57) | <.001 |
| Typical AP exposure | 3.65 (2.47–5.39) | <.001 |
| Exposure-only analysis | ||
| No AP exposure | 1 [Reference] | NA |
| AP use | 2.15 (1.82–2.55) | <.001 |
| AP exposure by type | ||
| No AP exposure | 1 [Reference] | NA |
| Atypical AP exposure | 2.09 (1.75–2.49) | <.001 |
| Typical AP exposure | 3.11 (1.72–5.60) | <.001 |
Abbreviations: AP, antipsychotic; HR, hazard ratio; NA, not applicable.
Obtained from testing for the significance of the covariate estimates obtained from the corresponding Cox proportional hazards regression model.
Figure. Mortality Rates in Antipsychotic (AP)-Exposed vs Non–AP-Exposed Patients.
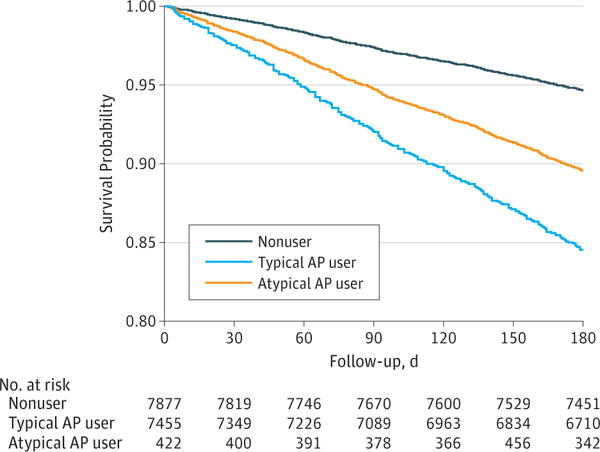
Covariate-adjusted 180-day survival estimates by baseline AP treatment status (intention-to-treat analysis) are shown. Graph is based on Cox proportional hazards regression modeling without pairing.
Because patients at imminent risk for mortality might be prescribed an AP as part of palliative care, we ran additional analyses excluding those patients who died within 4 weeks of the index date (eTable 2 in the Supplement). Excluding those patients who died within 4 weeks of the index date, the elevated mortality HR associated with AP exposure vs no exposure (HR, 2.30; 95% CI, 2.01–2.64), for typical AP exposure vs no exposure (HR, 3.98; 95% CI, 2.50–6.34), and atypical AP exposure vs no exposure (HR, 2.20; 95% CI, 1.90–2.55) were similar to the HRs presented for the entire cohort.
Mortality Risk by Specific AP
Increased mortality risk (compared with non–AP-exposed patients) was seen, in descending order, with use of haloperidol, olanzapine, risperidone, and quetiapine (Table 3). These results were seen for the ITT and exposure-only analyses. When we ran similar additional analyses excluding those patients who died within 4 weeks of the index data, an elevated HR associated with AP exposure remained for haloperidol, olanzapine, risperidone, and quetiapine specifically (eTable 3 in the Supplement).
Table 3.
Adjusted HRs for 180-Day Mortality by AP
| Group | HR (95% CI) | P Valuea |
|---|---|---|
| Intention-to-treat analysis | ||
| No AP exposure | 1 [Reference] | NA |
| Haloperidol | 5.08 (3.16–8.16) | <.001 |
| Other typical AP | 1.82 (0.94–3.50) | .07 |
| Olanzapine | 2.79 (1.97–3.96) | <.001 |
| Quetiapine fumarate | 2.16 (1.88–2.48) | <.001 |
| Risperidone | 2.46 (1.94–3.12) | <.001 |
| Other atypical AP | 1.19 (0.60–2.37) | .62 |
| Exposure-only analysis | ||
| No AP exposure | 1 [Reference] | NA |
| Haloperidol | 4.80 (2.41–9.57) | <.001 |
| Other typical AP | 0.82 (0.35–1.88) | .63 |
| Olanzapine | 2.76 (1.58–4.84) | <.001 |
| Quetiapine | 1.93 (1.59–2.33) | <.001 |
| Risperidone | 2.62 (1.83–3.76) | <.001 |
| Other atypical AP | 1.14 (0.41–3.18) | .80 |
Abbreviations: AP, antipsychotic; HR, hazard ratio; NA, not applicable.
Obtained from testing for the significance of the covariate estimates obtained from the corresponding Cox proportional hazards regression model.
