Abstract
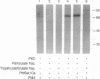
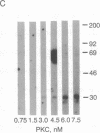
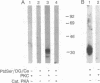
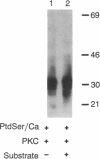
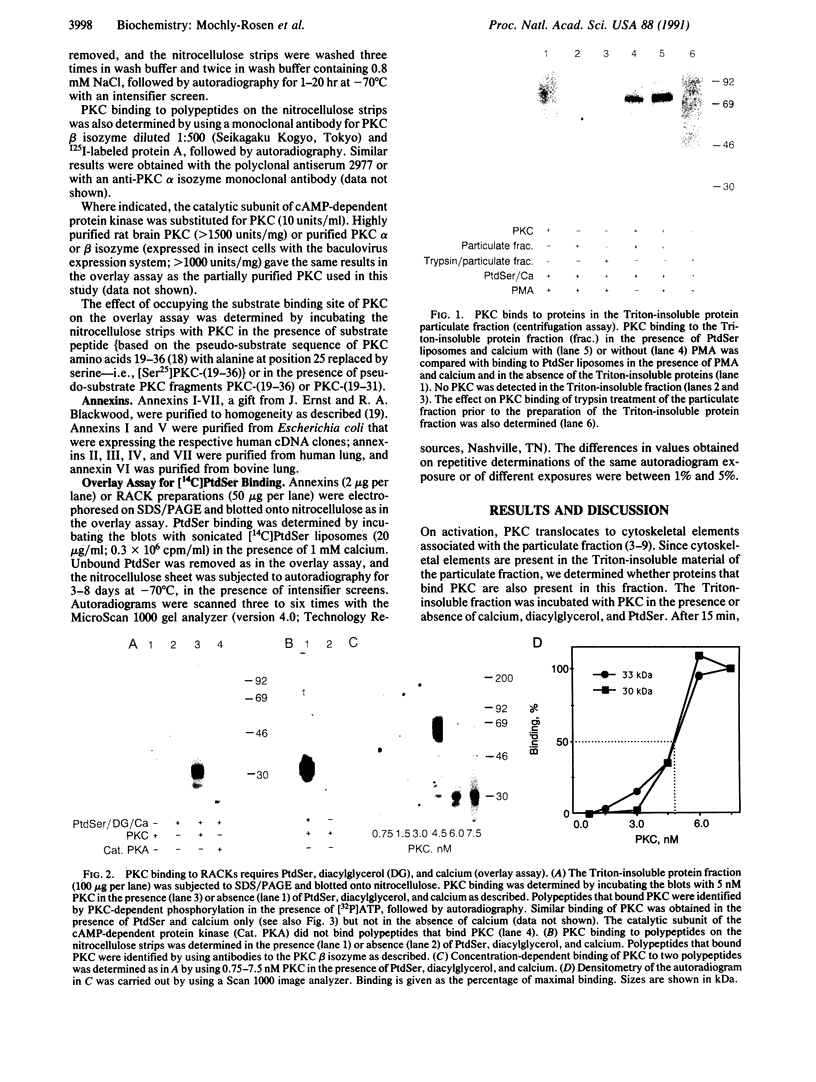
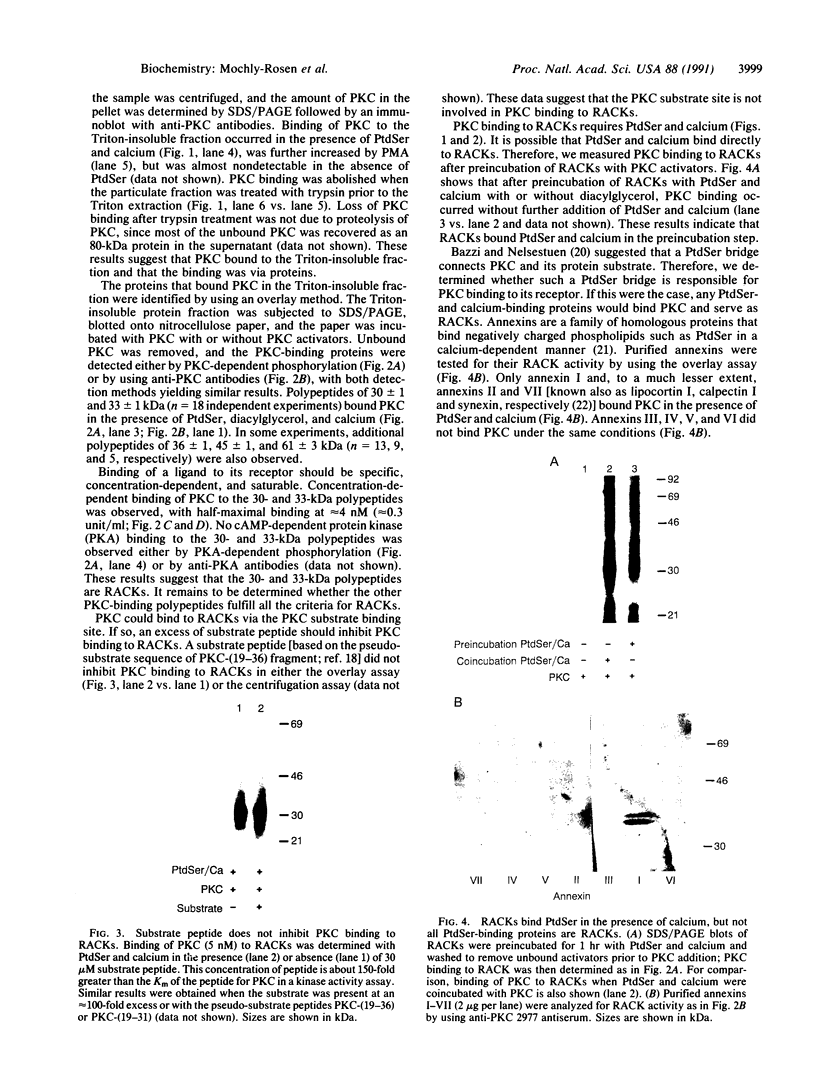
Protein kinase C (PKC) translocates from the cytosol to the particulate fraction on activation. This activation-induced translocation of PKC is thought to reflect PKC binding to the membrane lipids. However, immunological and biochemical data suggest that PKC may bind to proteins in the cytoskeletal elements in the particulate fraction and in the nuclei. Here we describe evidence for the presence of intracellular receptor proteins that bind activated PKC. Several proteins from the detergent-insoluble material of the particulate fraction bound PKC in the presence of phosphatidylserine and calcium; binding was further increased with the addition of diacylglycerol. Binding of PKC to two of these proteins was concentration-dependent, saturable, and specific, suggesting that these binding proteins are receptors for activated C-kinase, termed here "RACKs." PKC binds to RACKs via a site on PKC distinct from the substrate binding site. We suggest that binding to RACKs may play a role in activation-induced translocation of PKC.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bazzi M. D., Nelsestuen G. L. Role of substrate in determining the phospholipid specificity of protein kinase C activation. Biochemistry. 1987 Aug 11;26(16):5002–5008. doi: 10.1021/bi00390a018. [DOI] [PubMed] [Google Scholar]
- Bell R. M. Protein kinase C activation by diacylglycerol second messengers. Cell. 1986 Jun 6;45(5):631–632. doi: 10.1016/0092-8674(86)90774-9. [DOI] [PubMed] [Google Scholar]
- Blackwood R. A., Ernst J. D. Characterization of Ca2(+)-dependent phospholipid binding, vesicle aggregation and membrane fusion by annexins. Biochem J. 1990 Feb 15;266(1):195–200. doi: 10.1042/bj2660195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capitani S., Girard P. R., Mazzei G. J., Kuo J. F., Berezney R., Manzoli F. A. Immunochemical characterization of protein kinase C in rat liver nuclei and subnuclear fractions. Biochem Biophys Res Commun. 1987 Jan 30;142(2):367–375. doi: 10.1016/0006-291x(87)90283-x. [DOI] [PubMed] [Google Scholar]
- Chen Z. Z., McGuire J. C., Leach K. L., Cambier J. C. Transmembrane signaling through B cell MHC class II molecules: anti-Ia antibodies induce protein kinase C translocation to the nuclear fraction. J Immunol. 1987 Apr 1;138(7):2345–2352. [PubMed] [Google Scholar]
