Abstract
BACKGROUND
Obesity is a risk factor for atrial fibrillation, which in turn is associated with stroke, heart failure and increased all-cause mortality.
OBJECTIVE
We investigated whether weight loss through bariatric surgery may reduce the risk of new-onset atrial fibrillation.
METHODS
Swedish Obese Subjects (SOS) is a prospective matched cohort study conducted at 25 surgical departments and 480 primary healthcare centers in Sweden. The cohort was recruited between 1987 and 2001. Among 4021 obese individuals with sinus rhythm and no history of atrial fibrillation, 2000 underwent bariatric surgery (surgery group), and 2021 matched obese controls received usual care (control group). The outcome, first-time atrial fibrillation, was ascertained by crosschecking the SOS database with the Swedish National Patient Register on inpatient and outpatient diagnosis codes.
RESULTS
During a median follow-up of 19 years, first time atrial fibrillation occurred in 247 patients (12.4 %) in the surgical group, and in 340 (16.8 %) controls. The risk of developing atrial fibrillation was 29 % lower in the surgery group versus the control group (HR 0.71; 95 % CI 0.60-0.83; p < 0.001). Younger individuals benefited more from surgical intervention than those who were older (p-value for interaction 0.001). Also, those with a high diastolic blood pressure benefitted more from surgery than did those with a low diastolic blood pressure (p-value for interaction 0.028).
CONCLUSION
Compared with usual care, weight loss through bariatric surgery reduced the risk of atrial fibrillation among persons being treated for severe obesity. The risk reduction was more apparent in younger people and in those with higher blood pressure.
Keywords: Obesity, atrial fibrillation, bariatric surgery, weight loss
Introduction
Obesity is an increasing concern worldwide, with a global prevalence of 13 % (1). A high BMI is associated with hypertension, dyslipidemia and diabetes, and increases the risk of cardiovascular morbidity and mortality (2). Atrial fibrillation (AF) occurs in around 1 % of the adult population and is the most common type of cardiac arrhythmia requiring medical care (3). The consequences of AF are serious and include stroke, heart failure and increased all-cause mortality (4).
Large epidemiological studies have repeatedly demonstrated that obesity is a risk factor for AF (5,6). A meta-analysis of 16 studies found that a BMI above 30 kg/m2 increased the lifetime risk of developing AF by 49 % (7). It is likely that some of the increased morbidity and mortality attributed to excess body weight is mediated by this arrhythmia. Given the current global trends for obesity, the incidences of AF and related complications are likely to rise.
It is plausible to speculate that significant weight reduction would reduce the risk of new-onset AF, but there is little evidence to support such a belief. A clinically significant weight loss is difficult to achieve with lifestyle interventions and the results are often temporary. In comparison, bariatric surgery is an effective and safe treatment option resulting in large weight losses able to be maintained over time (8).
The Swedish Obese Subjects (SOS) study is an ongoing controlled intervention trial that compares the effects of bariatric surgery and conventional obesity care on morbidity and mortality (9). SOS has found that bariatric surgery, as a primary preventive strategy, reduces cardiovascular morbidity (10) and mortality (11) in obesity. The purpose of this report is to describe the effect of bariatric surgery on the incidence of AF.
Methods
The ongoing prospective controlled SOS intervention study comparing the effects of weight loss by bariatric surgery and conventional obesity care has previously been described in greater detail (12). In brief, 4047 obese participants were enrolled at 25 surgical departments and at 480 primary health care centers between September 1, 1987 and January 31, 2001. The surgery group was made up of 2010 individuals who expressed a preference for treatment with bariatric surgery. A matched control group of 2037 participants was created using an automatic matching program and 18 matching variables (sex, age, weight, height, waist-hip ratio, blood pressure, serum cholesterol and triglycerides, smoking, diabetes, menopause, 4 psychosocial variables associated with risk for death, and personality traits related to treatment preferences).
Eligible patients were 37 to 60 years of age and had a BMI ≥ 34 kg/m2 for men ≥ 38 kg/m2 for women. Patients were excluded if they had a history of AF at baseline, gastric surgery, ongoing malignancy, recent myocardial infarction, a bulimic eating pattern, alcohol/drug abuse, or psychiatric problems likely to impair study compliance. The bariatric surgical procedures used in the study included vertical banded gastroplasty (68%) gastric banding (19%), and gastric bypass (13%). The conventional treatment offered to control subjects was not predefined; instead it adhered to local routines of the health care centers. Seven regional ethics review boards in Sweden approved the study protocol. All participants gave written or oral consent.
