Unstructured Abstract
Chemical exposures during pregnancy can have a profound and life-long impact on human health. Due to the omnipresence of chemicals in our daily life, there is continuous contact with chemicals in food, water, air and consumer products. Consequently, human biomonitoring studies show that pregnant women around the globe are exposed to a variety of chemicals. In this review, we provide a summary of current data on maternal and fetal exposure as well as health consequences from these exposures. We review several chemical classes including polychlorinated biphenyls (PCBs), perfluoroalkyl substances (PFAS), polybrominated diphenyl ethers (PBDEs), phenols, phthalates, pesticides, and metals. Additionally, we discuss environmental disparities and vulnerable populations, and future research directions. We conclude by providing some recommendations for prevention of chemical exposure and its adverse reproductive health consequences.
Keywords: environmental chemical, environmental exposure, chemical, pregnancy, reproductive health
Introduction
Scientific evidence has shown the adverse impacts of exposure to toxic environmental chemicals to human reproduction (1). Chemical exposures, especially during critical and sensitive windows of development such as pregnancy, can lead to a myriad of health consequences that can manifest across individual’s lifespan and potentially be transmitted to future generations (1,2). Chemical exposures that occur during pregnancy can cross the placenta and can accumulate in the fetus (3). Accordingly, the next generations are born “pre-polluted” (4) due to these pre-conception and pre-birth exposures. Preventing harmful exposures to environmental chemicals is, therefore, a priority for reproductive health professionals around the world (5).
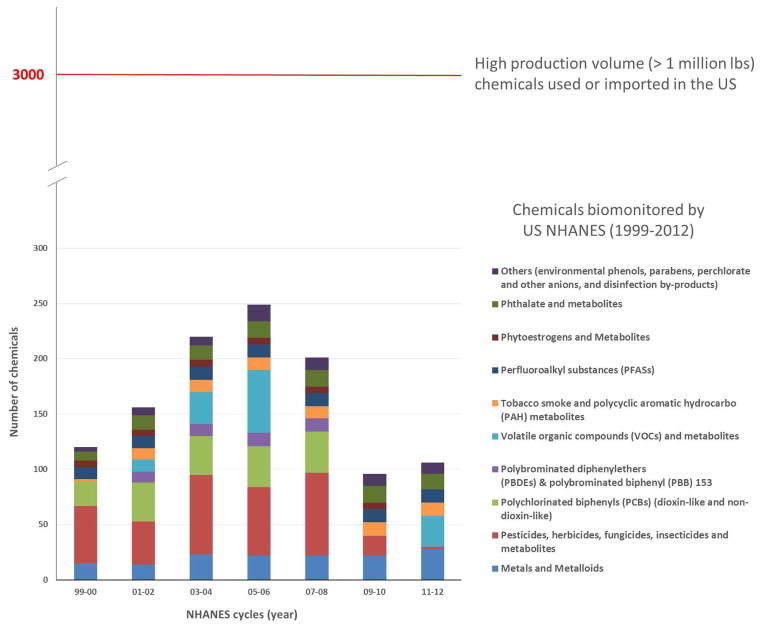
Industrial chemicals are present in our daily life and are ubiquitous in food, water, air, and consumer products. World chemical manufacturing has grown rapidly over the past few decades (6,7), with a projected 3.4% annual rate in production increase until 2030 (7). Among the estimated 70,000 to 100,000 commercially available chemicals, almost 5,000 of them are produced in volumes exceeding one million tons a year (8). In the US, the total reported production volume (domestically manufactured and imported) of industrial chemicals in 2012 was 9.5 trillion pounds (4.31 trillion kgs) – equivalent to more than 30,000 pounds (13,000 kg) for every American (5,9). As of 2016, more than 65,000 chemical substances are listed for use by the US Environmental Protection Agency (US EPA) (10). Around 3,000 of these chemicals have annual production and importation above 1 million pounds (11). Unlike pharmaceuticals that require extensive in-vitro and in-vivo toxicity testing as well as human experimental studies prior to entering marketplace and clinic, existing and new synthesized industrial chemicals currently can enter marketplace, homes, schools, workplaces, and communities with only limited or even no assessment on their reproductive or other related toxic effects (12,13). Further, there are not comprehensive data on where chemicals are used, so it is difficult to identify sources of exposures and the extent of exposures in the population. There are some data sources that allow characterization of certain exposures, such as air pollution monitoring, some monitoring of fish, and some portion of water and food supply (14). In the US, there is a national biomonitoring program run by the US Center for Disease Control and Prevention (CDC) using the National Health and Nutrition Examination Survey (NHANES), which has increased the number of chemicals biomonitored over the past 15 years (Figure 1) (15,16).
Figure 1.
Chemicals that are biomonitored by the US National Health and Nutrition Examination Survey (NHANES) from 1999 to 2012 based on the CDC 4th National Report on Human Exposure to Environmental Chemicals (Updated Tables, February 2015) and CDC NHANES website (http://www.cdc.gov/nchs/nhanes.htm) as of April 2016. Note: There will be more chemicals added for some biannual cycles in the future, especially later cycles, due to delay in data analyses and releasing. Not all the chemicals currently biomonitored by NHANES are high production volume chemicals.
While we have made great progress in understanding the importance of chemicals in reproductive health and understanding exposures to pregnancy, we still have yet to comprehensively understand the full scope of the exposures and outcomes that may affect reproductive health. Understanding exposures is critical to both identify potential health risks and identify opportunities for intervention and prevention of harmful chemical exposures. It is being increasingly recognized in numerous initiatives, including the Exposome (17), Precision Medicine Initiative (18), Genes, Environment and Health Initiative (19), and Children’s Health Exposure Analysis Resource (20). Thus, using illustrative examples, this article aims to review the current evidence on environmental chemical exposures including synthetic chemicals and metals in pregnant women. We first provide an updated view on the relationship between environmental chemical exposure during pregnancy and potential adverse health consequences. We then summarize the current knowledge on the maternal body burden and fetal exposure to different environmental chemicals. After discussion on environmental disparities and future research directions, we conclude the article with recommendations for prevention of chemical exposure and its adverse health consequences.
Health consequences of prenatal chemical exposure
Chemical exposures have been linked to a range of adverse reproductive and developmental outcomes, including fertility related affects, adverse pregnancy outcomes, and adverse health effects in childhood such as neurodevelopmental effects (Table 1). A key adverse health impact of concern to human reproduction and development is the endocrine disrupting property of many chemicals, particularly affecting hormones that are critical to proper development. These chemicals include polychlorinated biphenyls (PCBs), perfluoroalkyl substances (PFAS), polybrominated diphenyl ethers (PBDEs), bisphenol A (BPA), some current-use pesticides, metals and others (21). Exposure to environmental contaminants, especially during “critical” and “sensitive” periods of development such as during pregnancy, can heighten their potential impact. For example, prenatal exposure to lead and methyl mercury can lead to developmental neurotoxicity of the fetus (Table 1) (22). Sometimes, such negative health impact can be transgenerational and becomes apparent only decades after the initial exposure, as in the case of the drug diethylstilbestrol (DES), a potent synthetic estrogen (5). The daughters of pregnant women who took DES were found to have higher risks of infertility, poor pregnancy outcomes, and breast cancer (23) while the sons of these women have increased the risk of hypospadias (24,25). Such exposures during a window of vulnerability, even of small quantity, may trigger adverse health consequences that can manifest across the lifespan of individuals and generations (5). Despite these previous studies and discoveries, there is a paucity of studies on the effects of chemicals and reproductive outcomes, especially with regard to the number of chemicals and their potential harm.
Table 1.
Examples of exposure sources and pathways, and selected health impacts of prenatal exposure to environmental contaminants
| Chemical | Exposure sources and pathways | Selected health impact (reproduction, poor birth outcome, neurodevelopment, and cancer) |
|---|---|---|
| Polychlorinated biphenyls (PCBs) | Used as industrial insulators and lubricants; banned in the 1970s, but persistent in the aquatic and terrestrial food chains, which results in exposure by ingestion. | |
| Perfluoroalkyl substances (PFAS) | Widely used man-made organofluorine compounds with many diverse industrial and consumer product applications; examples are perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA), which are used in the manufacture of nonstick Teflon and other trademark cookware products and in food-contact packaging to provide grease, oil, and water resistance to plates, food containers, bags, and wraps that come into contact with food; persist in the environment; occupational exposure to workers and general population exposure by inhalation, ingestion, and dermal contact. | |
| Polybrominated diphenyl ethers (PBDEs) | Flame retardants that persist and bioaccumulate in the environment; found in furniture, textiles, carpeting, electronics and plastics that are mixed into, but not bound to, foam or plastic. | |
| Phenols | Examples are bisphenol A (BPA), triclosan, and parabens. | |
| BPA: Chemical intermediate for polycarbonate plastic and resins; found in consumer products and packaging; exposure through inhalation, ingestion, and dermal absorption. | ||
| Triclosan: Synthetic chlorinated aromatic compound with antibacterial properties; used in many consumer products such as antibacterial soaps, deodorants, toothpastes, cosmetics, fabrics, plastics, and other products; exposure through ingestion, dermal contact, and consumption of contaminated food and drinking water. |
||
| Parabens: Most commonly used preservatives in cosmetic products, including makeup, moisturizers, hair care products, and shaving products; also used in foods and drugs; exposure through dermal absorption and ingestion. |
|
|
| Phthalates | Synthetically derived; used in a variety of consumer goods such as medical devices, cleaning and building materials, personal care products, cosmetics, pharmaceuticals, food processing, and toys; exposure occurs through ingestion, inhalation, and dermal absorption. | |
| Heavy metals | Cadmium: used in batteries, pigments, metal coatings, and plastics; for non-smoking public, exposures mainly occur through diet (shellfish, organ meats, grains such as rice and wheat, leafy vegetables, and some root crops such as potato, carrot, and celeriac) (154,155); for smokers, exposure mainly occur through tobacco smoke. | |
| Lead: Occupational exposure occurs in battery manufacturing/recycling, smelting, car repair, welding, soldering, firearm cleaning/shooting, stained-glass ornament/jewelry making; nonoccupational exposure occurs in older homes where lead based paints were used, in or on some toys/children’s jewelry, water pipes, imported ceramics/pottery, herbal remedies, traditional cosmetics, hair dyes, contaminated soil, toys, costume jewelry. |
||
| Mercury: Coal-fired power plants are largest source in the United States; primary human exposure by consumption of contaminated seafood. |
||
| Perchlorate | Used to produce rocket fuel, fireworks, flares, and explosives and can also be present in bleach and in some fertilizers; primary pathway for exposure is through drinking water caused by contaminated runoff. |
|
| Pesticides | Applied in large quantities in agricultural, community, and household settings; in 2007, >1.1 billion pounds of active ingredients were used in the United States (163); can be ingested, inhaled, and absorbed by the skin; pathways of exposure include food, water, air, dust, and soil. |
|
| Solvents | Liquids or gases that can dissolve or extract other substances; used in manufacturing, service industries such as dry cleaning and printing, and consumer products including stain removers, paint thinners, nail polish removers, and hobby/craft products; examples are: benzene, gasoline, ethyl alcohol, methanol, phenol, styrene, toluene, trichloroethylene, and xylene; exposure occurs through inhalation, dermal absorption, and ingestion. |
Modified from American Journal of Obstetrics and Gynecology, volume 207, number 3, Sutton P, Woodruff TJ, Perron J, Stotland N, Conry JA, Miller MD, et al., Toxic environmental chemicals: the role of reproductive health professionals in preventing harmful exposures, Pages 164–73, Copyright 2012, with permission from Elsevier.
Based on animal studies
Maternal chemical body burden
Pregnant women can be exposed to environmental chemicals in food, water, air, consumer products as well as soil and dust; such exposure can happen via multiple pathways including ingestion, inhalation and dermal contact (Table 1). Biomonitoring of suitable human tissue, such as urine and blood, has been widely used for examining the chemical burden and provide a measure of the internal doses integrated across different exposure pathways (2,26). Starting from 1999/2000, the US Center for Disease Control and Prevention (CDC) has been biomonitoring several groups of environmental chemicals using the National Health and Nutrition Examination Survey (NHANES), including metals, pesticides, polychlorinated biphenyls, polybrominated diphenyl ethers, volatile organic compounds, tobacco smoke, polycyclic aromatic hydrocarbon metabolites, perfluoroalkyl substances, phthalate and metabolites, and many others (Figure 1) (15,16). The approximately 250 chemicals that we have biomonitoring data on over the years only make up a small fraction of the vast number of chemicals that we may be exposed to every day. In our own previous work, we have evaluated chemical exposures among pregnant women using the NHANES 2003–2004 data and found that virtually all pregnant women in the US are exposed to at least 43 different chemicals (27).
As illustrations, we have collected information on chemical concentrations measured in biomonitoring of pregnant women from more than 30 countries around the world on the most commonly measured chemicals or their metabolites (Tables 2–8). We focused on studies that have mothers’ biospecimens taken during pregnancy or at delivery and cord blood samples collected at delivery unless otherwise noted, and on studies published in the past 5 years for heavy metals as another study has summarized blood cadmium, lead and mercury levels from studies published from 2000 onward (28). We focus this review on common classes of chemicals and organize the following discussion according to chemical properties: persistent and bioaccumulative halogenated chemicals, less persistent and bioaccumulative chemicals, pesticides, and metals and organometallic chemicals. For each class of chemicals, we first briefly summarize the exposure sources and its health effect and then discuss its concentrations from biomonitoring studies.
Table 2.
