Abstract
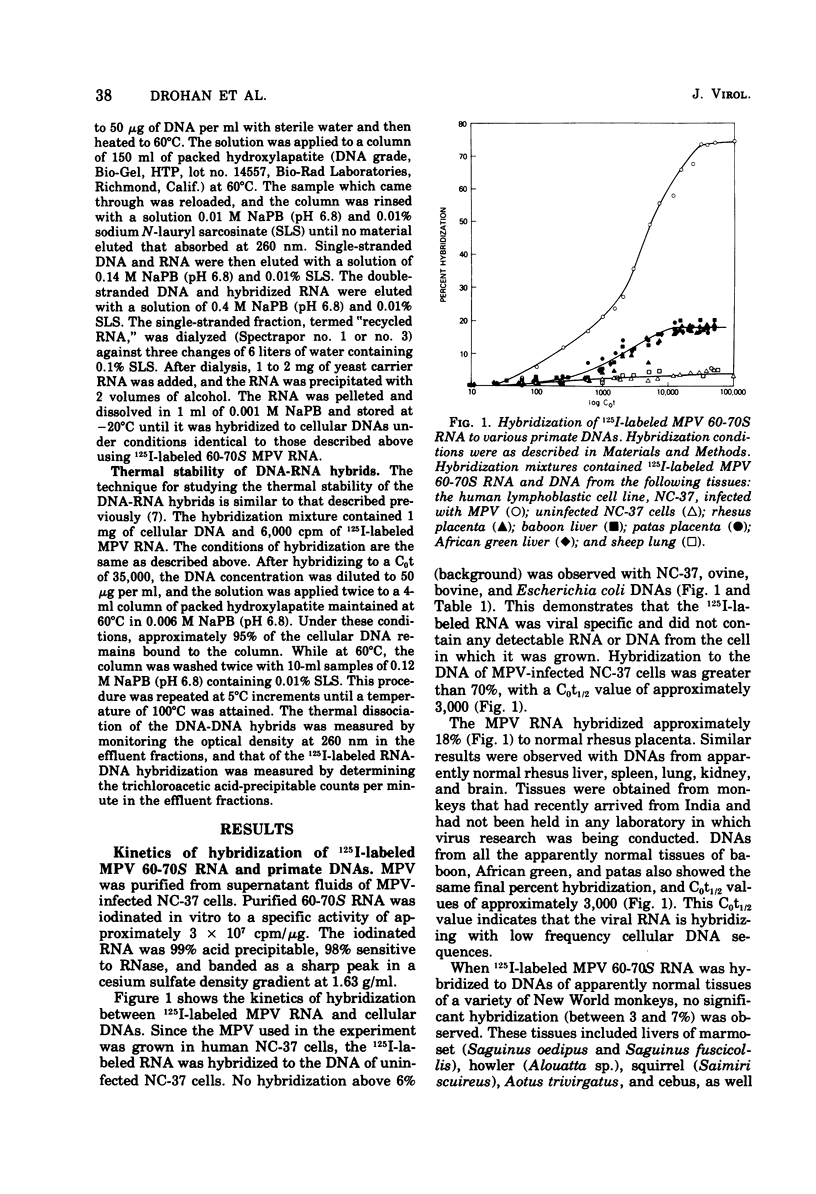
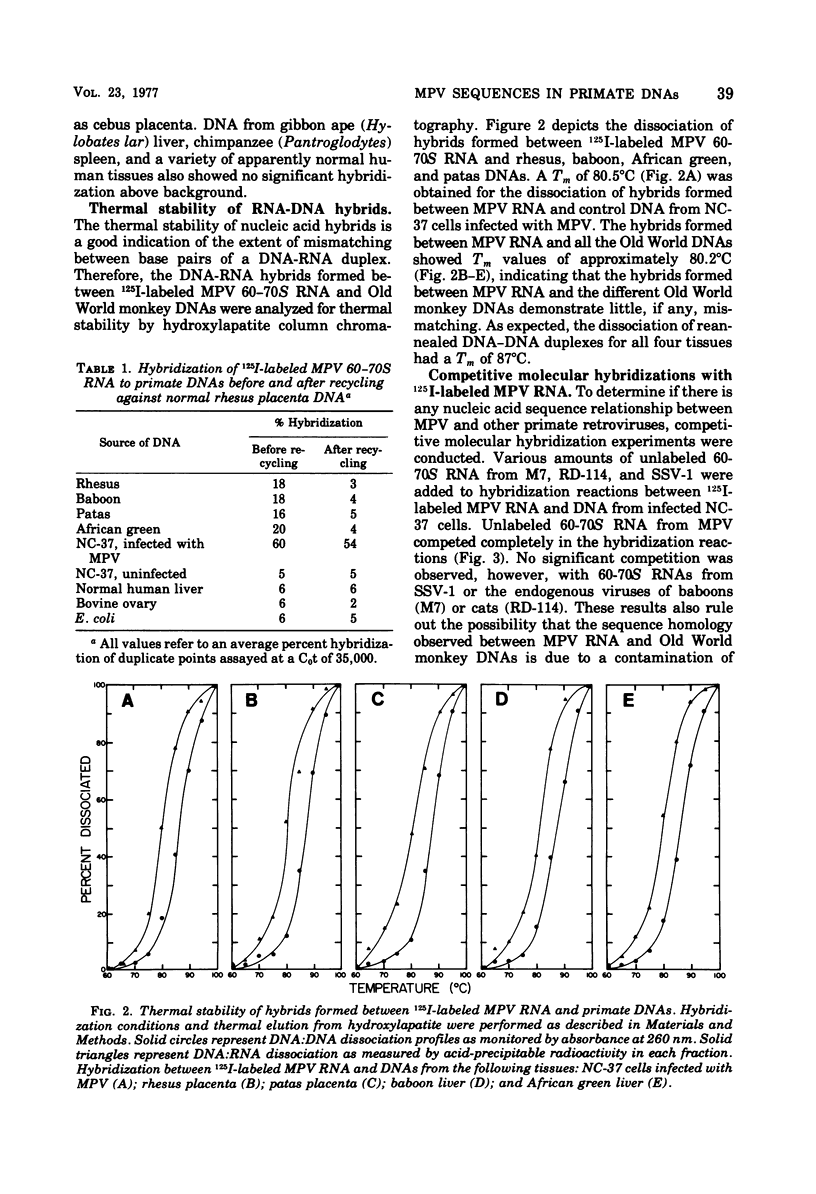
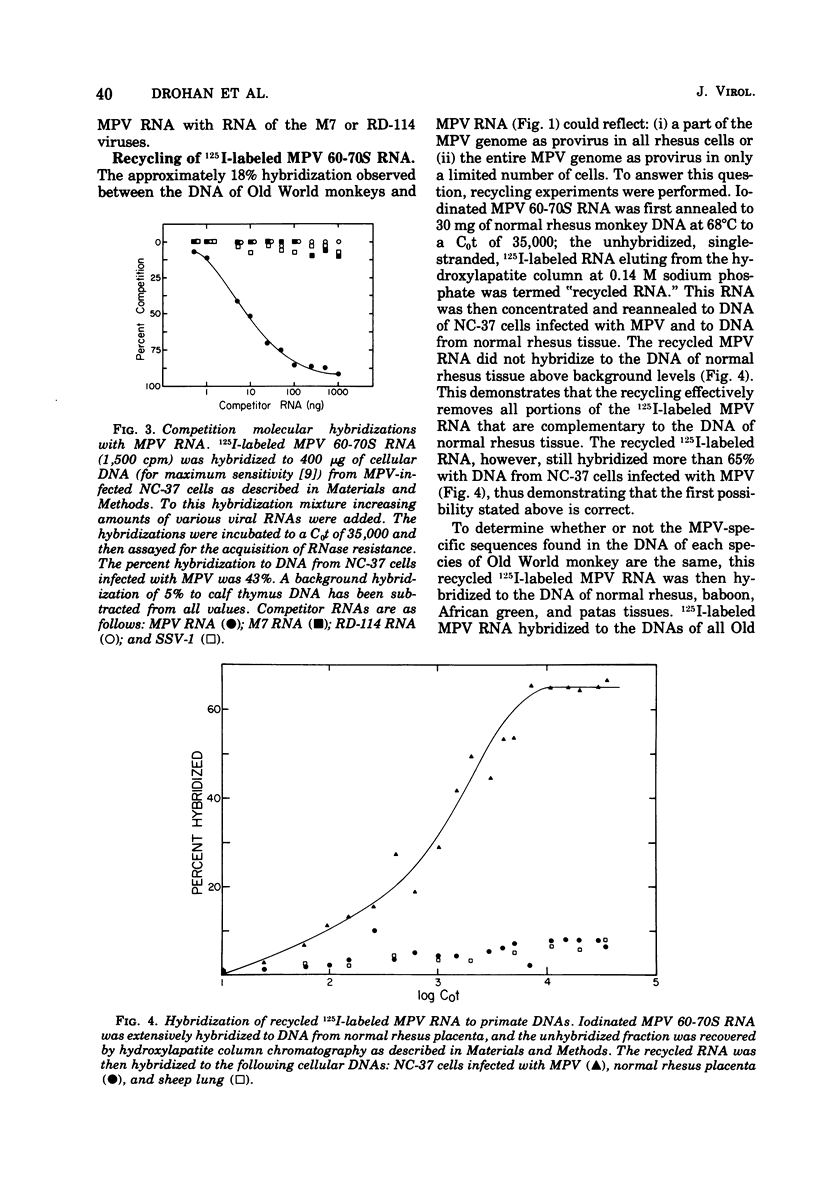
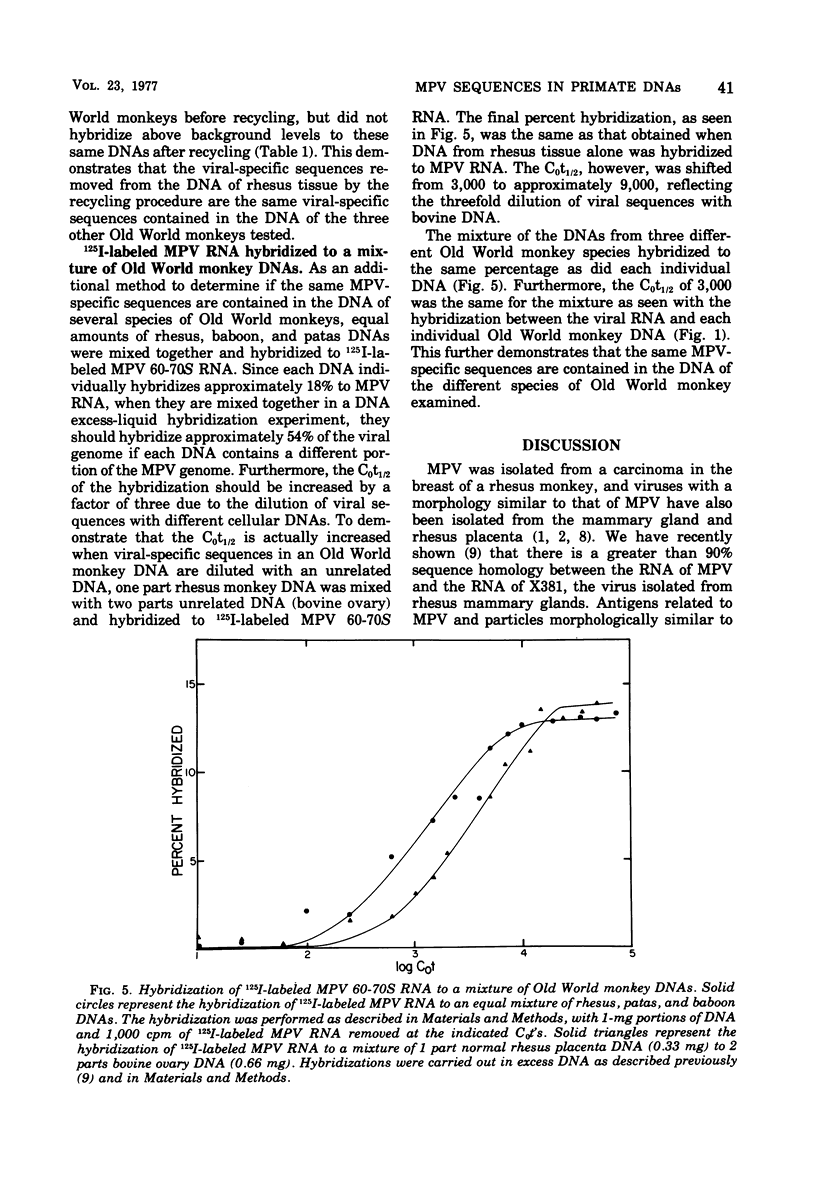
Iodinated Mason-Pfizer virus (MPV) 60-70S RNA has been used in molecular hybridization experiments to determine the distribution of MPV-specific proviral sequences in the DNAs of primates. Approximately 20% of the MPV genome is present as endogenous provirus in rhesus monkeys. Competitive hybridization experiments showed no homology between MPV 60-70S RNA and the 60-70S RNAs of M7, RD-114, and the simian sarcoma virus. No MPV-specific proviral sequences were detected in the DNAs of apparently normal tissues of various species of New World monkeys, apes, and humans. The part of the MPV genome that is endogenous to rhesus is also endogenous to the other species of Old World monkeys examined: baboon, African green, and patas. This was determined as a result of the following observations: (i) C0t1/2 values and final extent of hybridization were the same for all four species. (ii) Tm values of MPV 60-70S RNA and DNA of all four species were identical. (iii) The removal of MPV sequences endogenous to rhesus tissues by recycling against rhesus DNA resulted in the loss of any hybridizable MPV RNA to the DNAs of baboon, African green, and patas tissues. (iv) Mixing experiments of rhesus, African green, and baboon DNAs