Body weight was measured with electronic or calibrated scales at baseline and during follow-up at: 0.5, 1, 2, 3, 4, 6, 8, 10, 15, and 20 years. Blood samples were analyzed by the Central Laboratory at Sahlgrenska University Hospital (accredited according to European Norm EN45001). Self-reported information on previous cardiovascular disease, medication, smoking, and alcohol intake was obtained through a baseline questionnaire. Hypertension was defined as systolic pressure >140 mmHg, or diastolic pressure > 90 mmHg, or self-reported use of anti-hypertensive medication. Diabetes was defined as a fasting blood glucose level of at least 6.1 mmol/L (110 mg/dL) or self-reported use of a prescribed anti-diabetic medication. Sleep apnea was determined using a validated 8-item sleep questionnaire as described previously (13). All patients underwent a standard 12-lead ECG at baseline, which was read by a cardiologist.
Information regarding AF previous to baseline and during follow-up was obtained from the Swedish National Patient Register (NPR), which has been shown to be a valid and powerful tool to study health related outcomes in the Swedish population (14,15). The NPR has collected information on diagnoses for all inpatients in Sweden since 1987 and from all hospital-based outpatient visits since 2001. Primary care, however, is not included in the NPR. Every Swedish citizen has a unique Personal Identity Number (PIN), and almost all of all Swedish health care is included in the national public health care system. For public hospitals, reporting to the NPR is mandatory and the coverage is 99 % (15). Registrations consist of a principal diagnosis and up to 5 secondary diagnoses coded according to the International Classification of Diseases (ICD) system. We identified a first-time principal or secondary diagnosis of atrial fibrillation by cross-checking the SOS database with the NPR for the following diagnosis codes: 427D (ICD-9 until 1996) and I48 (ICD-10 from 1997).
Statistical Analysis
Patients who had a history of AF at baseline (n = 26) were excluded from all data analyses. Data is presented as mean values with standard deviations or as percentages. Comparisons between treatment groups used t-tests for continuous variables and a logistic-regression model for dichotomous variables. Participants were followed until the first diagnosis of AF, emigration or December 31, 2013 (the date on which the NPR was complete and the registers were linked). Cumulative incidence of AF was estimated with competing-risks regression models, in which deaths without AF were treated as competing events. Persons without AF who emigrated or were alive at the end of follow-up were treated as censored observations. Univariable and multivariable models were applied to obtain relative-risk estimates expressed as subhazard ratios. The treatment effect in the bariatric-surgery group compared to the control group was evaluated in a primary unadjusted analysis with a single covariate for treatment group (surgery or control) and in a secondary analysis that was adjusted for preselected baseline risk factors considered traditional for AF. The intention-to-treat principle was applied for all calculations. All statistical tests were 2-tailed and P-values < 0.05 were considered statistically significant.
Results
Baseline Characteristics and Changes in BMI during follow-up
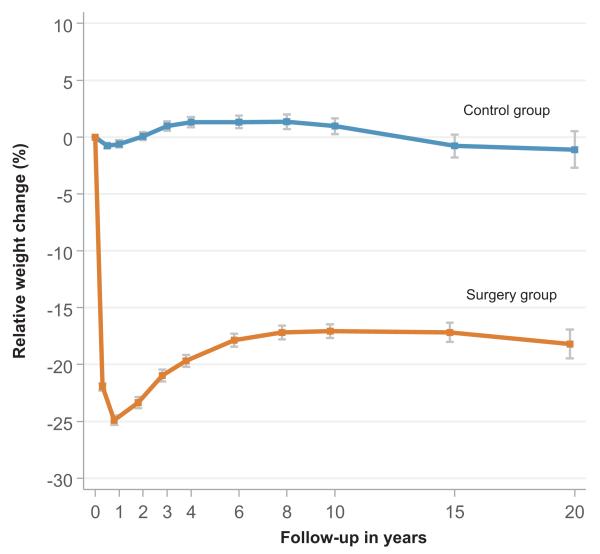
Among the 4047 patients in the SOS study, 4021 (99.4 %) had sinus rhythm and no history of AF at baseline and were included in the present analysis. Of these, 2000 underwent bariatric surgery (surgery group) and 2021 were treated conventionally (control group). Although the 2 study groups were fairly well balanced with respect to baseline characteristics, BMI was higher and several cardiovascular risk factors were less favorable in the surgery group (Table 1). The differences are likely explained by disparate weight changes occurring in the 2 groups during a delay between matching and baseline measurements caused by a long waiting time for bariatric surgery (> 1 year on average). Bariatric surgery lowered the mean weight of 121 kg by 25 % at year 1, by 20 % at year 4, by 17 % at year 10, and by 18 % at year 20, whereas the mean weight of 115 kg in the control group remained largely unchanged during follow-up (Figure 1).