Example studies of concentration of selected polychlorinated biphenyls (PCBs) in pregnant women, by location and study years
| Location | Study Years | N | Sample | Median concentration, ng/g lipid
|
Congeners included in Σ PCBs | 1st author, year | |
|---|---|---|---|---|---|---|---|
| PCB-153 | ΣPCBs | ||||||
| North America | |||||||
| US | 1959–1965 | 593 | MS | 1.7 mmol/L | 7.9 mmol/L | 28, 52, 74, 105, 118, 138, 153, 170, 180, 194, 203 | McGlynn, 2009 (187) |
|
| |||||||
| US (California) | 1960–1963 | 289 | MS | 0.79 μg/L | Cohn, 2011 (188) | ||
|
| |||||||
| US (California) | 1964–1967 | 399 | MS | 133 | 616 | 101, 105, 110, 118, 137, 138, 153, 156, 170, 180, 187 | James, 2002 (189) |
|
| |||||||
| US (Massachusetts) | 1993–1998 | 573 | CS | 0.19 ng/g | 118, 138, 153, 180 | Sagiv, 2010 (31) | |
|
| |||||||
| US (Illinois, Chicago) | 1993–1998 | 252 | MS | 86 | McGraw, 2009 (190) | ||
|
| |||||||
| Canada (Nunavik) | 1995–2001 | 159 | MP | 105.3 (GM) | 313.2 (GM) | 28, 52, 99, 101, 105, 118, 128, 138, 153, 156, 170, 180, 183, 187 | Muckle, 2001 (32) |
| 98 | CP | 86.9 (GM) | 279.9 (GM) | ||||
|
| |||||||
| Canada (Southwest Quebec) | N/A | 39 (1st Tri) | MP | 0.07 μg/L | 0.33 μg/L | 28, 52, 99, 101, 105, 118, 128, 138, 153, 156, 170, 180, 183, 187 | Takser, 2005 (191) |
| 145 (2nd Tri) | MP | 0.08 μg/L | 0.35 μg/L | ||||
| 101 (at delivery) | MP | 0.09 μg/L | 0.39 μg/L | ||||
| 92 (CP) | CP | 0.02 μg/L | 0.16 μg/L | ||||
|
| |||||||
| US (New York) | 1996–1997 | 79 | MS | 0.26 ng/g | Bloom, 2007 (192) | ||
|
| |||||||
| US (California, Salinas) | 1999–2001 | 24 | MP | 4.4 | Bradman, 2007 (193) | ||
|
| |||||||
| US (nationwide) | 2003–2004 | 75 | MS | 8.8 | 118, 138 and 158, 153, 180 | Woodruff, 2011 (27) | |
|
| |||||||
| US (Ohio) | 2003–2006 | 175 | MS | 11.0 | Braun, 2014 (194) | ||
|
| |||||||
| Canada | 2005–2007 | 173 | MS | 4.7 – 41 (GM) | Curren, 2014 (68) | ||
|
| |||||||
| Canada (Quebec) | 2007–2008 | 349 | MP | 8.0 | 18.9 | Serme-Gbedo, 2016 (195) | |
|
| |||||||
| US (California) | 2010–2011 | 77 | MS | 3.0 | Morello-Frosch, 2016 (in preparation) | ||
| 63 | CS | 4.4 | |||||
|
| |||||||
| Europe | |||||||
| The Netherlands & Germany (GRD cohort) | 1990–1995 | 523 | CP/CS | 150.0 ng/L | Casas, 2015 (196) | ||
|
| |||||||
| Faroe Islands (FAROES2 cohort) | 1994–1995 | 173 | MS | 394.4 ng/L | Casas, 2015 (196) | ||
|
| |||||||
| Sweden | 1994–1995 | 57 | MS | 430.0 | Fängström, 2005 (197) | ||
|
| |||||||
| Spain (INMA cohort) | 1997–2008 | 868 | MS | 93.8 ng/L | Casas, 2015 (196) | ||
| 1254 | CS | 135.2 ng/L | |||||
|
| |||||||
| The Netherlands | 1998–2000 | 97 | CS | 89.8 | 290.0 | 105, 118, 138, 146, 153, 156, 170, 180, 183, 187 | Berghuis, 2013 (198) |
|
| |||||||
| Germany (Duisburg) | 2000–2002 | 227 | MWB | 115.2 ng/L | Casas, 2015 (196) | ||
|
| |||||||
| The Netherlands | 2001–2002 | 62 | MS | 63.0 | Roze, 2009 (43) | ||
|
| |||||||
| Belgium (Flanders, FLEHSI cohort) | 2002–2004 | 1061 | CP | 60.0 ng/L | Casas, 2015 (196) | ||
|
| |||||||
| Eastern Slovakia | 2002–2004 | 966 | MS | 143.0 | 415.0 | 28, 52, 101, 105, 114, 118, 123, 138, 149, 153, 156, 157, 163, 167, 170, 171, 180, 189 | Jusko, 2012 (199) |
|
| |||||||
| Greenland | 2002–2004 | 546 | MS | 126.4 ng/L | Casas, 2015 (196) | ||
|
| |||||||
| Poland | 2002–2005 | 84 | CS | 43.4 ng/g fat (AM) | Hernik, 2013 (200) | ||
|
| |||||||
| France (Brittany) | 2002–2006 | 396 | CS | 110.0 ng/L | Casas, 2015 (196) | ||
|
| |||||||
| Poland (Warsaw) | 2003–2004 | 199 | MS | 15.3 ng/L | Casas, 2015 (196) | ||
|
| |||||||
| Ukraine (Kharkiv) | 2003–2004 | 575 | MS | 28.2 ng/L | Casas, 2015 (196) | ||
|
| |||||||
| Spain (Valencia) | 2003–2005 | 157 | MS | 137.0 | 28, 52, 101, 118, 138, 153, 180 | Lopez-Espinosa, 2009 (201) | |
|
| |||||||
| Spain (Valencia) | 2004–2006 | 494 | CS | 113.0 ng/L | 354.0 ng/L | 118, 138, 153, 180 | Lopez-Espinosa, 2011 (88) |
|
| |||||||
| Italy (Brescia) | 2006 | 70 | MS | 54.0 | 198.2 | 28, 31, 52, 74, 99, 101, 105, 114, 118, 123, 128, 138, 146, 153, 156, 157, 167, 170, 172, 177, 180, 183, 187, 189, 194, 196, 201, 203, 206, 209 | Bergonzi, 2009 (202) |
|
| |||||||
| Greece (RHEA cohort) | 2007–2008 | 1115 | MS | 47.4 ng/L | Casas, 2015 (196) | ||
|
| |||||||
| Northern Norway | 2007–2009 | 515 | MS | 24.8 (GM) | Veyhe, 2015 (203) | ||
|
| |||||||
| Greenland | 2010–2011, 2013 | 207 | MS | 57.0 | Long, 2015 (204) | ||
|
| |||||||
| The Netherlands | 2011–2013 | 51 | CP | 30.0 | Cock, 2014 (205) | ||
|
| |||||||
| Others | |||||||
| Mexico | 2005–2006 | 240 | MP | 3.6 (GM) | Adlard, 2014 (206) | ||
|
| |||||||
| Caribbean (10 countries) | 2008–2011 | 438 | MS | 7.0 (GM) | Forde, 2014 (207) | ||
|
| |||||||
| South Korea | 2011 | 104 | MS | 8.4 | 23.5 | 18, 28, 33, 44, 52, 70, 101, 105, 118, 128, 138, 153, 170, 180, 187, 194, 195, 199 and 206 | Kim, 2015 (89) |
| CS | 10.5 | 34.7 | |||||
|
| |||||||
| China (Wenling, e-waste area) | 2011 | 64 | MS | 8.3 | 26.2 | 28, 99, 118, 138, 153, 180 | Lv, 2015 (45) |
CP: cord plasma; CS: cord serum; MP: maternal plasma; MS: maternal serum; MWB: maternal whole blood.
Note: If several studies included participants from the same cohort, the one with the largest sample size was included. This table is for illustrative purpose and should not be taken as an exhaustive list of relevant biomonitored studies.
Table 8.
Example studies of concentration of blood cadmium (Cd), lead (Pb), and mercury (Hg) in pregnant women published in the past 5 years, by location and study years
| Location | Study Years | N | Sample | Median concentration
|
1st author, year | ||
|---|---|---|---|---|---|---|---|
| Cd, μg/L | Pb, μg/dL | Hg, μg/L | |||||
| North America | |||||||
| Canada (Nunavik) | 1995–2001 | 94 | CB | 3.5 | 17.0 | Boucher, 2014 (81) | |
|
| |||||||
| US (Boston) | 1998- | 50 | MRBCs | 0.86 (GM) | 3.93 (GM) | 2.35 (GM) | Chen, 2014 (83) |
| CRBCs | 0.06 (GM) | 2.41 (GM) | 3.58 (GM) | ||||
|
| |||||||
| US (nationwide) | 1999–2006 | 1183 | MWB | 0.71 (GM) | Razzaghi, 2014 (269) | ||
|
| |||||||
| US (Oklahoma) | 2002–2007 | 476 | MB | 0.61 (GM) | Karwowski, 2014 (136) | ||
| CB | 0.42 (GM) | ||||||
|
| |||||||
| US (nationwide) | 2003–2004 | 253 | MB | 0.2 | 0.6 | 0.7 (THg) | Woodruff, 2011 (27) |
|
| |||||||
| US (Baltimore) | 2004–2005 | 285 | CB | 0.66 | Wells, 2011 (270) | ||
|
| |||||||
| Canada | 2005–2007 | 16 (foreign-born) | MWB | 0.59 (GM) | 0.78 (GM) | 0.88 (THg, GM) | Adlard, 2014 (206) |
| 77 (Canadian-born) | 0.46 (GM) | 0.57 (GM) | 0.40 (THg, GM) | ||||
|
| |||||||
| Canada (Quebec) | 2007–2008 | 349 | MWB | 0.2 | 0.83 | 0.6 | Serme-Gbedo, 2016 (195) |
|
| |||||||
| US (New York City) | 2007–2009 | 78 | CB | 2.14 | Geer, 2012 (271) | ||
|
| |||||||
| Canada (10 cities) | 2008–2011 | 1260 | MWB | 0.32 | 0.88 | 0.86 | Ashley-Martin, 2015 (272) |
|
| |||||||
| US (North Carolina) | 2009–2011 | 211 | MWB | 0.18 (GM) | 0.89 (GM) | 0.45 (GM) | Sanders, 2012 (273) |
|
| |||||||
| US (Tennessee) | 2009–2011 | 98 (2nd Tri) | MB | 0.43 | Rabito, 2014 (274) | ||
| 88 (3rd Tri) | 0.43 | ||||||
| 69 (delivery) | 0.50 | ||||||
| 48 | CB | 0.37 | |||||
|
| |||||||
| US (Hawaii) | 2010–2011 | 107 | CB | 5.2 | Soon, 2014 (275) | ||
|
| |||||||
| US (California) | 2010–2011 | 77 | MWB | 0.22 | 0.60 | 0.46 | Morello-Frosch, 2016 (in preparation) |
| 59 | CWB | <LOD | 0.39 | 0.58 | |||
|
| |||||||
| Europe | |||||||
| Kosovo | 1985–1986 | 147 (Pristina) | MB | 5.6 | Kahn, 2014 (276) | ||
| 144 (Mitrovica) | 20.0 | ||||||
|
| |||||||
| Faroe Islands | 1986–1987 | 675 | CB | 23.3 (GM) | Grandjean, 2014 (80) | ||
|
| |||||||
| UK (Avon) | 1991–1992 | 4285 | MB | 0.29 | 3.41 | 1.86 | Taylor, 2014 (28) |
|
| |||||||
| Germany | 2000–2002 | 234 | MWB | 2.0 | Neugebauer, 2015 (277) | ||
|
| |||||||
| Poland (Krakow) | 2001–2004 | 379 | CB | 1.21 | 0.89 | Jedrychowski, 2015 (278) | |
|
| |||||||
| Spain | 2003–2004 | 140 | MB | 0.60 | 1.90 | 4.61 | García-Esquinas, 2013 (116) |
| 114 | CB | 0.27 | 1.38 | 7.66 | |||
|
| |||||||
| Spain | 2003–2008 | 1466; 1407 (Pb; Hg) | CWB | 1.1 (GM) | 7.7 (GM) | Llop, 2011 & 2015 (279,280) | |
|
| |||||||
| Germany | 2006 | 50 | MB | 0.34 | 1.15 | 0.44 | Kopp, 2012 (86) |
| CB | <LOD | 1.03 | 1.48 | ||||
|
| |||||||
| Northeastern Italy | 2007–2009 | 606 | MB | 2.35 ng/g | Valent, 2013 (281) | ||
| 457 | CB | 3.97 ng/g | |||||
|
| |||||||
| Northern Norway | 2007–2009 | 515 | MWB | 0.18 (GM) | 0.74 (GM) | 1.21 (GM) | Veyhe, 2015 (203) |
|
| |||||||
| Poland | 2007–2011 | 594 | CB | 1.1 (GM) | Polanska, 2014 (282) | ||
|
| |||||||
| Slovenia | 2007- | 446 | CB | 1.5 ng/g | Miklavcic, 2011 (283) | ||
|
| |||||||
| Belgium | 2008 | ||||||
|
| |||||||
| Poland (Upper Silesia) | 2010–2012 | 40 | MB | 8.0 | Kozikowska, 2013 (284) | ||
| CB | 0.008 μg/g | ||||||
|
| |||||||
| Greenland | 2010–2011, 2013 | 207 | MWB | 1.2 | 0.73 | 4.2 | Long, 2015 (204) |
|
| |||||||
| Turkey | 2011 | 93 | CB | 2.57 (AM) | Kayaalti, 2015 (285) | ||
|
| |||||||
| Eastern Slovakia | NA | 75 | MB | 0.50; 0.22 | Ursinyova, 2012 (286) | ||
| CB | 0.53; 0.32 (THg; MeHg) | ||||||
|
| |||||||
| Others | |||||||
| Mexico | 1994–2006 | 217 (1st Tri) | MB | 2.9 | Basu, 2014 (84) & Zhang, 2012 (287) | ||
| 264 (2nd Tri) | MB | 2.8 | |||||
| 248 (3rd Tri) | MB | 2.8 | |||||
| 457; 144 (Pb; Hg, at birth) | CB | 5.5 | 4.1 | ||||
|
| |||||||
| Japan | 2001- | 387 | CB | 1.0 | 10.1 ng/g (THg) | Tatsuta, 2014 (78) | |
|
| |||||||
| Taiwan | 2004–2005 | 230 | CWB | 1.14 | 12.27 | Lin, 2013 (288) | |
|
| |||||||
| Mexico | 2005–2006 | 233 | MWB | 0.36 (GM) | 2.5 (GM) | 0.86 (THg, GM) | Adlard, 2014 (206) |
|
| |||||||
| Saudi Arabia (Al-Kharj) | 2005–2006 | 247 | CB | 3.1 | Al-Saleh, 2014 (289) | ||
|
| |||||||
| China (Shanxi) | 2005–2009 | 215 | MB | 0.47 | 2.45 | 0.26 | Jin, 2014 (290) |
|
| |||||||
| South Korea | 2006–2010 | 1131 (< 20 gws) | MB | 1.29 | Hong, 2014 (291) | ||
| 914 (at delivery) | MB | 1.27 | |||||
| 897 (CB) | CB | 0.93 | |||||
|
| |||||||
| South Korea | 2006–2010 | 718–867 | MB | 1.4 (12–20 gws); 1.5 (28–42 gws) | 3.5 (12–20 gws); 3.1 (28–42 gws) | Kim, 2011 & 2013 (85,292) | |
| 797 | CB | 5.2 | |||||
|
| |||||||
| Iran (Tehran) | 2006–2011 | 174 (1st Tri) | MB | 4.15 (AM) | Vigeh, 2014 (293) | ||
| 148 (2nd Tri) | MB | 3.44 (AM) | |||||
| 145 (3rd Tri) | MB | 3.78 (AM) | |||||
| 150 | CB | 2.86 (AM) | |||||
|
| |||||||
| China (Chengdu) | 2007–2008 | 128 | MB | 5.95 (1st Tri) | Jiang, 2011 (294) | ||
| 5.51 (2nd Tri) | |||||||
| 5.57 (3rd Tri) | |||||||
|
| |||||||
| Mexico | 2007–2008 | 292 | MB | 2.79 (AM) | La-Llave-León, 2014 (295) | ||
|
| |||||||
| China | 2008 | 1323 | CB | 1.8 (GM) | Wu, 2013 (296) | ||
|
| |||||||
| Northeastern China | 2008 | 192 | MB | 1.24 (THg) | Li, 2014 (111) | ||
| 195 | CB | 2.15 (THg) | |||||
|
| |||||||
| South Africa | 2008 | 350 | MWB | 0.57 | Channa, 2013 (297) | ||
| CWB | 0.80 | ||||||
|
| |||||||
| Caribbean (10 countries) | 2008–2011 | 436 (Hg) | MB | 1.46 | 2.09 | Forde, 2014 (298) | |
| 102 (Pb) | |||||||
|
| |||||||
| Western Australia | 2008–2011 | 173 | MB | 0.38 | 0.37 | 0.46 | Hinwood, 2013 (299) |
|
| |||||||
| Eastern China | 2009–2010 | 213 | CB | 1.54 | Guo, 2013 (300) | ||
|
| |||||||
| Pakistan | 2009–2012 | 150; 120 (industrial area; domestic area) | MB | 19.0; 9.6 | Kazi, 2014 (301) | ||
| CB | 13.6; 8.6 | ||||||
|
| |||||||
| Northern China | 2010–2011 | 252; 258 (Pb; Hg) | MB | 3.20 | 0.90 | Ding, 2013 (302) & Xie, 2013 (303) | |
| CB | 2.52 | 1.50 | |||||
|
| |||||||
| Northern China | 2011 | 45 | MB | 2.17; 0.94 | Ou, 2015 (304) | ||
| 46 | CB | 2.81; 1.85 (THg; MeHg) | |||||
|
| |||||||
| Nigeria (Nnewi) | 2011 | 119; 95 (Pb; Hg) | MWB | 5.6 | 3.5 | Obi, 2014 & 2015 (87,305) | |
| CWB | 4.3 | 4.9 | |||||
|
| |||||||
| China (Guizhou) | 2011–2012 | 17 | MB | 3.0 (THg) | Rothenberg, 2013 (75) | ||
|
| |||||||
| China (Wuhan City) | 2012 | 234 (1st Tri) | MB | 1.93 | Shen, 2015 (306) | ||
| 249 (2nd Tri) | 1.36 | ||||||
| 248 (3rd Tri) | 1.29 | ||||||
|
| |||||||
| China | NA | 209 | MB | 0.48 | 4.05 | Sun, 2014 (115) | |
| CB | 0.15 | 3.23 | |||||
|
| |||||||
| India | NA | 60 | MB | 13.5 (AM) | Reddy, 2014 (307) | ||
| CB | 8.5 (AM) | ||||||
|
| |||||||
| Japan (Fukuoka) | NA | 81 | MRBCs | 1.97 ng/g (AM) | 26.4 ng/g (AM) | 9.4 ng/g (AM) | Sakamoto, 2010 (79) |
| CRBCs | 0.22 ng/g (AM) | 13.2 ng/g (AM) | 15.3 ng/g (AM) | ||||
|
| |||||||
| Kuwait | NA | 194 | MB | 5.8 (AM) | Rahman, 2012 (308) | ||
| CB | 10.9 (AM) | ||||||
|
| |||||||
| Nigeria | NA | 349 | MB | 36.4 (AM) | Ugwuja, 2013 (309) | ||
CB: cord blood; CRBCs: cord red blood cells; CWB: cord whole blood; MB: maternal blood; MRBCs: maternal red blood cells; MWB: maternal whole blood; THg: total mercury; MeHg: methyl mercury.