- Crompton M. R., Moss S. E., Crumpton M. J. Diversity in the lipocortin/calpactin family. Cell. 1988 Oct 7;55(1):1–3. doi: 10.1016/0092-8674(88)90002-5. [DOI] [PubMed] [Google Scholar]
- Crumpton M. J., Dedman J. R. Protein terminology tangle. Nature. 1990 May 17;345(6272):212–212. doi: 10.1038/345212a0. [DOI] [PubMed] [Google Scholar]
- Fields A. P., Pincus S. M., Kraft A. S., May W. S. Interleukin-3 and bryostatin 1 mediate rapid nuclear envelope protein phosphorylation in growth factor-dependent FDC-P1 hematopoietic cells. A possible role for nuclear protein kinase C. J Biol Chem. 1989 Dec 25;264(36):21896–21901. [PubMed] [Google Scholar]
- Gopalakrishna R., Barsky S. H., Thomas T. P., Anderson W. B. Factors influencing chelator-stable, detergent-extractable, phorbol diester-induced membrane association of protein kinase C. Differences between Ca2+-induced and phorbol ester-stabilized membrane bindings of protein kinase C. J Biol Chem. 1986 Dec 15;261(35):16438–16445. [PubMed] [Google Scholar]
- Halsey D. L., Girard P. R., Kuo J. F., Blackshear P. J. Protein kinase C in fibroblasts. Characteristics of its intracellular location during growth and after exposure to phorbol esters and other mitogens. J Biol Chem. 1987 Feb 15;262(5):2234–2243. [PubMed] [Google Scholar]
- House C., Kemp B. E. Protein kinase C contains a pseudosubstrate prototope in its regulatory domain. Science. 1987 Dec 18;238(4834):1726–1728. doi: 10.1126/science.3686012. [DOI] [PubMed] [Google Scholar]
- Ito M., Tanabe F., Sato A., Ishida E., Takami Y., Shigeta S. Possible involvement of microfilaments in protein kinase C translocation. Biochem Biophys Res Commun. 1989 May 15;160(3):1344–1349. doi: 10.1016/s0006-291x(89)80151-2. [DOI] [PubMed] [Google Scholar]
- Jaken S., Leach K., Klauck T. Association of type 3 protein kinase C with focal contacts in rat embryo fibroblasts. J Cell Biol. 1989 Aug;109(2):697–704. doi: 10.1083/jcb.109.2.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiley S. C., Jaken S. Activation of alpha-protein kinase C leads to association with detergent-insoluble components of GH4C1 cells. Mol Endocrinol. 1990 Jan;4(1):59–68. doi: 10.1210/mend-4-1-59. [DOI] [PubMed] [Google Scholar]
- Masmoudi A., Labourdette G., Mersel M., Huang F. L., Huang K. P., Vincendon G., Malviya A. N. Protein kinase C located in rat liver nuclei. Partial purification and biochemical and immunochemical characterization. J Biol Chem. 1989 Jan 15;264(2):1172–1179. [PubMed] [Google Scholar]