resulted in the same kinetics of hybridization as did rhesus DNA alone, when hybridized with MPV 60-70S RNA. These findings demonstrate that sequences that constitute an integral part of the MPV genome are conserved in the DNAs of several different species of Old World monkeys.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed M., Schidlovsky G., Korol W., Vidrine G., Cicmanec J. L. Occurrence of Mason-Pfizer monkey virus in healthy rhesus monkeys. Cancer Res. 1974 Dec;34(12):3504–3508. [PubMed] [Google Scholar]
- Baluda M. A., Drohan W. N. Distribution of deoxyribonucleic acid complementary to the ribonucleic acid of avian myeloblastosis virus in tissues of normal and tumor-bearing chickens. J Virol. 1972 Nov;10(5):1002–1009. doi: 10.1128/jvi.10.5.1002-1009.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste R. E., Lieber M. M., Livingston D. M., Sherr C. J., Todaro G. J., Kalter S. S. Infectious C-type virus isolated from a baboon placenta. Nature. 1974 Mar 1;248(5443):17–20. doi: 10.1038/248017a0. [DOI] [PubMed] [Google Scholar]
- Benveniste R. E., Sherr C. J., Todaro G. J. Evolution of type C viral genes: origin of feline leukemia virus. Science. 1975 Nov 28;190(4217):886–888. doi: 10.1126/science.52892. [DOI] [PubMed] [Google Scholar]