Note: If several studies included participants from the same cohort, the one with the largest sample size was included. This table is for illustrative purpose and should not be taken as an exhaustive list of relevant biomonitored studies.
Persistent and bioaccumulative halogenated chemicals
Many chemicals measured and found prevalently in pregnant women are persistent and bioaccumulative halogenated chemicals such as polychlorinated biphenyls (PCBs), perfluoroalkyl substances (PFAS), polybrominated diphenyl ethers (PBDEs), and organochlorine pesticides such as dichlorodiphenyltrichloroethane (DDT). Because of their chemical properties, they persist and can bioaccumulate up the food chain. Thus, they can remain in the environment and can be a source of exposure and pose risk to the health of humans and wildlife for many years. Even after their production and use are discontinued, it may take many years before their concentrations have sufficiently declined to minimal levels that are of less concern to human health (29). For example, despite being banned in the US after 1979, several PCBs (118, 138 and 158, 153, 180) were still detected in nearly 100% pregnant women from the NHANES 2003–2004 study (27). PCBs had once been used as industrial insulators and lubricants and have been shown to link to low birth weight (30) and poorer neurodevelopment outcomes (31) (Table 1). As they persistent in the aquatic and terrestrial food chains, high levels of PCBs were documented among Inuit pregnant women living in Nunavik (Arctic Quebec, Canada), due to high consumption of fish and marine mammals (32). Fortunately, the maternal concentration of PCB-153, the main contributor of the overall PCB level, has been declining over the years, though with some variability across countries (Table 2).
PFAS, especially perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA), were used in the manufacture of nonstick cookware products and in food-contact packaging and have been linked to reduced birth weight (33,34) and fetal growth (35) (Table 1). They are detected in the serum of 90–100% of pregnant women (3). One study examined the temporal changes in the levels of PFAS among California women over the past 50 years and found a significant drop of PFOS level from the 1960s to 2009, which is consistent with the phase-out of the perfluorooctyl manufacturing practice in the US in 2002 (36). The median concentration of PFOA was found to have increased approximately 10-fold from the 1960s to the 1980s but started to decline in 2009 (36). A similar decreasing trend for concentrations of PFOS and PFOA over time during the past two decades can be found in studies across the globe (Table 3). Other PFAS such as the perfluorononanoic acid (PFNA) and perfluorodecanoic acid (PFDA), though at a much lower concentration level compared to PFOS, have a high detection frequency (>90%) among pregnant women (37–39), and their serum concentrations have increased among California women from the 1960s to 2009 (36) and among Swedish women from 1996 to 2010 (40). The increasing blood levels of PFNA and PFDA most likely reflect the increased use of their precursors – fluorotelomer chemicals – in commercial products after banning PFOS (41). Perfluorobutane sulfonate (PFBS) – replacements for PFOS-based chemicals used as stain repellents – is also shown to increase in human blood with levels doubling every six years among Swedish women from 1996 to 2010 (40).
Table 3.
Example studies of concentration of selected perfluoroalkyl substances (PFAS) in pregnant women, by location and study years
| Location | Study Years | N | Sample | Median concentration, μg/L
|
1st author, year | |
|---|---|---|---|---|---|---|
| PFOA | PFOS | |||||
| North America | ||||||
| US (nationwide) | 2003–2004 | 76 | MS | 2.6 | 12.0 | Woodruff, 2011 (27) |
|
| ||||||
| US (Ohio) | 2003–2006 | 349 | MS | 5.5 (GM) | 13.3 (GM) | Donauer, 2015 (208) |
|
| ||||||
| USA (Maryland) | 2004–2005 | 293 | CS | 1.6 | 5.0 | Apelberg, 2007 (209) |
|
| ||||||
| Canada (Ontario) | 2004–2005 | 101 | MS (2nd Tri) | 2.1 | 16.6 | Monroy, 2008 (39) |
| MS (at delivery) | 1.8 | 14.5 | ||||
| 105 | CS | 1.6 | 6.1 | |||
|
| ||||||
| Canada (Alberta) | 2005–2006 | 252 | MS | 1.5 | 7.8 | Hamm, 2010 (210) |
|
| ||||||
| Canada (Ottawa) | 2005–2008 | 100 | CS | 1.6 | 5.0 | Arbuckle, 2013 (211) |
|
| ||||||
| Canada (10 cities) | 2008–2011 | 1743 | MP | 1.7 | 4.7 | Velez, 2015 (212) |
|
| ||||||
| US (California) | 2010–2011 | 77 | MS | 0.47 | 2.4 | Morello-Frosch, 2016 (in preparation) |
| 64 | CS | 0.39 | 2.2 | |||
|
| ||||||
| Europe | ||||||
| UK (Avon) | 1991–1992 | 447 | MS | 3.7 | 19.6 | Maisonet, 2012 (213) |
|
| ||||||
| Denmark (nationwide) | 1996–2002 | 1399 | MP (1st Tri) | 5.6 (AM) | 35.3 (AM) | Fei, 2007 (52) |
| 200 | MP (2nd Tri) | 4.5 (AM) | 29.9 (AM) | |||
| 50 | CP | 3.7 (AM) | 11.0 (AM) | |||
|
| ||||||
| Arctic Russia | 2001 | 7 | MB | wb: 0.89 pl: 1.61 |
wb: 5.79 pl: 11.0 |
Hanssen, 2013 (38) |
| CB | wb: 0.49 pl: 1.00 |
wb: 1.88 pl: 4.11 |
||||
| Uzbekistan | 2002 | 10 | MB | wb: 0.24; pl: 0.23 | ||
|
| ||||||
| Norway (nationwide) | 2003–2004 | 901 | MP | 2.2 | 13.0 | Whitworth, 2012 (33) |
|
| ||||||
| Greenland | 2010–2011, 2013 | 207 | MS | 1.2 | 10.2 | Long, 2015 (204) |
|
| ||||||
| Denmark (Odense) | 2011 | 200 | MS | 1.8 | 8.4 | Vorkamp, 2014 (44) |
|
| ||||||
| The Netherlands | 2011–2013 | 64 | CP | 1.6 | 0.9 | Cock, 2014 (205) |
|
| ||||||
| Others | ||||||
| Japan (Hokkaido) | 2002–2005 | 428 | MS | 1.3 | 5.2 | Washino, 2009 (34) |
|
| ||||||
| Taiwan | 2004–2005 | 429 | CP | 1.8 (GM) | 5.8 (GM) | Chen, 2012 (214) |
|
| ||||||
| China (Guiyu, e-waste recycling area) | 2007 | 108 | MS | 17.0 | Wu, 2012 (215) | |
| China (Chaonan) | 59 | 8.7 | ||||
|
| ||||||
| China (Jiangsu) | 2009 | 50 | MS | MS: 1.3 | MS: 2.9 | Liu, 2011 (37) |
| CS | CS: 1.1 | CS: 1.5 | ||||
|
| ||||||
| South Korea (Gyeongbok county) | 2011 | 59 | MS | MS: 2.6 | MS: 9.4 | Lee, 2013 (112) |
| CS | CS: 2.1 | CS: 3.2 | ||||
PFOA: perfluorooctanoate; PFOS: perfluorooctane sulfonate; CB: cord blood; CP: cord plasma; CS: cord serum; MB: maternal blood; MP: maternal plasma; MS: maternal serum; pl: plasma; wb: whole blood.
Note: If several studies included participants from the same cohort, the one with the largest sample size was included. This table is for illustrative purpose and should not be taken as an exhaustive list of relevant biomonitored studies.
PBDEs are a newer class of persistent chemicals that have been used as flame retardants in furniture, textiles, carpeting, electronics and plastics and linked to impaired neurodevelopment and poorer motor, cognitive, and behavioral performance at school age (42,43) (Table 1). Here we present data for the sum of different PBDE congeners and 2,2′,4,4′-tetrabromodiphenyl ether (BDE-47), one of the major congeners in the commercial mixture as it represents the most often found PBDE (Table 4). PBDEs (e.g. BDE-47) have been measured in populations around the globe (Table 4). BDE-47 is the most abundant PBDE detected in the serum of pregnant women from North America, Europe, and most places around the world, with exceptions in Denmark and China where the most abundant PBDE is BDE-209 (44) or BDE-153 (45) respectively. The concentration of BDE-47 and sum of PBDEs are also much higher among North American pregnant women compared to women from the rest of the world. The highest maternal serum concentration of BDE-47 was reported among pregnant women from Northern and Central California (46), which was in part due to regulatory standards in California requiring use of flame retardant chemicals in furniture (47). Levels of PBDEs have been shown to decline as a result of them being banned and phased out and the changed regulatory standard for flame retardants (48,49).
Table 4.
Example studies of concentration of selected polybrominated diphenyl ethers (PBDEs) in pregnant women, by location and study years
| Location | Study Years | N | Sample | Median concentration, ng/g lipid
|
Congeners included in Σ PBDEs | 1st author, year | |
|---|---|---|---|---|---|---|---|
| BDE-47 | Σ PBDEs | ||||||
| North America | |||||||
| US (California, Salinas) | 1999–2000 | 416 | MS | 15.2 | 24.6 | 47, 99, 100, 153 | Castorina, 2011 (216) |
| 26.9 | 17, 28, 47, 66, 85, 99, 100, 153, 154, 183 | ||||||
|
| |||||||
| US (Indianapolis) | 2001 | 12 | MS | 28.0 | 37.0 | 47, 99, 100, 153, 154, 183 | Mazdai, 2003 (217) |
| CS | 25.0 | 39.0 | |||||
|
| |||||||
| US | 2001 | 210 | CB | 11.2 | Herbstman, 2010 (42) | ||
|
| |||||||
| US (Ohio) | 2003–2006 | 349 | MS | 20.0 (GM) | 37.1 (GM) | 47, 99, 100, 153 | Donauer, 2015 (208) |
|
| |||||||
| US (nationwide) | 2003–2004 | 75 | MS | 23.7 | Woodruff, 2011 (27) | ||
|
| |||||||
| Canada (Ontario) | 2004–2005 | 97 | MS (24–28 gws; at delivery) | 24.2; 23.3 | 52.1; 50.1 | 17, 28, 47, 66, 99, 100, 153, 154, 183 | Foster, 2011 (218) |
| CS | 50.5 | 100.0 | |||||
|
| |||||||
| Canada | 2005–2007 | 173 | MS | 1.5 – 15.0 (GM) | Curren, 2014 (68) | ||
|
| |||||||
| Canada (Ottawa) | 2005–2008 | 98–114 | CB | <LOD | Arbuckle, 2013 (211) | ||
|
| |||||||
| Canada (Quebec) | 2007–2008 | 349 | MP | 21.2 | 33.0 | 47, 99, 100, 153 | Serme-Gbedo, 2016 (195) |
|
| |||||||
| US (North Carolina) | 2008–2010 | 140 | MS | 18.9 | 36.6 | 47, 99, 100, 153 | Stapleton, 2011 (219) |
|
| |||||||
| US (Northern and Central California) | 2008–2009 | 25 | MS | 43.1 | 82.9 | 28, 47, 99, 100, 153 | Zota, 2011 (46) |
|
| |||||||
| US (New York City) | 2009–2010 | 316 | MS | 7.9 | Horton, 2013 (220) | ||
|
| |||||||
| US (California) | 2010–2011 | 77 | MS | 8.3 | Morello-Frosch, 2016 (in preparation) | ||
| 63 | CS | 12.4 | |||||
|
| |||||||
| US (Ohio) | 2011 | 20 | MS | 11.0 | 28.6 | 28, 47, 99, 100, 153, 154, 209 | Chen, 2013 (221) |
|
| |||||||
| Europe | |||||||
| Sweden | 1994–1995 | 57 | MS | 1.3 | Fangstrom, 2005 (197) | ||
|
| |||||||
| Belgium (Stockholm) | 2000–2001 | 15 | MP | 0.8 | 2.1 | 17, 28, 47, 66, 99, 100, 153, 154, 183 | Guvenius, 2003 (222) |
| CP | 1.0 | 1.7 | |||||
|
| |||||||
| The Netherlands | 2001–2002 | 62 | MS | 0.9 | Roze, 2009 (43) | ||
|
| |||||||
| Poland | 2002–2005 | 84 | CS | 1.0 ng/g fat | Hernik, 2013 (200) | ||
|
| |||||||
| Spain (Valencia) | 2003–2005 | 174 | MS | 2.3 | 9.6 | 17, 28, 47, 66, 71, 85, 99, 100, 138, 153, 154, 183, 190, 209 | Vizcaino, 2011 (223) |
| CS | 2.3 | 9.6 | |||||
|
| |||||||
| Spain | 2003–2008 | 473 | MS | <LOD | 10.7 | 47, 99, 153, 154, 209 | Lopez-Espinosa, 2015 (224) |
| 486 | CS | 1.7 | 7.5 | ||||
|
| |||||||
| France | 2004–2006 | 91 | MS | 2.8 | Antignac, 2009 (225) | ||
| CS | <LOD | ||||||
|
| |||||||
| Sweden | 2005–2006 | 10 | MS | 3.0 pmol/g lipid | Jakobsson, 2012 (226) | ||
| CS | 3.4 pmol/g lipid | ||||||
|
| |||||||
| Spain | 2007 | 40 | CS | 30.0 pg/mL | Grimalt, 2010 (227) | ||
|
| |||||||
| Denmark (Copenhagen) | 2007 | 51 | MP | 0.4 | 1.8 | 28, 47, 99, 100, 153, 154, 209 | Frederiksen, 2010 (228) |
| 40 | CP | <0.07 | 1.0 | ||||
|
| |||||||
| Denmark (Odense) | 2011 | 100 | MS | 3.4 | 7.7 | 47, 99, 100, 153, 154, 183, 209 | Vorkamp, 2014 (44) |
|
| |||||||
| Others | |||||||
| China (Guiyu, e-waste recycling area) | 2007 | 75 | CS | 8.5 | 57.6 | 28, 47, 99, 100, 153, 154, 183, 209 | Xu, 2013 (229) |
| China (Chaonan) | 45 | CS | 2.0 | 8.2 | |||
|
| |||||||
| South Korea (Seoul) | 2007 | 108 | CS | 6.1 | 8.2 | 28, 47, 99, 100, 153, 154, 183 | Kim, 2009 (230) |
|
| |||||||
| Southern Taiwan | 2007–2008 | 54 | CS | 0.7 | 4.6 | 15, 28, 47, 49, 99, 100, 153, 154, 183, 196, 197 | Shy, 2011 (231) |
|
| |||||||
| South Korea (Seoul) | 2008 | 21 | MS | 3.2 | 7.8 | 28, 47, 99, 100, 153, 154, 183 | Kim, 2012 (232) |
| CS | 7.7 | 12.0 | |||||
|
| |||||||
| South Korea (Seoul) | 2008–2009 | 90 | CS | 7.4 | Kim, 2011 (233) | ||
|
| |||||||
| Caribbean (10 countries) | 2008–2011 | 438 | MS | 5.3 | Forde, 2014 (207) | ||
|
| |||||||
| Western Australia | 2008–2011 | 164 | MP | 4.0 | 10 | 47, 99, 100, 153, 154 | Stasinska, 2014 (234) |
|
| |||||||
| Northern China | 2010–2012 | 232 | CS | 3.7 | Ding, 2015 (235) | ||
|
| |||||||
| South Korea | 2011 | 148 | MS (at delivery) | 1.0 | 1.8 | 17, 28, 47, 49, 66, 71, 77, 85, 99, 100, 119, 126, 138, 153, 154, 156, 183, 184, 191 | Choi, 2014 (236) |
| 65 | MS (at 6 months) | 1.2 | 1.9 | ||||
| 118 | CS | 2.2 | 6.6 | ||||
|
| |||||||
| China (Wenling, e-waste area) | 2011 | 64 | MS | 1.0 | 9.8 | 28, 47, 99, 100, 153, 154, 183, 209 | Lv, 2015 (45) |
|
| |||||||
| Southern China (Guangdong) | 2012 | 30 | Placenta | 6.7 | 12.7 | 17, 28, 47, 85, 99, 100, 138, 153, 154, 183, 184, 191, 196, 197, 206, 207, 209 | Chen, 2014 (237) |
| CS | 3.8 | 9.7 | |||||
|
| |||||||
| China (Guiyu, e-waste recycling area) | 2012 | 69 | Placenta | 1.6 | 32.3 | 28, 47, 99, 100, 138, 153, 154, 183, 209 | Xu, 2015 (238) |
| China (Haojiang) | 86 | 0.4 | 5.1 | ||||
CP: cord plasma; CS: cord serum; MP: maternal plasma; MS: maternal serum.