Relative AP-Related Mortality Risks
In a subanalysis that included AP-exposed patients only and that used quetiapine exposure as a referent group (because quetiapine is the most commonly prescribed AP in patients with PD), increased mortality risks relative to quetiapine were seen (in descending order) for haloperidol, other typical APs, olanzapine, and risperidone (Table 4).
Table 4.
Individual AP Risks Relative to Quetiapine in AP-Exposed Patients
| Group | HR (95% CI) | P Valuea |
|---|---|---|
| Intention-to-treat analysis | ||
| Quetiapine fumarate | 1 [Reference] | NA |
| Haloperidol | 1.85 (1.41–2.43) | <.001 |
| Other typical AP | 1.54 (1.01–2.36) | .047 |
| Olanzapine | 1.47 (1.20–1.79) | <.001 |
| Risperidone | 1.30 (1.08–1.56) | .006 |
| Other atypical AP | 1.23 (0.69–2.19) | .49 |
| Exposure-only analysis | ||
| Quetiapine | 1 [Reference] | NA |
| Haloperidol | 2.33 (1.62–3.36) | <.001 |
| Other typical AP | 1.39 (0.70–2.79) | .35 |
| Olanzapine | 1.36 (1.01–1.83) | .04 |
| Risperidone | 1.42 (1.11–1.82) | .005 |
| Other atypical AP | 1.30 (0.58–2.89) | .52 |
Abbreviations: AP, antipsychotic; HR, hazard ratio; NA, not applicable.
Obtained from testing for the significance of the covariate estimates obtained from the corresponding Cox proportional hazards regression model.
Causes of Death
The leading causes of death in AP-exposed and non–AP-exposed patients (all causes of death in the ITT population) are given in eTable 4 in the Supplement. The leading cause of death in AP-exposed patients was PD (listed in 443 of 832 [53.2%] as a cause of death), 38% higher compared in non–AP-exposed patients (165 of 427 [38.6%]). The other causes of death that were listed in more than 10% of AP-exposed patients and more common in this group vs non–AP-exposed patients were influenza or pneumonia (127 of 832 [15.3%] vs 57 of 427 [13.3%]) and pneumonitis (85 of 832 [10.2%] vs 29 of 427 [6.8%]).
Discussion
Use of APs in patients with dementia is associated with an increased risk for morbidity and mortality,10,12,13 with differential risk among AP classes and specific APs.12,14 Our results extend this increased mortality risk to patients with PD and confirm preliminary research.17 We found a differential risk for typical vs atypical APs and among the atypical APs. Of particular note, the increased risk is not specific to dementia in this case because less than 10% of the patients studied herein were diagnosed as having dementia.
Some differences between patients with general dementia and PD preclude generalization. First, patients with PD have disease-related risk symptoms that may predispose them to or overlap with AP-associated adverse events (eg, falls, sedation, orthostatic hypotension, and parkinsonism). Second, the APs studied in dementia clinical trials (risperidone and olanzapine) are different than the most commonly used AP in patients with PD (quetiapine). Third, some general dementia studies have enrolled patients with broadly defined agitation or psychosis, whereas AP studies in PD have enrolled patients with psychosis specifically. Finally, psychosis in PD has a higher overall prevalence rate and presents differently than in general dementia; psychosis in patients with PD is associated with dopamine replacement therapy and is characterized by higher rates of hallucinations and less frequent delusions.2,23
The HR for mortality associated with any AP exposure was 2.35 (95% CI, 2.08–2.66), indicating a 6-month mortality rate 135% higher in AP-exposed patients compared with non–AP-exposed patients. Patients treated with atypical AP had an HR 62% higher than patients treated with an atypical AP. Given that two-thirds of typical AP exposure in this cohort was for haloperidol, this finding may suggest that APs that are potent dopamine receptor antagonists or have a high ratio of dopamine to serotonin receptor blocking24 pose the greatest risk to patients with PD.
Approximately 30% of treated patients were prescribed a high-potency AP (eg, risperidone, olanzapine, or haloperidol). These APs are most likely to worsen parkinsonism and potentially to increase mortality risks in patients with PD. Consistently, mortality rates were higher for haloperidol, risperidone, and olanzapine compared with lower-potency quetiapine.
