Abstract
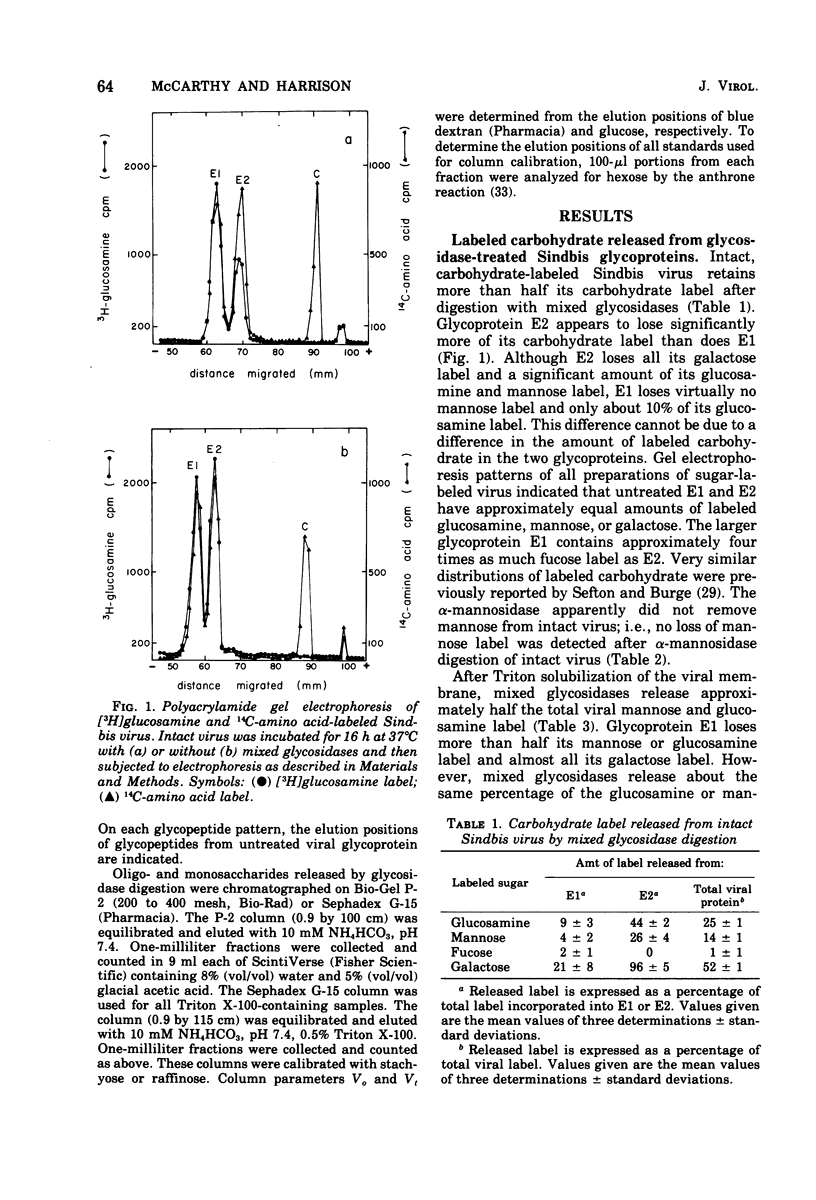
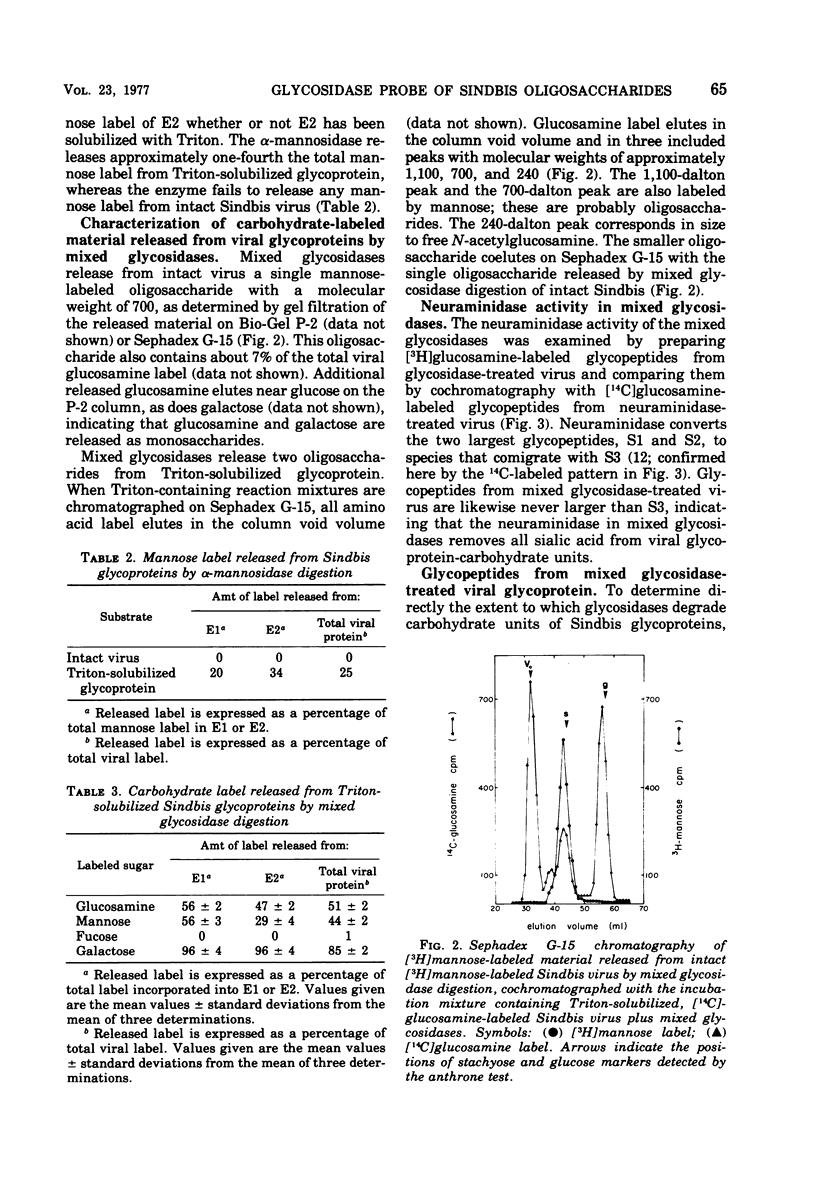
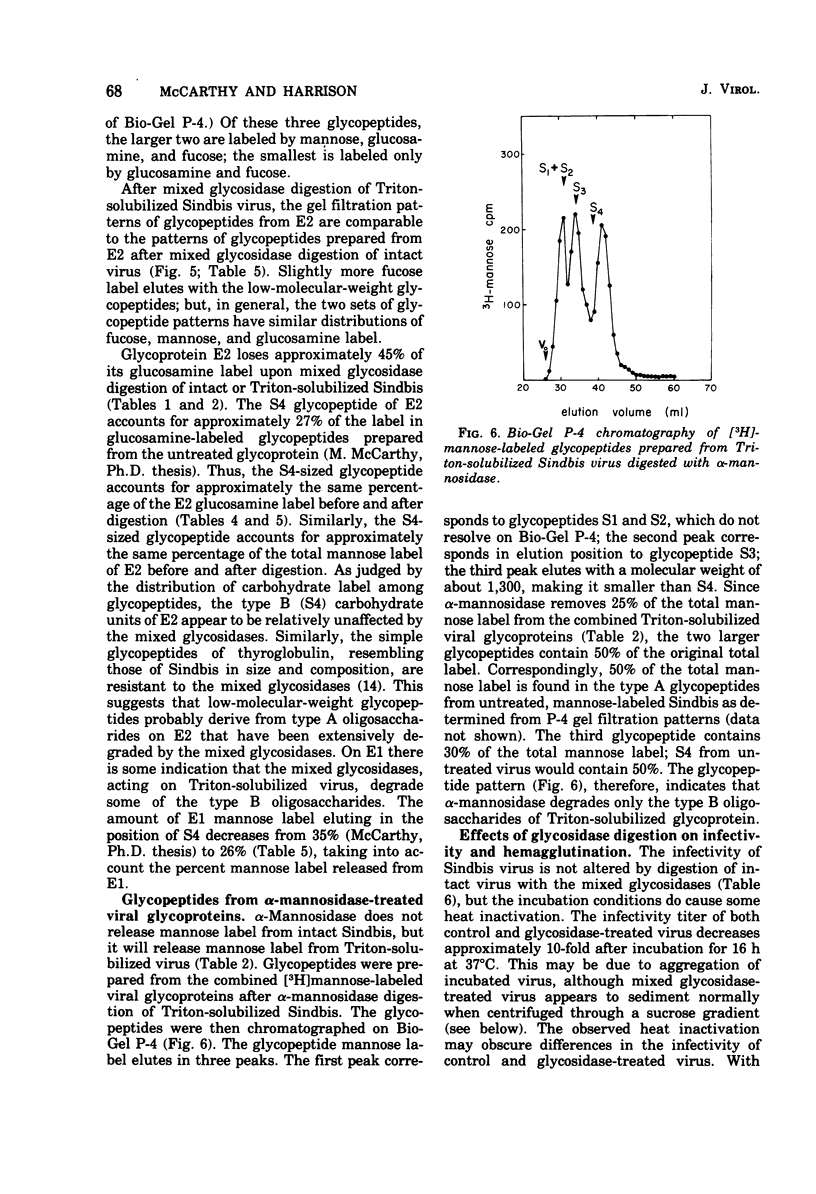
Intact Sindbis virus and Triton-solubilized viral glycoprotein were treated with alpha-mannosidase and with a preparation of mixed glycosidases from Diplococcus pneumoniae to probe the accesibility of carbohydrate units on the viral surface. The products of glycosidase attack on Triton-solubilized virus showed that mose carbohydrate units of the glycoproteins are good substrates for these enzymes. The relative resistance of most of the viral oligosaccharides in intact virus particles showed that much of the carbohydrate is not accessible to glycosidases, probably because it is not exposed at the viral surface. The only completely accessible carbohydrate units on Sindbis glycoproteins were the type A oligosaccharides of E2. This differential accessibility of Sindbis oligosaccharides is discussed in relation to the organization of the viral surface.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acheson N. H., Tamm I. Replication of Semliki Forest virus: an electron microscopic study. Virology. 1967 May;32(1):128–143. doi: 10.1016/0042-6822(67)90261-9. [DOI] [PubMed] [Google Scholar]
- Atkinson P. H. Synthesis and assembly of HeLa cell plasma membrane glycoproteins and proteins. J Biol Chem. 1975 Mar 25;250(6):2123–2134. [PubMed] [Google Scholar]
- Brown D. T., Waite M. R., Pfefferkorn E. R. Morphology and morphogenesis of Sindbis virus as seen with freeze-etching techniques. J Virol. 1972 Sep;10(3):524–536. doi: 10.1128/jvi.10.3.524-536.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLARKE D. H., CASALS J. Techniques for hemagglutination and hemagglutination-inhibition with arthropod-borne viruses. Am J Trop Med Hyg. 1958 Sep;7(5):561–573. doi: 10.4269/ajtmh.1958.7.561. [DOI] [PubMed] [Google Scholar]