Note: If several studies included participants from the same cohort, the one with the largest sample size was included. This table is for illustrative purpose and should not be taken as an exhaustive list of relevant biomonitored studies.
Less persistent and bioaccumulative chemicals
Many chemicals are nonpersistent and tend to be rapidly metabolized and eliminated, with half-lives in the human body within 24 hours. Two examples of these chemicals that are widely reported in biomonitoring studies are phenols, a type of carbolic acid and aromatic compounds including BPA, triclosan and parabens (3,50), and phthalates (51). In contrast to the persistent chemicals such as PFAS, where maternal concentrations across pregnancy are highly correlated (52), only low to moderate correlations were found between multiple measurements taken over pregnancy for less persistent chemicals such as BPA (53–55) and phthalate metabolites (55,56). Thus, multiple measurements of these chemicals across pregnancy, particularly for studies of small sample size, are recommended (55,57).
Phenols are (58) widely found in consumer products, packaging, and cosmetic products and also used in foods and drugs (Table 1). BPA is a female reproductive toxicant (59) and has been linked to adverse hormonal and behavioral outcome in childhood (Table 1). There is sufficient non-human evidence of an association between triclosan exposure and thyroxine concentrations decrements and triclosan is possibly toxic to reproductive and developmental health (60). Research also suggests that parabens have estrogenic activity (61). Detectable levels across multiple populations to these types of chemicals indicate recent and/or continuous exposure to the chemicals, and have been shown for BPA (Table 5), triclosan (Table 5), and three types of parabens (Table 6). Comparable BPA concentrations were found across different studies and geographic locations (Table 5). Fewer studies have documented the concentration of triclosan and parabens. There are variabilities in the maternal triclosan concentration across studies (Table 6). Methyl paraben had a much higher concentration than butyl paraben and propyl paraben, despite the small number of studies (Table 6).
Table 5.
Example studies of concentration of selected phenols in pregnant women, by location and study years
| Location | Study Years | N | Sample | Median concentration, μg/L
|
1st author, year | |
|---|---|---|---|---|---|---|
| bisphenol A | Triclosan | |||||
| North America | ||||||
| US (NYC) | 1998–2002 | 404 | MU | 1.3 | 11.0 | Wolff, 2008 (239) |
|
| ||||||
| US (African American and Dominican women) | 1998–2006 | 568 | MU | 1.8 | Donohue, 2013 (240) | |
|
| ||||||
| US (California, Salinas) | 1999–2000 | 364 | MU | 1.3 μg/g Cr | Chevrier, 2012 (150) | |
|
| ||||||
| US (nationwide) | 2003–2004 | 86 | MU | 2.7 | 8.2 | Woodruff, 2011 (27) |
|
| ||||||
| US (Ohio) | 2003–2006 | 330–363 | MU | 1.9 μg/g Cr (16 gws, GM) | Spanier, 2012 (53) | |
| 2.2 μg/g Cr (26 gws, GM) | ||||||
| 2.0 μg/g Cr (birth, GM) | ||||||
|
| ||||||
| US (Michigan) | 2006 | 40 | MS | 5.9 | Padmanabhan, 2008 (241) | |
|
| ||||||
| US (New York) | 2007–2009 | 181 | MU | 7.22 μg/g Cr | Pycke, 2014 (242) | |
|
| ||||||
| Canada | 2008–2011 | ~2000 | MU | 0.9, SG-adj (K-M median) | Arbuckle, 2014 (243) | |
|
| ||||||
| Canada | 2008–2011 | 1699 | MU | 8.3 | Velez, 2015 (212) | |
|
| ||||||
| Canada (Ontario) | 2009–2011 | 80 | MU | MU: 25.3 | Arbuckle, 2015 (244) | |
|
| ||||||
| Canada (Ottawa) | 2009–2010 | 66 | MU | 1.1 | Fisher, 2015 (55) | |
|
| ||||||
| US (nationwide) | 2009–2010 | 506 | MU | 1.3 | 15.6 | Mortensen, 2014 (245) |
|
| ||||||
| US (California) | 2010–2012 | 85 | CS (mid-gestation) | 0.16 (GM) | Gerona, 2013 (102) | |
|
| ||||||
| Puerto Rico | 2010–2012 | 105 | MU | 2.5 | 26.2 | Meeker, 2013 (246) |
|
| ||||||
| Europe | ||||||
| France | 2003–2006 | 520 | MU | 2.5 | 30.0 | Philippat, 2014 (247) |
|
| ||||||
| Spain | 2004–2006 | 402 | MU | 2.4 μg/g Cr (1st Tri) | Valvi, 2013 (54) | |
| 2.0 μg/g Cr (3rd Tri) | ||||||
|
| ||||||
| Spain | 2004–2008 | 120 | MU | 6.1 | Casas, 2011 (248) | |
|
| ||||||
| Greece (Crete) | 2007–2008 | 239 | MU | 1.1 μg/g Cr | Myridakis, 2015 (249) | |
|
| ||||||
| Denmark (Odense Municipality) | 2010–2012 | 200 | MU | 1.2 μg/g Cr | 0.6 μg/g Cr | Tefre de Renzy-Martin, 2014 (250) |
|
| ||||||
| Others | ||||||
| Mexico (Mexico City) | 2001–2003 | 60 | MU | 1.4 μg/g Cr | Cantonwine, 2010 (251) | |
|
| ||||||
| Taiwan | 2006–2007 | 97 | MP | MP: 2.5 (GM) | Chou, 2011 (106) | |
| CP | CP: 0.5 (GM) | |||||
|
| ||||||
| South Korea (Seoul) | 2007–2010 | 757 | MU | 1.6 μg/g Cr | Lee, 2014 (252) | |
|
| ||||||
| Western Australia | 2008–2011 | 24 | MU | 2.9 μg/g Cr | Callen, 2013 (253) | |
|
| ||||||
| China (Nanjing) | 2010–2012 | 567 | MU | 0.7 | Tang, 2013 (254) | |
CP: cord plasma; CS: cord serum; MP: maternal plasma; MU: maternal urine; SG-adj: specific gravity adjusted; K-M median: Kaplan-Meier median, censored method.
Note: If several studies included participants from the same cohort, the one with the largest sample size was included. This table is for illustrative purpose and should not be taken as an exhaustive list of relevant biomonitored studies.
Table 6.
Example studies of concentration of selected parabens in maternal urine of pregnant women, by location and study years
| Location | Study Years | N | Median concentration in maternal urine, μg/L
|
1st author, year | ||
|---|---|---|---|---|---|---|
| Butyl paraben | Methyl paraben | Propyl paraben | ||||
| North America | ||||||
| US (Boston) | 2005–2011 | 148 (1st Tri) | 1.1, SG-adj | 146.0, SG-adj | 39.9, SG-adj | Braun, 2014 (255) |
| 133 (2nd Tri) | 0.9, SG-adj | 141.0, SG-adj | 37.2, SG-adj | |||
| 97 (3rd Tri) | 0.8, SG-adj | 164.0, SG-adj | 31.8, SG-adj | |||
|
| ||||||
| US (nationwide) | 2009–2010 | 506 | 105.5 | 22.3 | Mortensen, 2014 (245) | |
|
| ||||||
| Puerto Rico | 2010–2012 | 105 | 0.4 | 153.0 | 36.7 | Meeker, 2013 (246) |
|
| ||||||
| Europe | ||||||
| France | 2003–2006 | 520 | 2.0 | 122.0 | 17.0 | Philippat, 2014 (247) |
|
| ||||||
| Spain | 2004–2008 | 120 | 2.4 | 191.0 | 29.8 | Casas, 2011 (248) |
|
| ||||||
| Greece (Crete) | 2007–2008 | 239 | 121.9 μg/g Cr | Myridakis, 2015 (249) | ||
|
| ||||||
| Denmark (Odense Municipality) | 2010–2012 | 200 | 20.5 μg/g Cr | Tefre de Renzy-Martin, 2014 (250) | ||
SG-adj: specific gravity adjusted.
Note: This table is for illustrative purpose and should not be taken as an exhaustive list of relevant biomonitored studies.
Phthalates are used in a variety of consumer goods such as medical devices, cleaning and building materials, personal care products, and cosmetics and have been linked to shortened gestational age (62) and impaired neurodevelopment in girls (63) (Table 1). Phthalate metabolites are detected in over 90% of the maternal urine samples during pregnancy among North American or European populations (3). Yet, there are variations in the MEP levels across different studies and populations (Table 7).
Table 7.
Example studies of concentration of selected phthalate metabolite in maternal urine of pregnant women, by location and study years
| Location | Study Years | N | Median MEP concentration in maternal urine, μg/L | 1st author, year |
|---|---|---|---|---|
| North America | ||||
| US (NYC) | 1998–2002 | 404 | 380 | Miodovnik, 2011 (256) |
|
| ||||
| US | 1999–2002 | 180 | 126.4 | Sathyanarayana, 2014 (257) |
|
| ||||
| US | 2000–2004 | 50 | 68.7 μg/g Cr | Buckley, 2012 (258) |
|
| ||||
| US (nationwide) | 2003–2004 | 91 | 265.7 | Woodruff, 2011 (27) |
|
| ||||
| US (Boston) | 2005–2011 | 155 | 39.5 (1st Tri), SG-adj | Braun, 2014 (255) |
| 138 | 48.2 (2nd Tri), SG-adj | |||
| 98 | 55.1 (3rd Tri), SG-adj | |||
|
| ||||
| US (Massachusetts) | 2006–2008 | 482 | 134.0, SG-adj | Ferguson, 2014 (259) |
|
| ||||
| Canada | 2008–2011 | ~2000 | 31.0, SG-adj | Arbuckle, 2014 (243) |
|
| ||||
| Canada (Ottawa) | 2009–2010 | 66 | 27.0 | Fisher, 2015 (55) |
|
| ||||
| Puerto Rico | 2010–2012 | 139 | 99.2 | Cantonwine, 2014 (260) |
|
| ||||
| Europe | ||||
| France | 2002–2006 | 287 | 110.2 | Philippat, 2011 (261) |
|
| ||||
| Spain | 2004–2006 | 390 | 389.1 μg/g Cr | Casas, 2016 (262) |
|
| ||||
| Central Poland | 2007 | 150 | 22.7 μg/g Cr | Polanska, 2014 (263) |
|
| ||||
| Greece (Crete) | 2007–2008 | 239 | 132.6 μg/g Cr | Myridakis, 2015 (249) |
|
| ||||
| Sweden | 2008–2009 | 196 | 60.6 | Bornehag, 2015 (249) |
|
| ||||
| Denmark (Odense Municipality) | 2010–2012 | 200 | 18.9 μg/g Cr | Tefre de Renzy-Martin, 2014 (250) |
|
| ||||
| Spain | 2011–2012 | 118 | 150.8 μg/g Cr | Cutanda, 2015 (264) |
|
| ||||
| Others | ||||
| Mexico (Mexico City) | 1997–2003 | 135 | 138.0, SG-adj | Téllez-Rojo, 2013 (265) |
|
| ||||
| Taiwan | 2000–2001 | 252 (children loss-to-follow-up) | 61.4 μg/g Cr | Ku, 2015 (266) |
| 136 (children followed-up) | 65.2 μg/g Cr | |||
|
| ||||
| Peru (Trujillo) | 2004 | 78 | 67.4 μg/g Cr | Irvin, 2010 (267) |
|
| ||||
| Japan (Tokyo) | 2005–2008 | 149 | 7.7 μg/g Cr | Suzuki, 2010 (268) |
SG-adj: specific gravity adjusted.
Note: If several studies included participants from the same cohort, the one with the largest sample size was included. This table is for illustrative purpose and should not be taken as an exhaustive list of relevant biomonitored studies.
Pesticides
Pesticides have been used to control a variety of pests, such as insects, weeds, rats and mice, bacteria and mold, and more, and are applied in agricultural, community, and household settings. Different pesticides have been associated with a range of reproductive and developmental outcomes including poorer birth outcomes, impaired cognitive and neurodevelopment, and childhood cancers (Table 1). Among pesticides, organochlorine pesticides (OCPs) such as hexachlorobenzene (HCB), dichlorodiphenyltrichloroethane (DDT), chlordane, and hexachlorocyclohexane (HCH) have been more widely studied, due to their earlier use and their persistent and bioaccumulative nature, endocrine disrupting properties, as well as their adverse health effects (3,64). Despite being banned in the US in the 1970s, some OCPs are still detected in US pregnant women (27). DDT is still used in some places around the world, most notably for controlling mosquito-borne diseases including malaria. As expected, in a study examining the regional difference of DDT exposure in South Africa, the authors found that levels of DDT isomers in plasma of delivering women were the highest in the endemic malaria sites where indoor residual spraying with DDT was taking place, among mining, urban, industrial, Atlantic, and rural sites (65). Women living in Latin America and other regions that use OCPs also have relatively higher maternal OCP levels (66–68). Current-use pesticides, characterized by shorter half-lives and chemical properties that do not promote bioaccumulation in sediments or organisms, are used to control a wide range of pests and in a variety of applications. For example, 2,4-dichlorophenol (2,4-DCP), 2,5-dichlorophenol (2,5-DCP), and 1-Naphthol have been detected in over 50% of the pregnant women from the Salinas Valley in California, US (agricultural regions) and with higher median concentrations than that from a US nationwide sample (NHANES study) (69).