- Britten R. J., Kohne D. E. Repeated sequences in DNA. Hundreds of thousands of copies of DNA sequences have been incorporated into the genomes of higher organisms. Science. 1968 Aug 9;161(3841):529–540. doi: 10.1126/science.161.3841.529. [DOI] [PubMed] [Google Scholar]
- Chopra H. C., Mason M. M. A new virus in a spontaneous mammary tumor of a rhesus monkey. Cancer Res. 1970 Aug;30(8):2081–2086. [PubMed] [Google Scholar]
- Colcher D., Drohan W., Schlom Mason-Pfizer virus RNA genome: relationship to the RNA of morphologically similar isolates and other oncornaviruses. J Virol. 1976 Mar;17(3):705–712. doi: 10.1128/jvi.17.3.705-712.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drohan W., Kettmann R., Colcher D., Schlom J. Isolation of the mouse mammary tumor virus sequences not transmitted as germinal provirus in the C3H and RIII mouse strains. J Virol. 1977 Mar;21(3):986–995. doi: 10.1128/jvi.21.3.986-995.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalter S. S., Helmke R. J., Panigel M., Heberling R. L., Felsburg P. J., Axelrod L. R. Observations of apparent C-type particles in baboon (Papio cynocephalus) placentas. Science. 1973 Mar 30;179(4080):1332–1333. doi: 10.1126/science.179.4080.1332. [DOI] [PubMed] [Google Scholar]
- McAllister R. M., Nicolson M., Gardner M. B., Rongey R. W., Rasheed S., Sarma P. S., Huebner R. J., Hatanaka M., Oroszlan S., Gilden R. V. C-type virus released from cultured human rhabdomyosarcoma cells. Nat New Biol. 1972 Jan 5;235(53):3–6. doi: 10.1038/newbio235003a0. [DOI] [PubMed] [Google Scholar]