- Dalrymple J. M., Schlesinger S., Russell P. K. Antigenic characterization of two sindbis envelope glycoproteins separated by isoelectric focusing. Virology. 1976 Jan;69(1):93–103. doi: 10.1016/0042-6822(76)90197-5. [DOI] [PubMed] [Google Scholar]
- Gahmberg C. G., Utermann G., Simons K. The membrane proteins of Semliki Forest virus have a hydrophobic part attached to the viral membrane. FEBS Lett. 1972 Dec 1;28(2):179–182. doi: 10.1016/0014-5793(72)80706-3. [DOI] [PubMed] [Google Scholar]
- Garoff H., Simons K. Location of the spike glycoproteins in the Semliki Forest virus membrane. Proc Natl Acad Sci U S A. 1974 Oct;71(10):3988–3992. doi: 10.1073/pnas.71.10.3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison S. C., Jack A., Goodenough D., Sefton B. M. Structural studies of spherical viruses. J Supramol Struct. 1974;2(2-4):486–495. doi: 10.1002/jss.400020233. [DOI] [PubMed] [Google Scholar]
- Helenius A., Söderlund H. Stepwise dissociation of the Semliki Forest Virus membrane with trition X-100. Biochim Biophys Acta. 1973 May 11;307(2):287–300. doi: 10.1016/0005-2736(73)90096-5. [DOI] [PubMed] [Google Scholar]
- Huber R., Deisenhofer J., Colman P. M., Matsushima M., Palm W. Crystallographic structure studies of an IgG molecule and an Fc fragment. Nature. 1976 Dec 2;264(5585):415–420. doi: 10.1038/264415a0. [DOI] [PubMed] [Google Scholar]
- Keegstra K., Sefton B., Burke D. Sindbis virus glycoproteins: effect of the host cell on the oligosaccharides. J Virol. 1975 Sep;16(3):613–620. doi: 10.1128/jvi.16.3.613-620.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy S. I. The effect of enzymes on structural and biological properties of Semliki forest virus. J Gen Virol. 1974 May;23(2):129–143. doi: 10.1099/0022-1317-23-2-129. [DOI] [PubMed] [Google Scholar]