Metals and organometallic chemicals
Cadmium, lead and mercury are three metals that have been widely used in a variety of applications over the past 50 years. They are also among the most well-studied industrial pollutants, and have been found collectively to have a number of adverse reproductive and developmental effects.
Cadmium are used in batteries, pigments, metal coatings, and plastics and have been found to alter epigenetic signatures in the DNA of both the placenta and the newborns (70), reduce IQ (71), and increase risk of emotional problems in boys (72) (Table 1). It can accumulate in liver and kidney and has a long half-life of 5–40 years in the body (73) and has been detected in both maternal and cord blood (Table 8).
Lead was widely used in gas, paint, water pipes and other applications. Although lead use in many applications has been banned for several decades, exposure can still occur in older homes where lead-based paints were used, and in or on toys, costume jewelry, and water pipes. Lead has been linked to alterations in genomic methylation (74) and impaired neurodevelopment (75,76) (Table 1). In human adults, around 94% of the total body burden of lead is found in the bones. During pregnancy, the mobilization of bone lead will increase, which contributes to 10–88% of the lead in blood in pregnant women(77). Environmental policies, including removing lead from gasoline, paint and water pipes, have led to significant reductions in blood lead levels in the US. The median level of blood lead level among pregnant women from the US tends to be lower than women from the rest of the world (Table 8) (28). Similarly, a gradual reduction in blood lead levels over time has been reported in pregnant women from the UK, which is in accordance with banning lead in petrol and paint, replacing lead water pipes, and reducing cigarette smoking in the UK (28).
Mercury, which comes primarily from coal-burning power plants, combustion of waste and industrial processes that use mercury, and from natural sources such as volcanoes (14), usually gets into human body through consumption of contaminated seafood or fresh water fish and can negatively affect cognitive performance and neurodevelopment (Table 1). Maternal blood mercury level can vary across geographic areas and populations (Table 8). Populations with high fish consumption tend to have higher maternal blood mercury level during pregnancy, for example, pregnant women in Japan (78,79) and Faroe Islands (80), and the Inuit population in Nunavik (Arctic Quebec, Canada) (81).
Challenges of biomonitoring studies
Biomonitoring studies, despite their usefulness, have their own challenges. Cautions should be taken when trying to compare maternal blood levels across biomonitoring studies due to differences in sample size, sample preparation and analytical techniques, study period, and gestational ages at which the blood is drawn (28). Additionally, chemical levels measured in biomonitoring studies do not provide information about the sources of exposure but are the sum of exposures through multiple pathways. Environmental monitoring (e.g. air, water, and soil) studies, on the other hand, can provide useful information on exposure sources identification, which is critical for prevention.
Placental transfer and fetal burden
Fetal chemical exposures result from maternal body burden of chemicals during pregnancy due to placental transfer. However, fetal exposure is difficult to measure directly and thus is usually achieved by measuring chemicals in maternal matrices as a surrogate, or by measuring chemicals in cord blood, placental tissue, amniotic fluid, or neonatal meconium (2,82). The pattern of placental transfer is determined by the specific structure, chemical composition, molecular weight, and relative persistence of xenobiotic chemicals (2). Past literature has documented that most classes of environmental chemicals can cross into the fetal environment. These chemicals include metals, PCBs, PFAS, PBDEs, pesticides, phenols and phthalates.
Some xenobiotic chemicals can bioaccumulate in the fetus and result in higher fetal exposure than maternal exposure while others are transferred in equal or less proportion (2,3). For example, studies have consistently found higher mercury concentration in cord blood than in maternal blood across different populations; the former being 1.5 to 3 times that of the latter (79,83–87). PCBs are widely detected in cord blood (88,89). Nearly all PCBs are found in higher levels in maternal than cord serum (90), with the exception of only a few congeners (91). One recent study suggested that lower chlorinated PCB congeners have a higher maternal-fetal transfer rate compared to higher chlorinated congeners (92) but this pattern is not supported by a systematic review and other studies (90,93,94). As PCB congener molecular weight (92,94) or lipophilicity (94) increase, placental transfer decreases. However, lipophilicity does not always predict bioaccumulation. PBDEs are lipophilic and also frequently detected in cord blood (3). Though the degree of bromination was suggested to influence placental transport, no apparent trend in ratios of cord:maternal concentration (>1 meaning concentration higher in cord blood than in maternal blood) was observed (90). The central estimates of this ratio also varied for the same PBDE congener across studies and depended on the measure (i.e., wet-weight basis or lipid-weight basis) (90). Organochlorine pesticides, similar to PBDEs, are also lipophilic chemicals and have been detected in placenta tissues (95) and cord serum (96–99). The majority of the studies included in a recent review reported central estimates of cord:maternal to be near or below 1 for most organochlorine pesticides, on the basis of both wet weight and lipid-adjusted concentrations (90).
Other chemicals tend to more evenly distribute between maternal-fetal compartments. For example, phenols, phthalates, and phthalate metabolites can cross the placenta but evidence suggests that they do not accumulate in the fetus (3). Studies in both human (100) and rats (101) indicated that both active BPA and its inactive form can cross the placenta into the fetus where most of the active form remains active and some of the inactive forms can be converted to the active form. Moreover, certain forms of the chemicals can distribute disproportionally in maternal versus fetal environment, due to immaturity of enzymes that conjugate or metabolize these chemicals. For example, levels of BPA and BPA in sulfate form were 2–3 times higher than levels of BPA in glucuronide form in cord sera collected during mid-gestation, possibly due to immaturity of the glucuronidation conjugation enzymes (102). Among the few human studies that have compared concentrations of BPA in fetal and maternal blood sera, the reported mean BPA level is lower in fetal serum in some studies (103–106) but higher in others (107). Phthalate levels in cord blood or newborns’ urine were found to be similar to or lower than that in maternal blood or urine respectively (108,109). Except for BPA, evidence is limited on placental transfer characteristics of triclosan, parabens, phthalates, and phthalate metabolites and the transfer patterns of these chemicals need further investigation.
For some chemicals, the levels found in the fetus are lower compared to maternal measurements. For example, lead is generally present in slightly lower levels in the cord blood relative to maternal blood (110,111). Lower levels of PFAS are also generally found in cord blood compared to maternal blood (37–39,112). Placental transfer of PFAS depends on the length of the carbon chain (2,3): shorter chain PFAS transferred more readily to cord blood than longer chain PFAS (113,114). Cadmium is another example, where the placenta appears to be a barrier, with much lower concentration in the cord blood being detected relative to maternal blood (79,86,115,116).
Studies have reported that PCBs, PFAS, PBDEs, OC pesticides, phenols such as BPA, TCS, and PBs, phthalates, and phthalate metabolites are detected in breast milk (3). This indicates that pre-birth exposures to the above chemicals and metals can continue to affect the offspring in postpartum, via breastfeeding.
Environmental disparities and vulnerable populations
The potential sources and amount of exposure to toxic chemicals are not the same for everyone. Women and men of reproductive age can encounter toxic chemicals at home, in the community, and in the workplace. Communities and individuals vary in their vulnerability and in their risk for exposure (5). The amount, duration and cumulative risk of exposure can depend on social, economic, geographic, occupational, medical and genetic factors (117).
Exposure to toxic environmental chemicals and related health outcomes are inequitably distributed among populations within countries and between countries. In the US, researchers and policy-makers have identified a higher frequency and magnitude of exposures to environmental stressors in communities of color and low-income communities (118,119). In addition, the consequences of exposure to toxic chemicals—including morbidity and mortality, loss of family income and productivity, and environmental degradation—are disproportionately borne by people with low incomes (120). For example, lower-income, ethnically diverse pregnant women in California were shown to have the highest level of PBDEs among pregnant women worldwide, mainly due to geography (e.g. California’s unique furniture flammability standards) and socioeconomic status (46). This combination and potential interaction of elevated environmental hazard exposures, on the one hand, and socioeconomic stressors, on the other, have been described as a form of “double jeopardy” (118,119).
Occupation can also add additional risk of exposure to toxic chemicals disparities which in turn impacts risk. For example, women employed as cosmetologists and manicurists are exposed to higher levels of volatile solvents (e.g., formaldehyde, methacrylates, ace- tone, and toluene), plasticizers (e.g., dibutyl phthalates), and other toxic substances. Pregnant women worked as cashiers had the highest urinary BPA concentrations (56). A recent study found that women in the nail and hair care industry were at higher risk of adverse birth outcomes (121). Farmworkers and their families are also at higher risk of exposure to pesticides with potential adverse reproductive and developmental outcomes (Table 1) (122).
Even after decades of basic science research and public health initiatives, disparities in pregnancy outcomes, such as preterm birth, remains relatively unchanged. Factors that underpin the disparity are elusive and likely derived in part from complex interactions between social, biologic and environmental factors including social inequality, genetics, neighborhood-level exposures, roles of infection and inflammation, and pre-conception health differentials. Better characterizing exposures has been recognized as a need in health disparities research and will provide important information in understanding the cumulative impacts of environmental and social stressors and promoting targeted policies to address these impacts (123).
Future directions
Non-targeted screening for novel chemicals
The chemicals discussed in the current review are merely the tip of the iceberg. There are tens of thousands of chemicals that we may be in contact with but know little or nothing about. Conventional studies used targeted approaches where the list of chemical analytes being measured is chosen a priori. There is research need to identify the “unknown” chemicals for future biomonitoring that are prevalent in and could potentially pose harm to the human body, using novel methods (124) such as non-targeted screening (publication in preparation).
Cumulative effects of multiple chemical exposures
Due to the wide application of various environmental chemicals, pregnant women are not exposed to a single chemical or a single class of chemicals, but a cocktail of chemicals from different classes. Research finds that simultaneous exposure to multiple chemicals can have an additive or synergistic effect on health, particularly for the same adverse health outcome (125–129). Analysis conducted on one chemical at a time is likely to underestimate its potential health effect in the presence of other chemicals. Thus, an increasing number of studies have measured cumulative exposures to multiple chemical classes. A recent review suggests that among these studies of the North American and European populations that had measured multiple chemical classes, few papers have attempted to capture a complete picture across classes and biological matrices (3). Certain classes are frequently measured simultaneously and in a single matrix (maternal urine or maternal serum): non-persistent phenols and phthalates are often measured in urine while persistent chemicals such as PFAS, PBDEs, PCBs and organochlorine pesticides are commonly measured in serum (3). Epidemiologic studies trying to examine the health effect of joint exposure to different chemicals are limited, possibly due to a high cost for multiple measurements and limited sample size. Cumulative risk assessment of multiple chemicals and other environmental stressors needs to account for the possible compounded effects on the outcomes of concern, as the appropriate statistical models become available. Meanwhile, more educational efforts are needed to reduce the cumulative chemical exposure load currently experienced by pregnant women (3).
Paternal exposure
Although the current review focuses on maternal exposure, paternal exposure to environmental chemicals also plays a critical role in the health of next generations. Especially for persistent chemicals like PCBs, PBDEs, and lead, fetal exposure can be a result of parental exposures prior to conception. Paternal lead exposure was found to affect the development of newborns (130). Paternal exposures may contribute to fetal risk through mutagenic and epigenetic mechanisms involving the sperm; and the chemical could also be carried in semen, leading to fetal exposure after intercourse (131–133).
Interactions between gene and environmental chemicals
Humans can vary in their susceptibility to the adverse effects of toxic chemicals due to genetic variability (134). For example, one study found that higher maternal blood levels of β-hexachlorocyclohexane (HCH), an organochlorine pesticide, is associated with increased risk of idiopathic preterm delivery in women with GSTM1 null polymorphisms, because they lack activity of the enzymes responsible for detoxification of xenobiotics (135). Some genetic variant may also modify placental transfer of chemicals, leading to differential levels of fetal exposure. For example, a maternal iron metabolism genotype was found to be a modifier of placental lead transfer in the US population: infants born to mothers with HFE C282Y gene variant have lower cord blood level concentrations relative to those born to mothers who were wild-type (prevailing among individuals in natural conditions) (136).
The relation between genetic profile and the external environment in affecting human health is not uni-directional, but bi-directional. Toxic environmental exposure could also induce changes in gene regulatory mechanisms that correlate strongly with disease etiology (e.g. cancer and infertility) (137). For example, PCBs may cause mutations in p53 and K-ras oncogenes and represent risk factors for colorectal and pancreatic cancers (138,139). The inclusion of gene-environmental interaction in risk assessment may help identify and thus safeguard vulnerable populations.
Recommendation for prevention
In clinical settings, obstetricians and gynecologists can provide authoritative and science-based guidance on how to avoid potentially adverse exposures (140). They are also uniquely poised to intervene to prevent harm before and during pregnancy, which is a critical window of human development (141). In 2015, the International Federation of Gynecology and Obstetrics (FIGO) released an opinion article on reproductive health impacts of exposure to toxic environmental chemicals, where FIGO joins ACOG/ASRM, the Royal College of Obstetricians and Gynecologists, the Endocrine Society, and the Society of Obstetricians and Gynecologists of Canada in “urging reproductive health professionals including obstetricians, gynecologists, midwives, nurses, women’s health nurses practitioners and others to take timely action to prevent exposure to toxic environmental chemicals” (5). Clinicians can adopt several tactics to incorporate environmental health into their patient-centered care, including (i) becoming knowledgeable about toxic environmental agents that are endemic to their specific geographic area, (ii) intervening as early as possible (preconception and during pregnancy), (iii) taking an exposure history (especially occupational exposures), (iv) providing anticipatory guidance on how to make healthier choices and avoid toxic exposures at home, in the community, and at work, and (v) reporting identified hazards. Detailed strategies and useful resources have been summarized elsewhere (1,140,141).
The role of reproductive health professionals in preventing harmful environmental exposures extends beyond the clinical setting. Advancing society-wide and prevention-oriented policy actions are essential for reducing toxic exposures to pregnant women and other vulnerable populations because many exposures are beyond individual’s control (i.e., from air and water) (140). To this end, clinicians play a crucial role in, for instance, initiating institutional-level interventions in support of a healthy food system and engaging in reducing pesticide use in institutional pest-control policies (140) and many more policy settings, including through their own professional organizations.
Understanding the sources and extent of exposures to environmental chemicals is a critical element in the efforts of reproductive health professionals to identify and prevent harmful chemical exposures to their patients and the population. In conclusion, to translate science into healthier pregnancy, healthier children, and healthy future generations, efforts are needed in advancing scientific research on characterizing chemical exposure in pregnant women and its health impact, synthesizing evidence to develop recommendations for prevention using systematic methods (142), and promoting policy change.
Acknowledgments
Funding
This work is supported in part by the National Institute of Environmental Health Sciences (NIEHS) grant PO1ES022841 and the United States Environmental Protection Agency (EPA) grant 83543301 [TW and AW], the March of Dimes Prematurity Research Center at Stanford, the Stanford Child Health Research Institute, and by the Stanford Clinical and Translational Science Award (CTSA to Spectrum (UL1 TR001085) [MS and AW], National Institutes of Health (R00ES021470, L40ES023163) [AP].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.The American College of Obstetricians and Gynecologists. [Accessed May 1, 2015];Companion Piece to Committee Opinion #575 “Exposure to Toxic Environmental Agents”. 2013 Available from: https://www.acog.org/-/media/Committee-Opinions/Committee-on-Health-Care-for-Underserved-Women/ExposuretoToxic.pdf.