Approximately 70% of APs prescribed in PD were quetiapine.9 Because no placebo-controlled studies demonstrate quetiapine’s efficacy for PD psychosis,25 clinicians often make it the first-line AP in PD based on clinical impressions of effectiveness, good tolerability compared with other APs, and complexities associated with use of the single AP approved by the US Food and Drug Administration that is efficacious for this condition (ie, clozapine). The concept of relatively good tolerability is supported by the finding that quetiapine had a lower mortality rate compared with all other commonly used APs, similar to what has been reported in patients with general dementia.26
We aimed to discern how AP exposure increases mortality in patients with PD. The most common cause of death in AP-exposed patients and the second most common cause of death in non–AP-exposed patients was PD, a nonspecific diagnosis in terms of determining the direct cause of death. However, patients treated with an AP were 38% more likely to have PD listed as their cause of death, and one possible explanation is that AP exposure was associated with worsening parkinsonism that led to serious adverse events subsumed under a diagnosis of PD.
Strengths of the research include our experience with analyzing VA pharmacoepidemiologic data, the exposure-matched design, the large sample size, and careful attention to inclusion and exclusion criteria and covariates. Limitations include our inability to verify the accuracy of medical record diagnoses (eg, not having a PD diagnosis made by a movement disorders neurologist, although a secondary analysis restricting the analyses to patients seen by a neurologist in the year before the index date yielded a similar result (HR, 2.61; 95% CI, 1.67–4.09; P < .001) and possible confounding by indication despite the inclusion and exclusion criteria, extensive matching, and inclusion of covariates (ie, patients prescribed an AP may have had more severe PD that predisposed to mortality, and we were unable to examine directly the severity of disease with this data set). As an additional measure to account for this latter possibility, additional analyses that excluded those patients who died within 4 weeks of initiation of AP treatment still showed a similar, elevated mortality HR associated with AP exposure in 180 days.
Future research should examine whether AP exposure is also associated with excess morbidity, compare risks in patients with PD vs Alzheimer disease and dementia with Lewy bodies, and determine whether clinical and demographic moderators of mortality exist. The latter analyses will help to inform clinical care as clinicians weigh the potential benefits and risks associated with AP prescribing to patients with PD. As new APs are introduced for PD psychosis, such as pimavanserin,27 whether they have a different mortality and morbidity risk profile needs to be determined. If newer APs with significantly different mechanisms of action are not similarly associated with increased mortality, this finding would more strongly implicate existing APs as associated with the observed increased mortality.
Conclusions
Given the more than 2-fold increased mortality risk during 6 months of AP exposure and limited evidence of their efficacy, APs need to be used cautiously in patients with PD. Evaluation for treatable comorbid medical conditions should be performed, and attempts to decrease use of PD medications that may contribute to psychosis should be considered.28 Off-label use of APs (eg, for insomnia) should be discouraged. Prescribing typical APs to patients with PD should be avoided. Research efforts are needed to develop APs that are efficacious but do not increase mortality risk in vulnerable patients, to test cognition-enhancing medications for AP properties (eg, cholinesterase inhibitors29,30), and to evaluate structured nonpharmacologic approaches to manage psychosis as has been done for behavioral symptoms in Alzheimer disease.31–33 Given that the incidence of PD is increasing worldwide and that psychosis is very common2 and distressing to patients and caregivers,34 the development of informed and improved treatment strategies for this condition remains a priority.
Supplementary Material
Key Points.
Question
Is an increased mortality risk associated with antipsychotic (AP) use in Parkinson disease (PD)?
Findings
In this retrospective cohort study of 7877 matched pairs of veterans with PD, AP use was associated with more than twice the hazard of death compared with nonuse. The hazard ratio was significantly higher for typical compared with atypical AP use.
Meaning
Because AP use is associated with significantly increased mortality risk in patients with PD, this medication class needs to be used cautiously in this population.
Acknowledgments
Funding/Support: This study was supported by merit review award IIR 12-144-2 from the Veterans Health Administration. Support for the Veterans Administration and Centers for Medicare & Medicaid data is provided by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Health Services Research and Development, VA Information Resource Center (project SDR 02-237 and 98-004).
Role of the Funder/Sponsor: The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Supplemental content at jamaneurology.com
Author Contributions: Dr Kim had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Weintraub, Kim, Wilkinson, Marras, Kales.
Acquisition, analysis, or interpretation of data: Weintraub, Chiang, Kim, Wilkinson, Stanislawski, Mamikonyan, Kales.
Drafting of the manuscript: Weintraub.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Weintraub, Chiang, Kim, Kales. Obtained funding: Kales.
Administrative, technical, or material support: Wilkinson, Stanislawski, Mamikonyan, Kales.
Conflict of Interest Disclosures: Dr Weintraub reported receiving honoraria from Acadia Pharmaceuticals, Inc, for participation on advisory board and continuing medical education activity. No other disclosures were reported.