Table 1.
Baseline characteristics of study participants
| Surgery group (n = 2000) |
Control group (n = 2021) |
p-Value | |
|---|---|---|---|
| Age (yrs.) | 47.2 ± 5.9 | 48.6 ± 6.2 | < 0.001 |
| Female sex (%) | 70.7 | 71.2 | 0.755 |
| Height (cm) | 169 ± 9.1 | 169 ± 9.2 | 0.688 |
| Weight (kg) | 121 ± 17 | 115 ± 16 | < 0.001 |
| Body mass index (kg/m2) | 42.4 ± 4.5 | 40.1 ± 4.7 | < 0.001 |
| Waist circumference (cm) | 126 ± 11 | 120 ± 11 | < 0.001 |
| Waist/Hip ratio | 0.99 ± 0.08 | 0.98 ± 0.07 | < 0.001 |
| Systolic blood pressure (mmHg) | 145 ± 19 | 138 ± 18 | < 0.001 |
| Diastolic blood pressure (mmHg) | 90 ± 11 | 85 ± 11 | < 0.001 |
| Total cholesterol (mmol/L) | 5.86 ± 1.12 | 5.61 ± 1.06 | < 0.001 |
| Apo B / Apo A1 ratio | 0.94 ± 0.28 | 0.91 ± 0.28 | < 0.001 |
| Blood glucose (mmol/L) | 5.18 ± 2.01 | 4.93 ± 1.82 | < 0.001 |
| Insulin (mU/L) | 21.5 ± 13.7 | 18.0 ± 11.4 | < 0.001 |
| Creatinine (μmo/L) | 69.2 ± 8.8 | 69.5 ± 9.6 | 0.245 |
| Urinary albumin excretion (μg/min) | 47.8 ± 297 | 37.1 ± 220 | 0.185 |
| Free thyroxin (pmol/L) | 15.6 ± 3.7 | 15.7 ± 3.6 | 0.812 |
| Thyroid-stimulating hormone (mIU/L) | 2.0 ± 2.4 | 2.04 ± 2.7 | 0.657 |
| Hypertension (%) | 78.3 | 63.6 | < 0.001 |
| Diabetes (%) | 17.2 | 12.7 | < 0.001 |
| Sleep apnea (%) | 24.4 | 21.7 | 0.047 |
| Smoking (%) | 25.8 | 20.9 | < 0.001 |
| Alcohol intake (g/daily) | 5.2 ± 7.3 | 5.3 ± 7.9 | 0.699 |
| Prevalent cardiovascular disease (%) | 3.0 | 2.9 | 0.853 |
Values are means ± standard deviation or percentages.
Figure 1. Relative weight changes in the two study groups.