- 2.Zota A, Atchley D, Woodruff T. The Intrauterine Environment and Early Infancy. In: Landrigan PJ, Etzel RA, editors. Textbook of Children’s Environmental Health. Oxford University Press; 2013. [Google Scholar]
- 3.Mitro SD, Johnson T, Zota AR. Cumulative Chemical Exposures During Pregnancy and Early Development. Curr Environ Heal reports. 2015;2(4):367–78. doi: 10.1007/s40572-015-0064-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Cancer Institute. Reducing environmental cancer risk: what we can do now. President’s Cancer Panel 2008–2009 annual report; Bethesda (MD). 2010. [Google Scholar]
- 5.Di Renzo GC, Conry JA, Blake J, DeFrancesco MS, DeNicola N, Martin JN, et al. International Federation of Gynecology and Obstetrics opinion on reproductive health impacts of exposure to toxic environmental chemicals. Int J Gynaecol Obstet. 2015;131(3):219–25. doi: 10.1016/j.ijgo.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Organisation for Economic Co-operation and Development. [Accessed May 1, 2015];40 Years of Chemical Safety at OECD: Quality and Efficiency. 2011 Available from: http://www.oecd.org/env/ehs/48153344.pdf.
- 7.OECD. [Accessed May 1, 2015];OECD Environmental Outlook to 2030. 2008 Available from: http://www.keepeek.com/Digital-Asset-Management/oecd/environment/oecd-environmental-outlook-to-2030_9789264040519-en#page1.
- 8.Ribeiro T, Volkery A, Pirc-Velkavrh A, Vos H, Hoogeveen Y. The European Environment, State and Outlook 2010: Assessment of Global Megatrends. Copenhagen: European Environment Agency; 2011. [Accessed May 1, 2015]. Available from: http://espas.eu/orbis/sites/default/files/generated/document/en/GlobalmegatrendsNEW(1).pdf. [Google Scholar]
- 9.United States Environmental Protection Agency. [Accessed May 1, 2015];Fact Sheet: Chemicals Snapshot. 2013 Available from: http://nepis.epa.gov/Exe/ZyPDF.cgi/P100G08T.PDF?Dockey=P100G08T.PDF.
- 10.USEPA. [Accessed May 1, 2015];TSCA Chemical Substance Inventory. 2016 Available from: https://www.epa.gov/tsca-inventory.
- 11.Scorecard. [Accessed May 1, 2015];High Production Volume (HPV) Chemicals. Available from: http://scorecard.goodguide.com/chemical-profiles/def/hpv.html.
- 12.Sutton P, Giudice LC, Woodruff TJ. Reproductive environmental health. Curr Opin Obstet Gynecol. 2010;22(6):517–24. doi: 10.1097/GCO.0b013e3283404e59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilson MP, Schwarzman MR. Toward a new U.S. chemicals policy: rebuilding the foundation to advance new science, green chemistry, and environmental health. Environ Health Perspect. 2009;117(8):1202–9. doi: 10.1289/ehp.0800404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.US Environmental Protection Agency. [Accessed May 1, 2015];America’s Children and the Environment. (3). 2013 Available from: http://nepis.epa.gov/Exe/ZyPDF.cgi/P100FU5Q.PDF?Dockey=P100FU5Q.PDF.
- 15.National Health and Nutrition Examination Survey (NHANES) [Accessed May 1, 2015]; Available from: http://www.cdc.gov/nchs/nhanes/index.htm.
- 16.CDC. Fourth National Report on Human Exposure to Environmental Chemicals (Updated Tables, February 2015) Atlanta (GA): 2015. [Accessed May 1, 2015]. Available from: http://www.cdc.gov/biomonitoring/pdf/FourthReport_UpdatedTables_Feb2015.pdf. [Google Scholar]
- 17.Wild CP. Complementing the genome with an “exposome”: the outstanding challenge of environmental exposure measurement in molecular epidemiology. Cancer Epidemiol Biomarkers Prev. 2005;14(8):1847–50. doi: 10.1158/1055-9965.EPI-05-0456. [DOI] [PubMed] [Google Scholar]
- 18.Collins FS, Varmus H. A New Initiative on Precision Medicine. N Engl J Med. 2015;372:793–5. doi: 10.1056/NEJMp1500523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Human Genome Research Institute. [Accessed May 1, 2015];The Genes, Environment and Health Initiative (GEI) Available from: https://www.genome.gov/19518663/
- 20.National Institute of Environmental Health Sciences. [Accessed May 1, 2015];Children’s Health Exposure Analysis Resource (CHEAR) Available from: http://www.niehs.nih.gov/research/supported/exposure/chear/index.cfm.
- 21.WHO/UNEP. [Accessed May 1, 2015];WHO | State of the science of endocrine disrupting chemicals - 2012. Available from: http://www.who.int/ceh/publications/endocrine/en/
- 22.Rice D, Barone S. Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ Health Perspect. 2000;108(Suppl):511–33. doi: 10.1289/ehp.00108s3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoover RN, Hyer M, Pfeiffer RM, Adam E, Bond B, Cheville AL, et al. Adverse health outcomes in women exposed in utero to diethylstilbestrol. N Engl J Med. 2011;365(14):1304–14. doi: 10.1056/NEJMoa1013961. [DOI] [PubMed] [Google Scholar]
- 24.Klip H, Verloop J, van Gool JD, Koster META, Burger CW, van Leeuwen FE. Hypospadias in sons of women exposed to diethylstilbestrol in utero: a cohort study. Lancet (London, England) 2002;359(9312):1102–7. doi: 10.1016/S0140-6736(02)08152-7. [DOI] [PubMed] [Google Scholar]
- 25.Brouwers MM, Feitz WFJ, Roelofs LAJ, Kiemeney LALM, de Gier RPE, Roeleveld N. Hypospadias: a transgenerational effect of diethylstilbestrol? Hum Reprod. 2006;21(3):666–9. doi: 10.1093/humrep/dei398. [DOI] [PubMed] [Google Scholar]
- 26.Committee on Human Biomonitoring for Environmental, Toxicants NRC. Human Biomonitoring for Environmental Chemicals. Washington, D.C: National Academies Press; 2006. [Accessed May 1, 2015]. Available from: http://www.nap.edu/catalog/11700.html. [Google Scholar]
- 27.Woodruff TJ, Zota AR, Schwartz JM. Environmental chemicals in pregnant women in the United States: NHANES 2003–2004. Environ Health Perspect. 2011;119(6):878–85. doi: 10.1289/ehp.1002727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taylor CM, Golding J, Emond AM. Lead, cadmium and mercury levels in pregnancy: the need for international consensus on levels of concern. J Dev Orig Health Dis. 2014;5(1):16–30. doi: 10.1017/S2040174413000500. [DOI] [PubMed] [Google Scholar]
- 29.Science and Environmental Health Network. [Accessed May 1, 2015];Persistent, Bioaccumulative and Toxic Chemicals (PBTs) Available from: http://saferchemicals.org/wp-content/uploads/sites/3/2014/07/PBT-Factsheet.pdf.
- 30.Baibergenova A, Kudyakov R, Zdeb M, Carpenter DO. Low birth weight and residential proximity to PCB-contaminated waste sites. Environ Health Perspect. 2003;111(10):1352–7. doi: 10.1289/ehp.6053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sagiv SK, Thurston SW, Bellinger DC, Tolbert PE, Altshul LM, Korrick SA. Prenatal organochlorine exposure and behaviors associated with attention deficit hyperactivity disorder in school-aged children. Am J Epidemiol. 2010;171(5):593–601. doi: 10.1093/aje/kwp427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muckle G, Ayotte P, Dewailly EE, Jacobson SW, Jacobson JL. Prenatal exposure of the northern Québec Inuit infants to environmental contaminants. Environ Health Perspect. 2001;109(12):1291–9. doi: 10.1289/ehp.011091291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Whitworth KW, Haug LS, Baird DD, Becher G, Hoppin JA, Skjaerven R, et al. Perfluorinated compounds in relation to birth weight in the Norwegian Mother and Child Cohort Study. Am J Epidemiol. 2012;175(12):1209–16. doi: 10.1093/aje/kwr459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Washino N, Saijo Y, Sasaki S, Kato S, Ban S, Konishi K, et al. Correlations between prenatal exposure to perfluorinated chemicals and reduced fetal growth. Environ Health Perspect. 2009;117(4):660–7. doi: 10.1289/ehp.11681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lam J, Koustas E, Sutton P, Johnson PI, Atchley DS, Sen S, et al. The Navigation Guide - evidence-based medicine meets environmental health: integration of animal and human evidence for PFOA effects on fetal growth. Environ Health Perspect. 2014;122(10):1040–51. doi: 10.1289/ehp.1307923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang M, Park J-S, Petreas M. Temporal changes in the levels of perfluorinated compounds in California women’s serum over the past 50 years. Environ Sci Technol. 2011;45(17):7510–6. doi: 10.1021/es2012275. [DOI] [PubMed] [Google Scholar]
- 37.Liu J, Li J, Liu Y, Chan HM, Zhao Y, Cai Z, et al. Comparison on gestation and lactation exposure of perfluorinated compounds for newborns. Environ Int. 2011;37(7):1206–12. doi: 10.1016/j.envint.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 38.Hanssen L, Dudarev AA, Huber S, Odland JØ, Nieboer E, Sandanger TM. Partition of perfluoroalkyl substances (PFASs) in whole blood and plasma, assessed in maternal and umbilical cord samples from inhabitants of arctic Russia and Uzbekistan. Sci Total Environ. 2013;447:430–7. doi: 10.1016/j.scitotenv.2013.01.029. [DOI] [PubMed] [Google Scholar]
- 39.Monroy R, Morrison K, Teo K, Atkinson S, Kubwabo C, Stewart B, et al. Serum levels of perfluoroalkyl compounds in human maternal and umbilical cord blood samples. Environ Res. 2008;108(1):56–62. doi: 10.1016/j.envres.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 40.Glynn A, Berger U, Bignert A, Ullah S, Aune M, Lignell S, et al. Perfluorinated alkyl acids in blood serum from primiparous women in Sweden: serial sampling during pregnancy and nursing, and temporal trends 1996–2010. Environ Sci Technol. 2012;46(16):9071–9. doi: 10.1021/es301168c. [DOI] [PubMed] [Google Scholar]
- 41.Butt C, Hessler W. New stain repellent chemical doubling in blood every 6 years. [Accessed May 1, 2015];Environ Heal News. 2012 Available from: http://www.environmentalhealthnews.org/ehs/newscience/2012/08/2012-1120-fluorinated-stain-repellent-rises-in-blood/
- 42.Herbstman JB, Sjödin A, Kurzon M, Lederman SA, Jones RS, Rauh V, et al. Prenatal exposure to PBDEs and neurodevelopment. Environ Health Perspect. 2010;118(5):712–9. doi: 10.1289/ehp.0901340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roze E, Meijer L, Bakker A, Van Braeckel KNJA, Sauer PJJ, Bos AF. Prenatal exposure to organohalogens, including brominated flame retardants, influences motor, cognitive, and behavioral performance at school age. Environ Health Perspect. 2009;117(12):1953–8. doi: 10.1289/ehp.0901015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vorkamp K, Nielsen F, Kyhl HB, Husby S, Nielsen LB, Barington T, et al. Polybrominated diphenyl ethers and perfluoroalkyl substances in serum of pregnant women: levels, correlations, and potential health implications. Arch Environ Contam Toxicol. 2014;67(1):9–20. doi: 10.1007/s00244-013-9988-z. [DOI] [PubMed] [Google Scholar]
- 45.Lv Q-X, Wang W, Li X-H, Yu L, Zhang Y, Tian Y. Polychlorinated biphenyls and polybrominated biphenyl ethers in adipose tissue and matched serum from an E-waste recycling area (Wenling, China) Environ Pollut. 2015;199:219–26. doi: 10.1016/j.envpol.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 46.Zota AR, Park J-S, Wang Y, Petreas M, Zoeller RT, Woodruff TJ. Polybrominated diphenyl ethers, hydroxylated polybrominated diphenyl ethers, and measures of thyroid function in second trimester pregnant women in California. Environ Sci Technol. 2011;45(18):7896–905. doi: 10.1021/es200422b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cone M. Californians have world’s highest levels of flame retardants. [Accessed May 1, 2015];Environ Heal News. 2008 Available from: http://www.environmentalhealthnews.org/ehs/news/californians-have-worlds-highest-levels-of-flame-retardants.
- 48.Zota AR, Linderholm L, Park J-S, Petreas M, Guo T, Privalsky ML, et al. Temporal comparison of PBDEs, OH-PBDEs, PCBs, and OH-PCBs in the serum of second trimester pregnant women recruited from San Francisco General Hospital, California. Environ Sci Technol. 2013;47(20):11776–84. doi: 10.1021/es402204y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Parry E, Park J-S, Zota AR, Petreas M, Woodruff TJ. Temporal trend of PBDEs in Northern California pregnant women [Abstract]. 36th Society of Environmental Toxicology and Chemistry (SETAC) Meeting; Salt Lake City, Utah. 2015. [Google Scholar]
- 50.Larsson K, Ljung Björklund K, Palm B, Wennberg M, Kaj L, Lindh CH, et al. Exposure determinants of phthalates, parabens, bisphenol A and triclosan in Swedish mothers and their children. Environ Int. 2014;73:323–33. doi: 10.1016/j.envint.2014.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.American Chemistry Council. [Accessed May 1, 2015];Bioaccumulation & Biomagnification. Available from: https://phthalates.americanchemistry.com/Science-Health/Bioaccumulation-Biomagnification.
- 52.Fei C, McLaughlin JK, Tarone RE, Olsen J. Perfluorinated chemicals and fetal growth: a study within the Danish National Birth Cohort. Environ Health Perspect. 2007;115(11):1677–82. doi: 10.1289/ehp.10506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Spanier AJ, Kahn RS, Kunselman AR, Hornung R, Xu Y, Calafat AM, et al. Prenatal exposure to bisphenol A and child wheeze from birth to 3 years of age. Environ Health Perspect. 2012;120(6):916–20. doi: 10.1289/ehp.1104175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Valvi D, Casas M, Mendez MA, Ballesteros-Gómez A, Luque N, Rubio S, et al. Prenatal bisphenol a urine concentrations and early rapid growth and overweight risk in the offspring. Epidemiology. 2013;24(6):791–9. doi: 10.1097/EDE.0b013e3182a67822. [DOI] [PubMed] [Google Scholar]
- 55.Fisher M, Arbuckle TE, Mallick R, LeBlanc A, Hauser R, Feeley M, et al. Bisphenol A and phthalate metabolite urinary concentrations: Daily and across pregnancy variability. J Expo Sci Environ Epidemiol. 2015;25(3):231–9. doi: 10.1038/jes.2014.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Braun JM, Kalkbrenner AE, Calafat AM, Bernert JT, Ye X, Silva MJ, et al. Variability and predictors of urinary bisphenol A concentrations during pregnancy. Environ Health Perspect. 2011;119(1):131–7. doi: 10.1289/ehp.1002366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Braun JM, Smith KW, Williams PL, Calafat AM, Berry K, Ehrlich S, et al. Variability of urinary phthalate metabolite and bisphenol A concentrations before and during pregnancy. Environ Health Perspect. 2012;120(5):739–45. doi: 10.1289/ehp.1104139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Agency for Toxic Substances and Disease Registry (ATSDR) [Accessed May 1, 2015];Toxicological Profile for Phenol. 2008 Available from: http://www.atsdr.cdc.gov/ToxProfiles/tp115.pdf. [PubMed]
- 59.State of California Environmental Protection Agency OEHHA. [Accessed May 1, 2015];The Proposition 65 List: Chemicals Known to the State to Cause Cancer or Reproductive Toxicity. 2016 Available from: http://oehha.ca.gov/media/downloads/crnr/p65single05202016.pdf.