- Koide N., Muramatsu T. Endo-beta-N-acetylglucosaminidase acting on carbohydrate moieties of glycoproteins. Purification and properties of the enzyme from Diplococcus pneumoniae. J Biol Chem. 1974 Aug 10;249(15):4897–4904. [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. Comparative aspects of glycoprotein structure. Annu Rev Biochem. 1976;45:217–237. doi: 10.1146/annurev.bi.45.070176.001245. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lenard J., Compans R. W. The membrane structure of lipid-containing viruses. Biochim Biophys Acta. 1974 Apr 8;344(1):51–94. doi: 10.1016/0304-4157(74)90008-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer S. A., Summers D. F. Vesicular stomatitis virus envelope glycoprotein alterations induced by host cell transformation. Cell. 1974 May;2(1):63–70. doi: 10.1016/0092-8674(74)90009-9. [DOI] [PubMed] [Google Scholar]
- Moyer S. A., Tsang J. M., Atkinson P. H., Summers D. F. Oligosaccharide moieties of the glycoprotein of vesicular stomatitis virus. J Virol. 1976 Apr;18(1):167–175. doi: 10.1128/jvi.18.1.167-175.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muramatsu T., Atkinson P. H., Nathenson S. G., Ceccarini C. Cell-surface glycopeptides: growth-dependent changes in the carbohydrate-peptide linkage region. J Mol Biol. 1973 Nov 15;80(4):781–799. doi: 10.1016/0022-2836(73)90210-6. [DOI] [PubMed] [Google Scholar]
- Muramatsu T. Demonstration of an endo-glycosidase acting on a glycoprotein. J Biol Chem. 1971 Sep 10;246(17):5535–5537. [PubMed] [Google Scholar]