Bariatric surgery lowered the mean weight of 121 in the surgery group kg by 25 % at year 1, by 20 % at year 4, by 17 % at year 10, and by 18 % at year 20, whereas the mean weight of 115 kg in the control group remained largely unchanged during follow-up
Incidence of AF
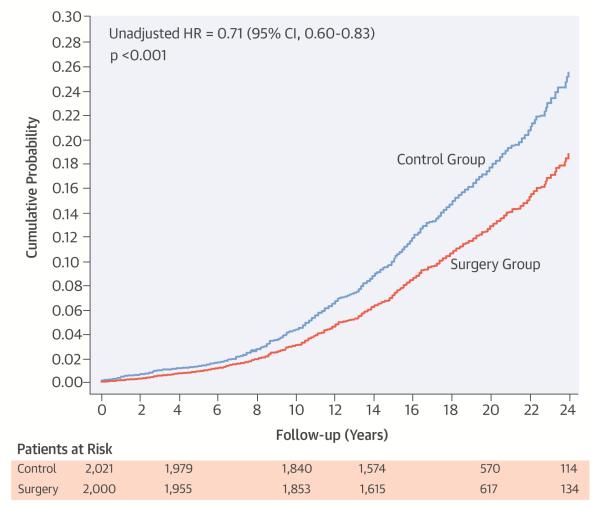
During a median follow-up of 19 years, first time AF diagnosed during inpatient hospital admission or hospital-based outpatient consultations occurred in 247 (12.4 %) patients in the surgery group and in 340 (16.8 %) patients in the control group (Central Illustration). Patients treated with bariatric surgery had a 29 % lower risk for being diagnosed with first time AF than patients in the control group (HR 0.71; 95% CI 0.60 to 0.83; p < 0.001)
Central Illustration. Bariatric Surgery and the Risk of Atrial Fibrillation.
Cumulative incidence estimates of first time atrial fibrillation in the surgery and control groups showing reduced risk of atrial fibrillation following weight loss through bariatric surgery.
Multivariate Analyses
After multivariable adjustments for selected baseline conditions, weight loss by bariatric surgery remained associated with reduced incidence of AF (adjusted HR 0.69; 95% CI 0.58 to 0.82; p < 0.001) (Table 2). Baseline conditions that were independently associated with increased risk of AF included advancing age, greater height, increasing BMI, hypertension, increasing thyroxin levels, and higher alcohol intake.
Table 2.
Association of bariatric surgery and selected baseline characteristics with risk of atrial fibrillation*
| Hazard ratio | 95 % CI | p-Value | |
|---|---|---|---|
| Surgery | 0.69 | 0.58-0.82 | < 0.001 |
| Age per 5 years | 1.52 | 1.42-1.64 | < 0.001 |
| Sex (male vs. female) | 1.26 | 0.95-1.68 | 0.105 |
| Height per 10 cm | 1.19 | 1.10-1.28 | < 0.001 |
| Body mass index per 5 kg/m2 | 1.33 | 1.14-1.55 | < 0.001 |
| Waist circumference per 5 cm | 0.97 | 0.90-1.03 | 0.282 |
| Hypertension | 1.57 | 1.25-1.98 | < 0.001 |
| Total cholesterol per mmol/L | 0.98 | 0.90-1.06 | 0.604 |
| Apo B / Apo A1 ratio | 1.17 | 0.82-1.67 | 0.378 |
| Diabetes | 1.12 | 0.90-1.38 | 0.305 |
| Urinary albumin excretion per 100 μg/min | 1.01 | 0.00-1.03 | 0.145 |
| Free thyroxin per 5 pmol/L | 1.18 | 1.07-1.29 | 0.001 |
| Smoking | 0.86 | 0.70-1.06 | 0.170 |
| Alcohol intake per 10 g/daily | 1.12 | 1.02-1.24 | 0.016 |
| Presence of cardiovascular disease | 1.02 | 0.67-1.56 | 0.914 |
Hazards ratios were estimated with a multivariate competing-risks regression model.