- 60.Johnson PI, Koustas E, Vesterinen HM, Sutton P, Atchley DS, Kim AN, et al. Application of the Navigation Guide Systematic Review Methodology to the Evidence for Developmental and Reproductive Toxicity of Triclosan. Environ Int. 2016 doi: 10.1016/j.envint.2016.03.009. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wielogórska E, Elliott CT, Danaher M, Connolly L, Connolly L. Endocrine disruptor activity of multiple environmental food chain contaminants. Toxicol Vitr. 2015;29(1):211–20. doi: 10.1016/j.tiv.2014.10.014. [DOI] [PubMed] [Google Scholar]
- 62.Latini G, De Felice C, Presta G, Del Vecchio A, Paris I, Ruggieri F, et al. In utero exposure to di-(2-ethylhexyl)phthalate and duration of human pregnancy. Environ Health Perspect. 2003;111(14):1783–5. doi: 10.1289/ehp.6202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Engel SM, Zhu C, Berkowitz GS, Calafat AM, Silva MJ, Miodovnik A, et al. Prenatal phthalate exposure and performance on the Neonatal Behavioral Assessment Scale in a multiethnic birth cohort. Neurotoxicology. 2009;30(4):522–8. doi: 10.1016/j.neuro.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.WWF. [Accessed May 1, 2015];Chain of Contamination: The Food Link (Fact Sheet) Available from: http://www.wwf.org.uk/filelibrary/pdf/contamination.pdf.
- 65.Röllin HB, Sandanger TM, Hansen L, Channa K, Odland JØ. Concentration of selected persistent organic pollutants in blood from delivering women in South Africa. Sci Total Environ. 2009;408(1):146–52. doi: 10.1016/j.scitotenv.2009.08.049. [DOI] [PubMed] [Google Scholar]
- 66.Vizcaino E, Grimalt JO, Lopez-Espinosa M-J, Llop S, Rebagliato M, Ballester F. Maternal origin and other determinants of cord serum organochlorine compound concentrations in infants from the general population. Environ Sci Technol. 2010;44(16):6488–95. doi: 10.1021/es101397e. [DOI] [PubMed] [Google Scholar]
- 67.Rudge CVC, Sandanger T, Röllin HB, Calderon IMP, Volpato G, Silva JLP, et al. Levels of selected persistent organic pollutants in blood from delivering women in seven selected areas of São Paulo State, Brazil. Environ Int. 2012;40:162–9. doi: 10.1016/j.envint.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 68.Curren MS, Davis K, Liang CL, Adlard B, Foster WG, Donaldson SG, et al. Comparing plasma concentrations of persistent organic pollutants and metals in primiparous women from northern and southern Canada. Sci Total Environ. 2014;479–480:306–18. doi: 10.1016/j.scitotenv.2014.01.017. [DOI] [PubMed] [Google Scholar]
- 69.Castorina R, Bradman A, Fenster L, Barr DB, Bravo R, Vedar MG, et al. Comparison of current-use pesticide and other toxicant urinary metabolite levels among pregnant women in the CHAMACOS cohort and NHANES. Environ Health Perspect. 2010;118(6):856–63. doi: 10.1289/ehp.0901568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vilahur N, Vahter M, Broberg K. The Epigenetic Effects of Prenatal Cadmium Exposure. Curr Environ Heal reports. 2015;2(2):195–203. doi: 10.1007/s40572-015-0049-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sanders AP, Claus Henn B, Wright RO. Perinatal and Childhood Exposure to Cadmium, Manganese, and Metal Mixtures and Effects on Cognition and Behavior: A Review of Recent Literature. Curr Environ Heal reports. 2015;2(3):284–94. doi: 10.1007/s40572-015-0058-8. [cited 2016 Apr 14] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sioen I, Den Hond E, Nelen V, Van de Mieroop E, Croes K, Van Larebeke N, et al. Prenatal exposure to environmental contaminants and behavioural problems at age 7–8 years. Environ Int. 2013;59:225–31. doi: 10.1016/j.envint.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 73.Agency for Toxic Substances and Disease Registry (ATSDR) [Accessed May 1, 2015];Toxicological Profile: Cadmium. 2012 Available from: http://www.atsdr.cdc.gov/ToxProfiles/tp5.pdf. [PubMed]
- 74.Pilsner JR, Hu H, Ettinger A, Sánchez BN, Wright RO, Cantonwine D, et al. Influence of prenatal lead exposure on genomic methylation of cord blood DNA. Environ Health Perspect. 2009;117(9):1466–71. doi: 10.1289/ehp.0800497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schnaas L, Rothenberg SJ, Flores M-F, Martinez S, Hernandez C, Osorio E, et al. Reduced intellectual development in children with prenatal lead exposure. Environ Health Perspect. 2006;114(5):791–7. doi: 10.1289/ehp.8552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.National Toxicology Program. NTP Monograph on Health Effects of Low-level Lead. Research Triangle Park, NC: National Institute of Environmental Health Sciences, National Toxicology Program; 2012. [Accessed May 1, 2015]. Available from: http://ntp.niehs.nih.gov/go/36443. [Google Scholar]
- 77.Agency for Toxic Substances and Disease Registry (ATSDR) [Accessed May 1, 2015];Toxicological Profile: Lead. 2007 Available from: http://www.atsdr.cdc.gov/ToxProfiles/tp.asp?id=96&tid=22. [PubMed]
- 78.Tatsuta N, Nakai K, Murata K, Suzuki K, Iwai-Shimada M, Kurokawa N, et al. Impacts of prenatal exposures to polychlorinated biphenyls, methylmercury, and lead on intellectual ability of 42-month-old children in Japan. Environ Res. 2014;133:321–6. doi: 10.1016/j.envres.2014.05.024. [DOI] [PubMed] [Google Scholar]
- 79.Sakamoto M, Murata K, Kubota M, Nakai K, Satoh H. Mercury and heavy metal profiles of maternal and umbilical cord RBCs in Japanese population. Ecotoxicol Environ Saf. 2010;73(1):1–6. doi: 10.1016/j.ecoenv.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 80.Grandjean P, Weihe P, Debes F, Choi AL, Budtz-Jørgensen E. Neurotoxicity from prenatal and postnatal exposure to methylmercury. Neurotoxicol Teratol. 2014;43:39–44. doi: 10.1016/j.ntt.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Boucher O, Muckle G, Jacobson JL, Carter RC, Kaplan-Estrin M, Ayotte P, et al. Domain-specific effects of prenatal exposure to PCBs, mercury, and lead on infant cognition: results from the Environmental Contaminants and Child Development Study in Nunavik. Environ Health Perspect. 2014;122(3):310–6. doi: 10.1289/ehp.1206323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Barr DB, Bishop A, Needham LL. Concentrations of xenobiotic chemicals in the maternal-fetal unit. Reprod Toxicol. 23(3):260–6. doi: 10.1016/j.reprotox.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 83.Chen Z, Myers R, Wei T, Bind E, Kassim P, Wang G, et al. Placental transfer and concentrations of cadmium, mercury, lead, and selenium in mothers, newborns, and young children. J Expo Sci Environ Epidemiol. 2014;24(5):537–44. doi: 10.1038/jes.2014.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Basu N, Tutino R, Zhang Z, Cantonwine DE, Goodrich JM, Somers EC, et al. Mercury levels in pregnant women, children, and seafood from Mexico City. Environ Res. 2014;135:63–9. doi: 10.1016/j.envres.2014.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kim B-M, Lee B-E, Hong Y-C, Park H, Ha M, Kim Y-J, et al. Mercury levels in maternal and cord blood and attained weight through the 24 months of life. Sci Total Environ. 2011;410–411:26–33. doi: 10.1016/j.scitotenv.2011.08.060. [DOI] [PubMed] [Google Scholar]
- 86.Kopp RS, Kumbartski M, Harth V, Brüning T, Käfferlein HU. Partition of metals in the maternal/fetal unit and lead-associated decreases of fetal iron and manganese: an observational biomonitoring approach. Arch Toxicol. 2012;86(10):1571–81. doi: 10.1007/s00204-012-0869-4. [DOI] [PubMed] [Google Scholar]
- 87.Obi E, Okafor C, Igwebe A, Ebenebe J, Afonne OJ, Ifediata F, et al. Elevated prenatal methylmercury exposure in Nigeria: evidence from maternal and cord blood. Chemosphere. 2015;119:485–9. doi: 10.1016/j.chemosphere.2014.07.038. [DOI] [PubMed] [Google Scholar]
- 88.Lopez-Espinosa M-J, Murcia M, Iñiguez C, Vizcaino E, Llop S, Vioque J, et al. Prenatal exposure to organochlorine compounds and birth size. Pediatrics. 2011;128(1):e127–34. doi: 10.1542/peds.2010-1951. [DOI] [PubMed] [Google Scholar]
- 89.Kim S, Park J, Kim H-J, Lee JJ, Choi G, Choi S, et al. Association between Several Persistent Organic Pollutants and Thyroid Hormone Levels in Cord Blood Serum and Bloodspot of the Newborn Infants of Korea. PLoS One. 2015;10(5):e0125213. doi: 10.1371/journal.pone.0125213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Aylward LL, Hays SM, Kirman CR, Marchitti SA, Kenneke JF, English C, et al. Relationships of chemical concentrations in maternal and cord blood: a review of available data. J Toxicol Environ Health B Crit Rev. 2014;17(3):175–203. doi: 10.1080/10937404.2014.884956. [DOI] [PubMed] [Google Scholar]
- 91.Soechitram SD, Athanasiadou M, Hovander L, Bergman A, Sauer PJJ. Fetal exposure to PCBs and their hydroxylated metabolites in a Dutch cohort. Environ Health Perspect. 2004;112(11):1208–12. doi: 10.1289/ehp.6424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mori C, Nakamura N, Todaka E, Fujisaki T, Matsuno Y, Nakaoka H, et al. Correlation between human maternal-fetal placental transfer and molecular weight of PCB and dioxin congeners/isomers. Chemosphere. 2014;114:262–7. doi: 10.1016/j.chemosphere.2014.04.095. [DOI] [PubMed] [Google Scholar]
- 93.Patayová H, Wimmerová S, Lancz K, Palkovičová L, Drobná B, Fabišiková A, et al. Anthropometric, socioeconomic, and maternal health determinants of placental transfer of organochlorine compounds. Environ Sci Pollut Res Int. 2013;20(12):8557–66. doi: 10.1007/s11356-013-1786-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lancz K, Murínová L, Patayová H, Drobná B, Wimmerová S, Sovčíková E, et al. Ratio of cord to maternal serum PCB concentrations in relation to their congener-specific physicochemical properties. Int J Hyg Environ Health. 2015;218(1):91–8. doi: 10.1016/j.ijheh.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tyagi V, Garg N, Mustafa MD, Banerjee BD, Guleria K. Organochlorine pesticide levels in maternal blood and placental tissue with reference to preterm birth: a recent trend in North Indian population. Environ Monit Assess. 2015;187(7):471. doi: 10.1007/s10661-015-4369-x. [DOI] [PubMed] [Google Scholar]
- 96.Lopez-Espinosa M-J, Vizcaino E, Murcia M, Fuentes V, Garcia A-M, Rebagliato M, et al. Prenatal exposure to organochlorine compounds and neonatal thyroid stimulating hormone levels. J Expo Sci Environ Epidemiol. 2010;20(7):579–88. doi: 10.1038/jes.2009.47. [DOI] [PubMed] [Google Scholar]
- 97.Guo H, Jin Y, Cheng Y, Leaderer B, Lin S, Holford TR, et al. Prenatal exposure to organochlorine pesticides and infant birth weight in China. Chemosphere. 2014;110:1–7. doi: 10.1016/j.chemosphere.2014.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mariscal-Arcas M, Lopez-Martinez C, Granada A, Olea N, Lorenzo-Tovar ML, Olea-Serrano F. Organochlorine pesticides in umbilical cord blood serum of women from Southern Spain and adherence to the Mediterranean diet. Food Chem Toxicol. 2010;48(5):1311–5. doi: 10.1016/j.fct.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 99.Asawasinsopon R, Prapamontol T, Prakobvitayakit O, Vaneesorn Y, Mangklabruks A, Hock B. The association between organochlorine and thyroid hormone levels in cord serum: a study from northern Thailand. Environ Int. 2006;32(4):554–9. doi: 10.1016/j.envint.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 100.Balakrishnan B, Henare K, Thorstensen EB, Ponnampalam AP, Mitchell MD. Transfer of bisphenol A across the human placenta. Am J Obstet Gynecol. 2010;202(4):393e1–7. doi: 10.1016/j.ajog.2010.01.025. [DOI] [PubMed] [Google Scholar]
- 101.Nishikawa M, Iwano H, Yanagisawa R, Koike N, Inoue H, Yokota H. Placental transfer of conjugated bisphenol A and subsequent reactivation in the rat fetus. Environ Health Perspect. 2010;118(9):1196–203. doi: 10.1289/ehp.0901575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gerona RR, Woodruff TJ, Dickenson CA, Pan J, Schwartz JM, Sen S, et al. Bisphenol-A (BPA), BPA glucuronide, and BPA sulfate in midgestation umbilical cord serum in a northern and central California population. Environ Sci Technol. 2013;47(21):12477–85. doi: 10.1021/es402764d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lee YJ, Ryu H-Y, Kim H-K, Min CS, Lee JH, Kim E, et al. Maternal and fetal exposure to bisphenol A in Korea. Reprod Toxicol. 2008;25(4):413–9. doi: 10.1016/j.reprotox.2008.05.058. [DOI] [PubMed] [Google Scholar]
- 104.Schönfelder G, Wittfoht W, Hopp H, Talsness CE, Paul M, Chahoud I. Parent bisphenol A accumulation in the human maternal-fetal-placental unit. Environ Health Perspect. 2002;110(11):A703–7. doi: 10.1289/ehp.110-1241091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wan Y, Choi K, Kim S, Ji K, Chang H, Wiseman S, et al. Hydroxylated polybrominated diphenyl ethers and bisphenol A in pregnant women and their matching fetuses: placental transfer and potential risks. Environ Sci Technol. 2010;44(13):5233–9. doi: 10.1021/es1002764. [DOI] [PubMed] [Google Scholar]
- 106.Chou W-C, Chen J-L, Lin C-F, Chen Y-C, Shih F-C, Chuang C-Y. Biomonitoring of bisphenol A concentrations in maternal and umbilical cord blood in regard to birth outcomes and adipokine expression: a birth cohort study in Taiwan. Environ Health. 2011;10:94. doi: 10.1186/1476-069X-10-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ikezuki Y, Tsutsumi O, Takai Y, Kamei Y, Taketani Y. Determination of bisphenol A concentrations in human biological fluids reveals significant early prenatal exposure. Hum Reprod. 2002;17(11):2839–41. doi: 10.1093/humrep/17.11.2839. [DOI] [PubMed] [Google Scholar]
- 108.Enke U, Schleussner E, Pälmke C, Seyfarth L, Koch HM. Phthalate exposure in pregnant women and newborns - the urinary metabolite excretion pattern differs distinctly. Int J Hyg Environ Health. 2013;216(6):735–42. doi: 10.1016/j.ijheh.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 109.Li L-X, Chen L, Meng X-Z, Chen B-H, Chen S-Q, Zhao Y, et al. Exposure levels of environmental endocrine disruptors in mother-newborn pairs in China and their placental transfer characteristics. PLoS One. 2013;8(5):e62526. doi: 10.1371/journal.pone.0062526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kim J-T, Son M-H, Lee D-H, Seong WJ, Han S, Chang Y-S. Partitioning behavior of heavy metals and persistent organic pollutants among feto-maternal bloods and tissues. Environ Sci Technol. 2015;49(12):7411–22. doi: 10.1021/es5051309. [DOI] [PubMed] [Google Scholar]
- 111.Li M-M, Wu M-Q, Xu J, Du J, Yan C-H. Body burden of Hg in different bio-samples of mothers in Shenyang city, China. PLoS One. 2014;9(5):e98121. doi: 10.1371/journal.pone.0098121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lee YJ, Kim M-K, Bae J, Yang J-H. Concentrations of perfluoroalkyl compounds in maternal and umbilical cord sera and birth outcomes in Korea. Chemosphere. 2013;90(5):1603–9. doi: 10.1016/j.chemosphere.2012.08.035. [DOI] [PubMed] [Google Scholar]
- 113.Gützkow KB, Haug LS, Thomsen C, Sabaredzovic A, Becher G, Brunborg G. Placental transfer of perfluorinated compounds is selective--a Norwegian Mother and Child sub-cohort study. Int J Hyg Environ Health. 2012;215(2):216–9. doi: 10.1016/j.ijheh.2011.08.011. [DOI] [PubMed] [Google Scholar]
- 114.Needham LL, Grandjean P, Heinzow B, Jørgensen PJ, Nielsen F, Patterson DG, et al. Partition of environmental chemicals between maternal and fetal blood and tissues. Environ Sci Technol. 2011;45(3):1121–6. doi: 10.1021/es1019614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sun H, Chen W, Wang D, Jin Y, Chen X, Xu Y. The effects of prenatal exposure to low-level cadmium, lead and selenium on birth outcomes. Chemosphere. 2014;108:33–9. doi: 10.1016/j.chemosphere.2014.02.080. [DOI] [PubMed] [Google Scholar]
- 116.García-Esquinas E, Pérez-Gómez B, Fernández-Navarro P, Fernández MA, de Paz C, Pérez-Meixeira AM, et al. Lead, mercury and cadmium in umbilical cord blood and its association with parental epidemiological variables and birth factors. BMC Public Health. 2013;13:841. doi: 10.1186/1471-2458-13-841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.National Research Council. Science and Decisions: Advancing Risk Assessment. Washington, D.C: National Academies Press; 2009. [Accessed May 1, 2015]. Available from: http://www.nap.edu/catalog/12209/science-and-decisions-advancing-risk-assessment. [PubMed] [Google Scholar]
- 118.Committee on Environmental Justice; Institute of Medicine (IOM) Toward Environmental Justice: Research, Education, and Health Policy Needs. Washington, D.C: National Academies Press; 1999. [Accessed May 1, 2015]. Available from: http://www.nap.edu/catalog/6034/toward-environmental-justice-research-education-and-health-policy-needs. [Google Scholar]
- 119.Morello-Frosch R, Shenassa ED. The environmental “riskscape” and social inequality: implications for explaining maternal and child health disparities. Environ Health Perspect. 2006;114(8):1150–3. doi: 10.1289/ehp.8930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Goldman L, Tran N. [Accessed May 1, 2015];Toxics and poverty: the impact of toxic substances on the poor in developing countries. 2002 Available from: http://documents.worldbank.org/curated/en/2002/08/9689786/toxics-poverty-impact-toxic-substances-poor-developing-countries.