References
- 1.Weintraub D, Burn DJ. Parkinson’s disease: the quintessential neuropsychiatric disorder. Mov Disord. 2011;26(6):1022–1031. doi: 10.1002/mds.23664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fénelon G, Soulas T, Zenasni F, Cleret de Langavant L. The changing face of Parkinson’s disease–associated psychosis: a cross-sectional study based on the new NINDS-NIMH criteria. Mov Disord. 2010;25(6):763–766. doi: 10.1002/mds.22839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Forsaa EB, Larsen JP, Wentzel-Larsen T, et al. A 12-year population-based study of psychosis in Parkinson disease. Arch Neurol. 2010;67(8):996–1001. doi: 10.1001/archneurol.2010.166. [DOI] [PubMed] [Google Scholar]
- 4.Aarsland D, Andersen K, Larsen JP, Lolk A, Kragh-Sørensen P. Prevalence and characteristics of dementia in Parkinson disease: an 8-year prospective study. Arch Neurol. 2003;60(3):387–392. doi: 10.1001/archneur.60.3.387. [DOI] [PubMed] [Google Scholar]
- 5.Hely MA, Reid WGJ, Adena MA, Halliday GM, Morris JGL. The Sydney Multicenter Study of Parkinson’s Disease: the inevitability of dementia at 20 years. Mov Disord. 2008;23(6):837–844. doi: 10.1002/mds.21956. [DOI] [PubMed] [Google Scholar]
- 6.Aarsland D, Larsen JP, Lim NG, et al. Range of neuropsychiatric disturbances in patients with Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1999;67(4):492–496. doi: 10.1136/jnnp.67.4.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marras C, Kopp A, Qiu F, et al. Antipsychotic use in older adults with Parkinson’s disease. Mov Disord. 2007;22(3):319–323. doi: 10.1002/mds.21192. [DOI] [PubMed] [Google Scholar]
- 8.Wang M-T, Lian P-W, Yeh C-B, Yen C-H, Ma K-H, Chan ALF. Incidence, prescription patterns, and determinants of antipsychotic use in patients with Parkinson’s disease. Mov Disord. 2011;26(9):1663–1669. doi: 10.1002/mds.23719. [DOI] [PubMed] [Google Scholar]
- 9.Weintraub D, Chen P, Ignacio RV, Mamikonyan E, Kales HC. Patterns and trends in antipsychotic prescribing for Parkinson disease psychosis. Arch Neurol. 2011;68(7):899–904. doi: 10.1001/archneurol.2011.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.US Food Drug Administration. Safety: atypical antipsychotic drugs. http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm150688.htm. Published April 11, 2005. Accessed February 9, 2016.
- 11.US Food Drug Administration. FDA requests boxed warnings on older class of antipsychotic drugs. http://www.fda.gov/newsevents/newsroom/pressannouncements/2008/ucm116912.htm. Published June 16, 2008. Accessed February 9, 2016.
- 12.Huybrechts KF, Rothman KJ, Silliman RA, Brookhart MA, Schneeweiss S. Risk of death and hospital admission for major medical events after initiation of psychotropic medications in older adults admitted to nursing homes. CMAJ. 2011;183(7):E411–E419. doi: 10.1503/cmaj.101406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liperoti R, Onder G, Landi F, et al. All-cause mortality associated with atypical and conventional antipsychotics among nursing home residents with dementia: a retrospective cohort study. J Clin Psychiatry. 2009;70(10):1340–1347. doi: 10.4088/JCP.08m04597yel. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kales HC, Kim HM, Zivin K, et al. Risk of mortality among individual antipsychotics in patients with dementia. Am J Psychiatry. 2012;169(1):71–79. doi: 10.1176/appi.ajp.2011.11030347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen Y-Y, Cheng P-Y, Wu S-L, Lai C-H. Parkinson’s disease and risk of hip fracture: an 8-year follow-up study in Taiwan. Parkinsonism Relat Disord. 2012;18(5):506–509. doi: 10.1016/j.parkreldis.2012.01.014. [DOI] [PubMed] [Google Scholar]
- 16.Dore DD, Trivedi AN, Mor V, Friedman JH, Lapane KL. Atypical antipsychotic use and risk of fracture in persons with parkinsonism. Mov Disord. 2009;24(13):1941–1948. doi: 10.1002/mds.22679. [DOI] [PubMed] [Google Scholar]