Interaction Analysis of Subgroups
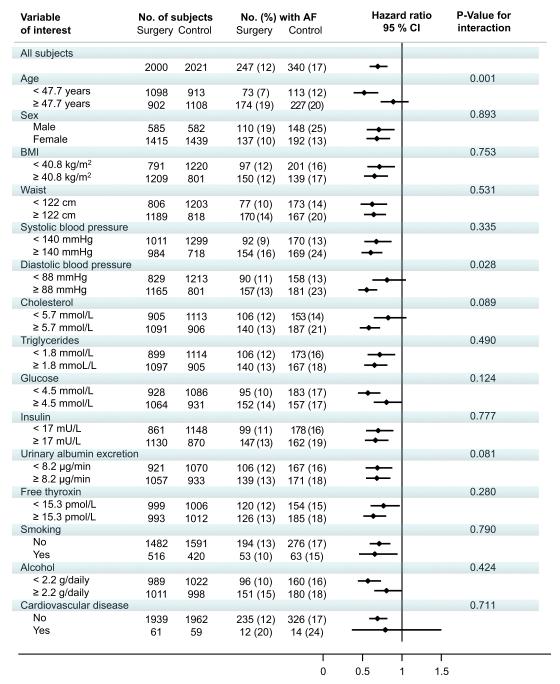
The effect of bariatric surgery on AF across subgroups is shown in Figure 2. Age and diastolic blood pressure at baseline influenced the treatment benefit of bariatric surgery. Younger individuals benefitted more from surgery than did those who were older (p-value for interaction 0.002). Also, those with high diastolic blood pressure benefitted more from surgery than those with low diastolic blood pressure (p-value for interaction 0.024). Otherwise, there were no significant interactions between treatment and subgroups.
Figure 2. Hazard ratios for the risk of atrial fibrillation in subgroups.
A reduced risk of atrial fibrillation following bariatric surgery was more pronounced in younger subjects (HR 0.52; 95 % CI 0.39-0.70) as compared to older (HR 0.91, 95 % CI 0.74-1.10) and also more apparent in individuals with higher diastolic blood pressure (HR 0.57; 95 % CI 0.46-0.70) as compared to those with lower (HR 0.82; 95 % CI 0.64-1.06). Otherwise, there were no significant interactions between treatment and subgroups.
Adverse Events
Adverse events in the SOS study have been described previously (9). Bariatric procedures were performed with open surgery in 89 % of cases. There were 5 individuals (0.2%) in the surgery group and 2 (0.1%) in the control group who died within 90 days of surgery/inclusion. In the surgery group, 151 (13.0 %) of the participants had 193 postoperative complications. Of these, 46 persons, (2.8 %) needed additional surgery.
Discussion
Among persons with severe obesity, those treated with bariatric surgery had a lower rate of new-onset AF than did those receiving usual care. The risk of AF was reduced by 29 % in the surgery group, despite a less favorable cardiovascular risk factor profile at baseline. The benefit took several years to become apparent and was more pronounced in younger patients and in those with higher blood pressure.
To our knowledge, this is the first time weight loss has been reported as reducing the risk of new-onset AF among persons with obesity. In this respect, it is important to emphasize that the findings relate to men with BMI ≥ 34 kg/m2 and to women with BMI ≥ 38 kg/m2 treated with bariatric surgery. The Look AHEAD (Action for HEAlth in Diabetes) trial (16) is a large randomized study of lifestyle intervention in overweight and obese people with type 2 diabetes. In a recent report (17), the Look AHEAD investigators found that a modest weight reduction (8.6% in year 1 and 6.0 % in year 9) did not reduce the risk of AF, which suggests that larger weight losses are necessary to attain a primary preventive anti-arrhythmic effect. This is consistent with observations from the SOS study, in which long-standing risk factor improvements required weight reductions that are larger and more sustainable than those typically seen after life style intervention (18). On the other hand, non-surgical weight management treatment has been shown to lower symptomatic arrhythmia burden among obese persons with established AF (19,20), implying that lifestyle modification can be successful as a secondary preventive measure.
The present findings support the causal role of obesity in the development of AF. The mechanism by which obesity is likely to increase the risk for AF includes hypertension (21), left ventricular diastolic dysfunction (22) and left atrial enlargement (23). Other probable pathways involve diabetes (24), obstructive sleep apnea (25) and systemic inflammation (26). We speculate that weight loss related alleviation of various hemodynamic, metabolic and inflammatory stimuli (9) may affect cardiac geometry (27) and function (28) in such a favorable way that the risk of arrhythmia is lowered. Weight reduction has also been shown to improve cardiac autonomic function (29), which would be expected to be protective. The beneficial effect of bariatric surgery on development of AF appeared late in the trial, with a separation of the cumulative incidence curves only after 6 years. This is not unexpected considering that SOS is essentially a primary prevention study for middle-aged persons, of whom only 3% had cardiovascular disease at inclusion.