- Muramatsu T., Egami F. Alpha-mannosidase and beta-mannosidase from the liver of Tubo cortunus: purfication, properties and application of carbohydrate research. J Biochem. 1967 Dec;62(6):700–709. doi: 10.1093/oxfordjournals.jbchem.a128726. [DOI] [PubMed] [Google Scholar]
- NEUBERGER A., PAPKOFF H. Carbohydrates in protein. 7. The nature of the carbohydrate in ovomucoid. Biochem J. 1963 Jun;87:581–585. doi: 10.1042/bj0870581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PFEFFERKORN E. R., CLIFFORD R. L. PRECIPITATION AND RECOVERY OF SINDBIS VIRUS FROM SOLUTIONS OF LOW IONIC STRENGTH. Virology. 1963 Oct;21:273–274. doi: 10.1016/0042-6822(63)90270-8. [DOI] [PubMed] [Google Scholar]
- PFEFFERKORN E. R., HUNTER H. S. PURIFICATION AND PARTIAL CHEMICAL ANALYSIS OF SINDBIS VIRUS. Virology. 1963 Jul;20:433–445. doi: 10.1016/0042-6822(63)90092-8. [DOI] [PubMed] [Google Scholar]
- Scheid A., Choppin P. W. Isolation and purification of the envelope proteins of Newcastle disease virus. J Virol. 1973 Feb;11(2):263–271. doi: 10.1128/jvi.11.2.263-271.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger S., Gottlieb C., Feil P., Gelb N., Kornfeld S. Growth of enveloped RNA viruses in a line of chinese hamster ovary cells with deficient N-acetylglucosaminyltransferase activity. J Virol. 1975 Jan;17(1):239–246. doi: 10.1128/jvi.17.1.239-246.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sefton B. M., Burge B. W. Biosynthesis of the Sindbis virus carbohydrates. J Virol. 1973 Dec;12(6):1366–1374. doi: 10.1128/jvi.12.6.1366-1374.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sefton B. M., Keegstra K. Glycoproteins of Sindbis virus: priliminary characterization of the oligosaccharides. J Virol. 1974 Sep;14(3):522–530. doi: 10.1128/jvi.14.3.522-530.