- 17.Marras C, Gruneir A, Wang X, et al. Antipsychotics and mortality in parkinsonism. Am J Geriatr Psychiatry. 2012;20(2):149–158. doi: 10.1097/JGP.0b013e3182051bd6. [DOI] [PubMed] [Google Scholar]
- 18.Kales HC, Valenstein M, Kim HM, et al. Mortality risk in patients with dementia treated with antipsychotics versus other psychiatric medications. Am J Psychiatry. 2007;164(10):1568–1576. doi: 10.1176/appi.ajp.2007.06101710. [DOI] [PubMed] [Google Scholar]
- 19.Cowper DC, Kubal JD, Maynard C, Hynes DM. A primer and comparative review of major US mortality databases. Ann Epidemiol. 2002;12(7):462–468. doi: 10.1016/s1047-2797(01)00285-x. [DOI] [PubMed] [Google Scholar]
- 20.McKeith IG, Dickson DW, Lowe J, et al. Consortium on DLB Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65(12):1863–1872. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- 21.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 22.Lin D, Wei J. The robust inference for the Cox proportional hazards model. J Am Stat Assoc. 1989;84(408):1074–1078. [Google Scholar]
- 23.Lyketsos CG, Sheppard J-ME, Steinberg M, et al. Neuropsychiatric disturbance in Alzheimer’s disease clusters into three groups: the Cache County study. Int J Geriatr Psychiatry. 2001;16(11):1043–1053. doi: 10.1002/gps.448. [DOI] [PubMed] [Google Scholar]
- 24.Richtand NM, Welge JA, Logue AD, Keck PE, Jr, Strakowski SM, McNamara RK. Dopamine and serotonin receptor binding and antipsychotic efficacy. Neuropsychopharmacology. 2007;32(8):1715–1726. doi: 10.1038/sj.npp.1301305. [DOI] [PubMed] [Google Scholar]
- 25.Seppi K, Weintraub D, Coelho M, et al. The Movement Disorder Society Evidence-Based Medicine Review Update: treatments for the non-motor symptoms of Parkinson’s disease. Mov Disord. 2011;26(suppl 3):S42–S80. doi: 10.1002/mds.23884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rossom RC, Rector TS, Lederle FA, Dysken MW. Are all commonly prescribed antipsychotics associated with greater mortality in elderly male veterans with dementia? J Am Geriatr Soc. 2010;58(6):1027–1034. doi: 10.1111/j.1532-5415.2010.02873.x. [DOI] [PubMed] [Google Scholar]
- 27.Cummings J, Isaacson S, Mills R, et al. Pimavanserin for patients with Parkinson’s disease psychosis: a randomised, placebo-controlled phase 3 trial. Lancet. 2014;383(9916):533–540. doi: 10.1016/S0140-6736(13)62106-6. [DOI] [PubMed] [Google Scholar]
- 28.Olanow CW, Stern MB, Sethi K. The scientific and clinical basis for the treatment of Parkinson disease (2009) Neurology. 2009;72(21 suppl 4):S1–S136. doi: 10.1212/WNL.0b013e3181a1d44c. [DOI] [PubMed] [Google Scholar]
- 29.Burn D, Emre M, McKeith I, et al. Effects of rivastigmine in patients with and without visual hallucinations in dementia associated with Parkinson’s disease. Mov Disord. 2006;21(11):1899–1907. doi: 10.1002/mds.21077. [DOI] [PubMed] [Google Scholar]
- 30.Mori E, Ikeda M, Kosaka K, Donepezil-DLB Study Investigators Donepezil for dementia with Lewy bodies: a randomized, placebo-controlled trial. Ann Neurol. 2012;72(1):41–52. doi: 10.1002/ana.23557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gitlin LN, Kales HC, Lyketsos CG. Nonpharmacologic management of behavioral symptoms in dementia. JAMA. 2012;308(19):2020–2029. doi: 10.1001/jama.2012.36918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kales HC, Gitlin LN, Lyketsos CG, Detroit Expert Panel on Assessment and Management of Neuropsychiatric Symptoms of Dementia Management of neuropsychiatric symptoms of dementia in clinical settings: recommendations from a multidisciplinary expert panel. J Am Geriatr Soc. 2014;62(4):762–769. doi: 10.1111/jgs.12730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kales HC, Gitlin LN, Lyketsos CG. Assessment and management of behavioral and psychological symptoms of dementia. BMJ. 2015;350:h369. doi: 10.1136/bmj.h369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goetz CG, Stebbins GT. Risk factors for nursing home placement in advanced Parkinson’s disease. Neurology. 1993;43(11):2227–2229. doi: 10.1212/wnl.43.11.2227. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