After adjusting for baseline conditions, bariatric surgery remained significantly associated with a reduced risk for AF (HR 0.69; 95 % CI, 0.58–0.82; p < 0.001). The associations of baseline characteristics with risk of AF were similar to those observed in other populations (30) and included: advancing age, greater height, increasing BMI, hypertension, and higher alcohol intake. We also observed that higher free thyroxin was independently associated with risk for AF, suggesting that subclinical hyperthyroidism may be of importance with respect to the development of supraventricular arrhythmia in obesity.
Younger obese individuals and those with a higher diastolic blood pressure appeared to benefit more from surgical obesity treatment than those who were older and had lower diastolic blood pressure. This is reasonable, since obesity-related changes in cardiac structure and function are expected to be more reversible in younger patients with a shorter duration of obesity. Similarly, the antihypertensive effect of weight loss (31) is likely to be more advantageous among those with high blood pressure at baseline. Otherwise the effect of surgical obesity treatment was consistent across subgroups. Hence, bariatric surgery did not show a greater benefit among patients with a higher BMI at baseline as compared to those with a lower number. The finding that the obesity level does not influence the treatment advantage of bariatric surgery has been consistent throughout the SOS study and applies to both risk factors and cardiovascular events (9).
AF is a growing public health concern, not least due to its relationship to comorbidities and death (3). The rising prevalence of AF can, at least in part, be attributed to the continuing increase in obesity (30). A high BMI is among the largest modifiable risk factors for this arrhythmia and weight reduction has been proposed as a potential preventive measure (32). In this context, the findings of the present study are important. The successful prevention of AF through obesity surgery suggests that this treatment may have a positive impact on arrhythmia related complications and health care utilization.
Although the SOS study has several strengths there are also certain limitations. Allocation to surgical treatment was not random. Swedish ethical review boards did not approve this design, because of a high postoperative mortality in the 1980s. Therefore, the surgery group was made up of individuals who expressed a preference for treatment with bariatric surgery. Also, the matching procedure used here resulted in 2 slightly different study groups at baseline. However, the risk profile for AF was, if anything, less advantageous in the surgery group. AF was not a pre-specified endpoint of the trial and the diagnosis was collected by crosslinking the SOS database with the National Patient Register on inpatient and outpatient diagnosis codes. Still, ascertainment of newly diagnosed AF using the NPR has shown high validity with a sensitivity and positive predictive value exceeding 95% (14). Although the NPR does not cover primary care, it is unlikely that we missed many cases; in Sweden persons with newly diagnosed AF are customarily referred to a hospital for diagnostic work-up.
Conclusions
Surgically induced weight loss in severe obesity is associated with a reduced incidence of new-onset AF during long-term follow-up. The benefit is more pronounced in younger individuals and in those with higher blood pressure. Primary prevention of AF by bariatric surgery is likely to decrease arrhythmia related cardiovascular morbidity and mortality.
Perspectives.
Competency in Patient Care
Patients with severe obesity undergoing bariatric surgery develop atrial fibrillation less often than comparably obese patients managed without surgery.
Translational Outlook
Further studies are needed to understand the underlying mechanisms contributing to atrial fibrillation in obese patients and identify the amount of weight loss necessary to reduce risk of this type of arrhythmia.
Acknowledgments
Jonathan D. Stubbs, M.Sc., C.R.C. reviewed the English used in this article.
Funding sources: Research reported in this publication was supported by the Swedish Heart-Lung Foundation; and the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health [Award Number R01DK105948]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The SOS study is supported by the Swedish Research Council [K2013-99X-22279-01, K2013-54X-11285-19, K2015-55X-22082-04]; the Swedish federal government under the LUA/ALF agreement; and the Swedish Diabetes Foundation.
Abbreviations
- AF
Atrial fibrillation
- BMI
Body mass index
- CI
Confidence interval
- ECG
Electrocardiogram
- HR
Hazard ratio
- ICD
International Classification of Diseases
- NPR
National Patient Register
- SOS
Swedish Obese Subjects
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interest: LC has received lecture fees from AstraZeneca and Johnson & Johnson. KK has received lecture fees from AstraZeneca and Orion Pharma. There are no financial conflicts of interests among the other authors.