- Parks W. P., Gilden R. V., Bykovsky A. F., Miller G. G., Zhdanov V. M., Soloviev V. D., Scolnick E. M. Mason-Pfizer virus characterization: a similar virus in a human amniotic cell line. J Virol. 1973 Dec;12(6):1540–1547. doi: 10.1128/jvi.12.6.1540-1547.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlom J., Colcher D., Spiegelman S., Gillespie S., Gillespie D. Quantitation of RNA tumor viruses and viruslike particles in human milk by hybridization to polyadenylic acid sequences. Science. 1973 Feb 16;179(4074):696–698. doi: 10.1126/science.179.4074.696. [DOI] [PubMed] [Google Scholar]
- Schlom J., Spiegelman S. DNA polymerase activities and nucleic acid components of virions isolated from a spontaneous mammary carcinoma from a rhesus monkey. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1613–1617. doi: 10.1073/pnas.68.7.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoyab M., Evans R. M., Baluda M. A. Presence in leukemic cells of avian myeloblastosis virus-specific DNA sequences absent in normal chicken cells. J Virol. 1974 Jul;14(1):47–49. doi: 10.1128/jvi.14.1.47-49.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong-Staal F., Gallo R. C., Gillespie D. Genetic relationship of a primate RNA tumour virus genome to genes in normal mice. Nature. 1975 Aug 21;256(5519):670–672. doi: 10.1038/256670a0. [DOI] [PubMed] [Google Scholar]