- Mochly-Rosen D., Henrich C. J., Cheever L., Khaner H., Simpson P. C. A protein kinase C isozyme is translocated to cytoskeletal elements on activation. Cell Regul. 1990 Aug;1(9):693–706. doi: 10.1091/mbc.1.9.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochly-Rosen D., Koshland D. E., Jr A general procedure for screening inhibitory antibodies: application for identifying anti-protein kinase C antibodies. Anal Biochem. 1988 Apr;170(1):31–37. doi: 10.1016/0003-2697(88)90085-1. [DOI] [PubMed] [Google Scholar]
- Mochly-Rosen D., Koshland D. E., Jr Domain structure and phosphorylation of protein kinase C. J Biol Chem. 1987 Feb 15;262(5):2291–2297. [PubMed] [Google Scholar]
- Morrison D. K., Kaplan D. R., Escobedo J. A., Rapp U. R., Roberts T. M., Williams L. T. Direct activation of the serine/threonine kinase activity of Raf-1 through tyrosine phosphorylation by the PDGF beta-receptor. Cell. 1989 Aug 25;58(4):649–657. doi: 10.1016/0092-8674(89)90100-1. [DOI] [PubMed] [Google Scholar]
- Papadopoulos V., Hall P. F. Isolation and characterization of protein kinase C from Y-1 adrenal cell cytoskeleton. J Cell Biol. 1989 Feb;108(2):553–567. doi: 10.1083/jcb.108.2.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resh M. D., Ling H. P. Identification of a 32K plasma membrane protein that binds to the myristylated amino-terminal sequence of p60v-src. Nature. 1990 Jul 5;346(6279):84–86. doi: 10.1038/346084a0. [DOI] [PubMed] [Google Scholar]
- Thomas T. P., Talwar H. S., Anderson W. B. Phorbol ester-mediated association of protein kinase C to the nuclear fraction in NIH 3T3 cells. Cancer Res. 1988 Apr 1;48(7):1910–1919. [PubMed] [Google Scholar]
- Wolf M., Baggiolini M. Identification of phosphatidylserine-binding proteins in human white blood cells. Biochem J. 1990 Aug 1;269(3):723–728. doi: 10.1042/bj2690723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf M., Sahyoun N. Protein kinase C and phosphatidylserine bind to Mr 110,000/115,000 polypeptides enriched in cytoskeletal and postsynaptic density preparations. J Biol Chem. 1986 Oct 5;261(28):13327–13332. [PubMed] [Google Scholar]
- Zalewski P. D., Forbes I. J., Valente L., Apostolou S., Hurst N. P. Translocation of protein kinase C to a Triton-insoluble sub-cellular compartment induced by the lipophilic gold compound auranofin. Biochem Pharmacol. 1988 Apr 1;37(7):1415–1417. doi: 10.1016/0006-2952(88)90802-7. [DOI] [PubMed] [Google Scholar]