References
- 1.Ng M, Fleming T, Robinson M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:766–81. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lavie CJ, Milani RV, Ventura HO. Obesity and cardiovascular disease: risk factor, paradox, and impact of weight loss. J Am Coll Cardiol. 2009;53:1925–32. doi: 10.1016/j.jacc.2008.12.068. [DOI] [PubMed] [Google Scholar]
- 3.Lip GY, Tse HF, Lane DA. Atrial fibrillation. Lancet. 2012;379:648–61. doi: 10.1016/S0140-6736(11)61514-6. [DOI] [PubMed] [Google Scholar]
- 4.Camm AJ, Kirchhof P, Lip GY, et al. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC) Eur Heart J. 2010;31:2369–429. doi: 10.1093/eurheartj/ehq278. [DOI] [PubMed] [Google Scholar]
- 5.Heeringa J, van der Kuip DA, Hofman A, et al. Prevalence, incidence and lifetime risk of atrial fibrillation: the Rotterdam study. Eur Heart J. 2006;27:949–53. doi: 10.1093/eurheartj/ehi825. [DOI] [PubMed] [Google Scholar]
- 6.Lloyd-Jones DM, Wang TJ, Leip EP, et al. Lifetime risk for development of atrial fibrillation: the Framingham Heart Study. Circulation. 2004;110:1042–6. doi: 10.1161/01.CIR.0000140263.20897.42. [DOI] [PubMed] [Google Scholar]
- 7.Wanahita N, Messerli FH, Bangalore S, Gami AS, Somers VK, Steinberg JS. Atrial fibrillation and obesity--results of a meta-analysis. Am Heart J. 2008;155:310–5. doi: 10.1016/j.ahj.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 8.DeMaria EJ. Bariatric surgery for morbid obesity. N Engl J Med. 2007;356:2176–83. doi: 10.1056/NEJMct067019. [DOI] [PubMed] [Google Scholar]
- 9.Sjostrom L. Review of the key results from the Swedish Obese Subjects (SOS) trial - a prospective controlled intervention study of bariatric surgery. J Intern Med. 2013;273:219–34. doi: 10.1111/joim.12012. [DOI] [PubMed] [Google Scholar]
- 10.Sjostrom L, Peltonen M, Jacobson P, et al. Bariatric surgery and long-term cardiovascular events. JAMA. 2012;307:56–65. doi: 10.1001/jama.2011.1914. [DOI] [PubMed] [Google Scholar]
- 11.Sjostrom L, Narbro K, Sjostrom CD, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357:741–52. doi: 10.1056/NEJMoa066254. [DOI] [PubMed] [Google Scholar]
- 12.Sjostrom L, Larsson B, Backman L, et al. Swedish obese subjects (SOS). Recruitment for an intervention study and a selected description of the obese state. Int J Obe Relat Metab Disord. 1992;16:465–79. [PubMed] [Google Scholar]
- 13.Grunstein RR, Stenlof K, Hedner JA, Peltonen M, Karason K, Sjöström L. Two year reduction in sleep apnea symptoms and associated diabetes incidence after weight loss in severe obesity. Sleep. 2007;30:703–10. doi: 10.1093/sleep/30.6.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith JG, Platonov PG, Hedblad B, Engstrom G, Melander O. Atrial fibrillation in the Malmo Diet and Cancer study: a study of occurrence, risk factors and diagnostic validity. Eur J Epidemiol. 2010;25:95–102. doi: 10.1007/s10654-009-9404-1. [DOI] [PubMed] [Google Scholar]
- 15.Ludvigsson JF, Andersson E, Ekbom A, et al. External review and validation of the Swedish national inpatient register. BMC Public Health. 2011;11:450. doi: 10.1186/1471-2458-11-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wing RR, Bolin P, Brancati FL, et al. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. New Eng J Med. 2013;369:145–54. doi: 10.1056/NEJMoa1212914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alonso A, Bahnson JL, Gaussoin SA, et al. Effect of an intensive lifestyle intervention on atrial fibrillation risk in individuals with type 2 diabetes: The Look AHEAD randomized trial. Am Heart J. 2015;170:770–7. doi: 10.1016/j.ahj.2015.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sjostrom L, Lindroos AK, Peltonen M, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351:2683–93. doi: 10.1056/NEJMoa035622. [DOI] [PubMed] [Google Scholar]