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sefton B. M. Virus-dependent glycosylation. J Virol. 1975 Jan;17(1):85–93. doi: 10.1128/jvi.17.1.85-93.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sefton B. M., Wickus G. G., Burge B. W. Enzymatic iodination of Sindbis virus proteins. J Virol. 1973 May;11(5):730–735. doi: 10.1128/jvi.11.5.730-735.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons K., Helenius A., Garoff H. Solubilization of the membrane proteins from Semliki Forest virus with Triton X100. J Mol Biol. 1973 Oct 15;80(1):119–133. doi: 10.1016/0022-2836(73)90236-2. [DOI] [PubMed] [Google Scholar]
- Strauss J. H., Jr, Burge B. W., Darnell J. E. Sindbis virus infection of chick and hamster cells: synthesis of virus-specific proteins. Virology. 1969 Mar;37(3):367–376. doi: 10.1016/0042-6822(69)90220-7. [DOI] [PubMed] [Google Scholar]
- Tai T., Yamashita K., Ogata-Arakawa M., Koide N., Muramatsu T., Iwashita S., Inoue Y., Kobata A. Structural studies of two ovalbumin glycopeptides in relation to the endo-beta-N-acetylglucosaminidase specificity. J Biol Chem. 1975 Nov 10;250(21):8569–8575. [PubMed] [Google Scholar]
- Utermann G., Simons K. Studies on the amphipathic nature of the membrane proteins in Semliki Forest virus. J Mol Biol. 1974 Jan 5;85(4):569–587. doi: 10.1016/0022-2836(74)90316-7. [DOI] [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]
- YAMASHINA I., MAKINO M. The properties of a carbohydrate-amino acid complex from ovalbumin. J Biochem. 1962 May;51:359–364. [PubMed] [Google Scholar]
- von Bonsdorff C. H., Harrison S. C. Sindbis virus glycoproteins form a regular icosahedral surface lattice. J Virol. 1975 Jul;16(1):141–145. doi: 10.1128/jvi.16.1.141-145.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]