- 19.Abed HS, Wittert GA, Leong DP, et al. Effect of weight reduction and cardiometabolic risk factor management on symptom burden and severity in patients with atrial fibrillation: a randomized clinical trial. JAMA. 2013;310:2050–60. doi: 10.1001/jama.2013.280521. [DOI] [PubMed] [Google Scholar]
- 20.Pathak RK, Middeldorp ME, Meredith M, et al. Long-term effect of goal-directed weight management in an atrial fibrillation cohort: a long-term follow-up study (LEGACY) J Am Coll Cardiol. 2015;65:2159–69. doi: 10.1016/j.jacc.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 21.Healey JS, Connolly SJ. Atrial fibrillation: hypertension as a causative agent, risk factor for complications, and potential therapeutic target. Am J Cardiol. 2003;91:9G–14G. doi: 10.1016/s0002-9149(03)00227-3. [DOI] [PubMed] [Google Scholar]
- 22.Russo C, Jin Z, Homma S, et al. Effect of obesity and overweight on left ventricular diastolic function: a community-based study in an elderly cohort. J Am Coll Cardiol. 2011;57:1368–74. doi: 10.1016/j.jacc.2010.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang TJ, Parise H, Levy D, et al. Obesity and the risk of new-onset atrial fibrillation. JAMA. 2004;292:2471–7. doi: 10.1001/jama.292.20.2471. [DOI] [PubMed] [Google Scholar]
- 24.Zethelius B, Gudbjornsdottir S, Eliasson B, Eeg-Olofsson K, Svensson AM, Cederholm J. Risk factors for atrial fibrillation in type 2 diabetes: report from the Swedish National Diabetes Register (NDR) Diabetologia. 2015;58:2259–68. doi: 10.1007/s00125-015-3666-9. [DOI] [PubMed] [Google Scholar]
- 25.Gami AS, Pressman G, Caples SM, et al. Association of atrial fibrillation and obstructive sleep apnea. Circulation. 2004;110:364–7. doi: 10.1161/01.CIR.0000136587.68725.8E. [DOI] [PubMed] [Google Scholar]
- 26.Aviles RJ, Martin DO, Apperson-Hansen C, et al. Inflammation as a risk factor for atrial fibrillation. Circulation. 2003;108:3006–10. doi: 10.1161/01.CIR.0000103131.70301.4F. [DOI] [PubMed] [Google Scholar]
- 27.Kardassis D, Bech-Hanssen O, Schonander M, Sjöström L, Karason K. The influence of body composition, fat distribution, and sustained weight loss on left ventricular mass and geometry in obesity. Obesity (Silver Spring) 2012;20:605–11. doi: 10.1038/oby.2011.101. [DOI] [PubMed] [Google Scholar]
- 28.Kardassis D, Grote L, Sjostrom L, Hedner J, Karason K. Sleep apnea modifies the long-term impact of surgically induced weight loss on cardiac function and inflammation. Obesity (Silver Spring) 2013;21:698–704. doi: 10.1002/oby.20115. [DOI] [PubMed] [Google Scholar]
- 29.Karason K, Molgaard H, Wikstrand J, Sjostrom L. Heart rate variability in obesity and the effect of weight loss. Am J Cardiol. 1999;83:1242–7. doi: 10.1016/s0002-9149(99)00066-1. [DOI] [PubMed] [Google Scholar]
- 30.Schnabel RB, Yin X, Gona P, et al. 50 year trends in atrial fibrillation prevalence, incidence, risk factors, and mortality in the Framingham Heart Study: a cohort study. Lancet. 2015;386:154–62. doi: 10.1016/S0140-6736(14)61774-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neter JE, Stam BE, Kok FJ, Grobbee DE, Geleijnse JM. Influence of weight reduction on blood pressure: a meta-analysis of randomized controlled trials. Hypertension. 2003;42:878–84. doi: 10.1161/01.HYP.0000094221.86888.AE. [DOI] [PubMed] [Google Scholar]
- 32.Nalliah CJ, Sanders P, Kottkamp H, Kalman JM. The role of obesity in atrial fibrillation. Eur Heart J. 2015;37:1565–72. doi: 10.1093/eurheartj/ehv486. [DOI] [PubMed] [Google Scholar]