- 121.Quach T, Von Behren J, Goldberg D, Layefsky M, Reynolds P. Adverse birth outcomes and maternal complications in licensed cosmetologists and manicurists in California. Int Arch Occup Environ Health. 2015;88(7):823–33. doi: 10.1007/s00420-014-1011-0. [DOI] [PubMed] [Google Scholar]
- 122.Flocks J, Kelley M, Economos J, McCauley L. Female farmworkers’ perceptions of pesticide exposure and pregnancy health. J Immigr Minor Health. 2012;14(4):626–32. doi: 10.1007/s10903-011-9554-6. [DOI] [PubMed] [Google Scholar]
- 123.Morello-Frosch R, Zuk M, Jerrett M, Shamasunder B, Kyle AD. Understanding the cumulative impacts of inequalities in environmental health: implications for policy. Health Aff (Millwood) 2011;30(5):879–87. doi: 10.1377/hlthaff.2011.0153. [DOI] [PubMed] [Google Scholar]
- 124.Sciences B on L, Studies D on E and L, National Academies of Sciences E and M. Use of Metabolomics to Advance Research on Environmental Exposures and the Human Exposome. Washington, D.C: National Academies Press; 2016. [Accessed May 1, 2015]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK355519/ [PubMed] [Google Scholar]
- 125.Sobolewski M, Conrad K, Allen JL, Weston H, Martin K, Lawrence BP, et al. Sex-specific enhanced behavioral toxicity induced by maternal exposure to a mixture of low dose endocrine-disrupting chemicals. Neurotoxicology. 2014;45:121–30. doi: 10.1016/j.neuro.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tardif R, Laparé S, Krishnan K, Brodeur J. A descriptive and mechanistic study of the interaction between toluene and xylene in humans. Int Arch Occup Environ Health. 1993;65(1 Suppl):S135–7. doi: 10.1007/BF00381325. [DOI] [PubMed] [Google Scholar]
- 127.Sarigiannis DA, Hansen U. Considering the cumulative risk of mixtures of chemicals - a challenge for policy makers. Environ Health. 2012;11(Suppl 1):S18. doi: 10.1186/1476-069X-11-S1-S18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Carpenter DO, Arcaro K, Spink DC. Understanding the human health effects of chemical mixtures. Environ Health Perspect. 2002;110(Suppl):25–42. doi: 10.1289/ehp.02110s125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.National Research Council. Phthalates and Cumulative Risk Assessment: the Task Ahead. Washington, D.C: National Academies Press; 2008. [Accessed May 1, 2015]. Available from: http://www.nap.edu/catalog/12528/phthalates-and-cumulative-risk-assessment-the-task-ahead. [PubMed] [Google Scholar]
- 130.García-Esquinas E, Aragonés N, Fernández MA, García-Sagredo JM, de León A, de Paz C, et al. Newborns and low to moderate prenatal environmental lead exposure: might fathers be the key? Environ Sci Pollut Res Int. 2014;21(13):7886–98. doi: 10.1007/s11356-014-2738-6. [DOI] [PubMed] [Google Scholar]
- 131.Cordier S. Evidence for a role of paternal exposures in developmental toxicity. Basic Clin Pharmacol Toxicol. 2008;102(2):176–81. doi: 10.1111/j.1742-7843.2007.00162.x. [DOI] [PubMed] [Google Scholar]
- 132.Woodruff TJ, Carlson A, Schwartz JM, Giudice LC. Proceedings of the Summit on Environmental Challenges to Reproductive Health and Fertility: executive summary. Fertil Steril. 2008;89(2):281–300. doi: 10.1016/j.fertnstert.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mattison DR. Environmental exposures and development. Curr Opin Pediatr. 2010;22(2):208–18. doi: 10.1097/MOP.0b013e32833779bf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Eckerman DA. Human Variability in Response to Chemical Exposures Measures, Modeling, and Risk Assessment. CRC Press; 1998. [Google Scholar]
- 135.Mustafa MD, Banerjee BD, Ahmed RS, Tripathi AK, Guleria K. Gene-environment interaction in preterm delivery with special reference to organochlorine pesticides. Mol Hum Reprod. 2013;19(1):35–42. doi: 10.1093/molehr/gas039. [DOI] [PubMed] [Google Scholar]
- 136.Karwowski MP, Just AC, Bellinger DC, Jim R, Hatley EL, Ettinger AS, et al. Maternal iron metabolism gene variants modify umbilical cord blood lead levels by gene-environment interaction: a birth cohort study. Environ Health. 2014;13(1):77. doi: 10.1186/1476-069X-13-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Edwards TM, Myers JP. Environmental exposures and gene regulation in disease etiology. Environ Health Perspect. 2007;115(9):1264–70. doi: 10.1289/ehp.9951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Howsam M, Grimalt JO, Guinó E, Navarro M, Martí-Ragué J, Peinado MA, et al. Organochlorine exposure and colorectal cancer risk. Environ Health Perspect. 2004;112(15):1460–6. doi: 10.1289/ehp.7143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Tabrez S, Priyadarshini M, Priyamvada S, Khan MS, Na A, Zaidi SK. Gene-environment interactions in heavy metal and pesticide carcinogenesis. Mutat Res Genet Toxicol Environ Mutagen. 2014;760:1–9. doi: 10.1016/j.mrgentox.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 140.Sutton P, Woodruff TJ, Perron J, Stotland N, Conry JA, Miller MD, et al. Toxic environmental chemicals: the role of reproductive health professionals in preventing harmful exposures. Am J Obstet Gynecol. 2012;207(3):164–73. doi: 10.1016/j.ajog.2012.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.The American College of Obstetricians and Gynecologists. Exposure to toxic environmental agents. Committee Opinion No 575. Obstet Gynecol. 2013;122(4):931–5. doi: 10.1097/01.AOG.0000435416.21944.54. [DOI] [PubMed] [Google Scholar]
- 142.Woodruff TJ, Sutton P. The Navigation Guide systematic review methodology: a rigorous and transparent method for translating environmental health science into better health outcomes. Environ Health Perspect. 2014;122(10):1007–14. doi: 10.1289/ehp.1307175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Dallinga JW. Decreased human semen quality and organochlorine compounds in blood. Hum Reprod. 2002;17(8):1973–9. doi: 10.1093/humrep/17.8.1973. [DOI] [PubMed] [Google Scholar]
- 144.Jacobson JL, Jacobson SW. Intellectual impairment in children exposed to polychlorinated biphenyls in utero. N Engl J Med. 1996;335(11):783–9. doi: 10.1056/NEJM199609123351104. [DOI] [PubMed] [Google Scholar]
- 145.C8 Science Panel. [Accessed May 1, 2015];C8 Probable Link Reports: Probable Link Evaluation of Pregnancy-Induced Hypertension and Preeclampsia. 2011 Available from: http://www.c8sciencepanel.org/pdfs/Probable_Link_C8_PIH_5Dec2011.pdf.
- 146.C8 Science Panel. [Accessed May 1, 2015];C8 Probable Link Reports: Probable Link Evaluation of Thyroid Disease. 2012 Available from: http://www.c8sciencepanel.org/pdfs/Probable_Link_C8_Thyroid_30Jul2012.pdf.
- 147.Sugiura-Ogasawara M, Ozaki Y, Sonta S, Makino T, Suzumori K. Exposure to bisphenol A is associated with recurrent miscarriage. Hum Reprod. 2005;20(8):2325–9. doi: 10.1093/humrep/deh888. [DOI] [PubMed] [Google Scholar]
- 148.Braun JM, Yolton K, Dietrich KN, Hornung R, Ye X, Calafat AM, et al. Prenatal bisphenol A exposure and early childhood behavior. Environ Health Perspect. 2009;117(12):1945–52. doi: 10.1289/ehp.0900979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Braun JM, Kalkbrenner AE, Calafat AM, Yolton K, Ye X, Dietrich KN, et al. Impact of Early-Life Bisphenol A Exposure on Behavior and Executive Function in Children. Pediatrics. 2011;128(5):873–82. doi: 10.1542/peds.2011-1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Chevrier J, Gunier RB, Bradman A, Holland NT, Calafat AM, Eskenazi B, et al. Maternal urinary bisphenol a during pregnancy and maternal and neonatal thyroid function in the CHAMACOS study. Environ Health Perspect. 2013;121(1):138–44. doi: 10.1289/ehp.1205092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Paul KB, Hedge JM, Bansal R, Zoeller RT, Peter R, DeVito MJ, et al. Developmental triclosan exposure decreases maternal, fetal, and early neonatal thyroxine: a dynamic and kinetic evaluation of a putative mode-of-action. Toxicology. 2012;300(1–2):31–45. doi: 10.1016/j.tox.2012.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Chronic Hazard Advisory Panel (CHAP) Chronic Hazard Advisory Panel on Phthalates and Phthalate Alternatives Final Report. Bethesda, MD: 2014. [Accessed May 1, 2015]. Available from: https://www.cpsc.gov/PageFiles/169902/CHAP-REPORT-With-Appendices.pdf. [Google Scholar]
- 153.Engel SM, Miodovnik A, Canfield RL, Zhu C, Silva MJ, Calafat AM, et al. Prenatal phthalate exposure is associated with childhood behavior and executive functioning. Environ Health Perspect. 2010;118(4):565–71. doi: 10.1289/ehp.0901470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Agency for Toxic Substances and Disease Registry (ATSDR) [Accessed May 1, 2015];Toxicological profile for cadmium. 2012 Available from: http://www.atsdr.cdc.gov/toxprofiles/tp5.pdf. [PubMed]
- 155.Järup L, Akesson A. Current status of cadmium as an environmental health problem. Toxicol Appl Pharmacol. 2009;238(3):201–8. doi: 10.1016/j.taap.2009.04.020. [DOI] [PubMed] [Google Scholar]
- 156.Grandjean P, Weihe P, White RF, Debes F, Araki S, Yokoyama K, et al. Cognitive deficit in 7-year-old children with prenatal exposure to methylmercury. Neurotoxicol Teratol. 19(6):417–28. doi: 10.1016/s0892-0362(97)00097-4. [DOI] [PubMed] [Google Scholar]
- 157.Grandjean P, Weihe P, White RF, Debes F. Cognitive performance of children prenatally exposed to “safe” levels of methylmercury. Environ Res. 1998;77(2):165–72. doi: 10.1006/enrs.1997.3804. [DOI] [PubMed] [Google Scholar]
- 158.Lederman SA, Jones RL, Caldwell KL, Rauh V, Sheets SE, Tang D, et al. Relation between cord blood mercury levels and early child development in a World Trade Center cohort. Environ Health Perspect. 2008;116(8):1085–91. doi: 10.1289/ehp.10831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Davidson PW, Strain JJ, Myers GJ, Thurston SW, Bonham MP, Shamlaye CF, et al. Neurodevelopmental effects of maternal nutritional status and exposure to methylmercury from eating fish during pregnancy. Neurotoxicology. 2008;29(5):767–75. doi: 10.1016/j.neuro.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Symeonides C. Environmental Chemical Contributions to ADHD and the Externalising Disorders of Childhood – A Review of Epidemiological Evidence. J Enviromental Immunol Toxicol. 2013;1(2):92. [Google Scholar]
- 161.Grandjean P, Weihe P, Nielsen F, Heinzow B, Debes F, Budtz-Jørgensen E. Neurobehavioral deficits at age 7 years associated with prenatal exposure to toxicants from maternal seafood diet. Neurotoxicol Teratol. 2012;34(4):466–72. doi: 10.1016/j.ntt.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Steinmaus C, Miller MD, Smith AH. Perchlorate in drinking water during pregnancy and neonatal thyroid hormone levels in California. J Occup Environ Med. 2010;52(12):1217–24. doi: 10.1097/JOM.0b013e3181fd6fa7. [DOI] [PubMed] [Google Scholar]
- 163.Grube A, Donaldson D, Kiely T, Wu L. Pesticides Industry Sales and Usage 2006 and 2007 Market Estimates. Washington, DC: 2011. [Accessed May 1, 2015]. Available from: https://www.epa.gov/sites/production/files/2015-10/documents/market_estimates2007.pdf. [Google Scholar]
- 164.Whyatt RM, Rauh V, Barr DB, Camann DE, Andrews HF, Garfinkel R, et al. Prenatal insecticide exposures and birth weight and length among an urban minority cohort. Environ Health Perspect. 2004;112(10):1125–32. doi: 10.1289/ehp.6641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Bouchard MF, Chevrier J, Harley KG, Kogut K, Vedar M, Calderon N, et al. Prenatal exposure to organophosphate pesticides and IQ in 7-year-old children. Environ Health Perspect. 2011;119(8):1189–95. doi: 10.1289/ehp.1003185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Engel SM, Wetmur J, Chen J, Zhu C, Barr DB, Canfield RL, et al. Prenatal exposure to organophosphates, paraoxonase 1, and cognitive development in childhood. Environ Health Perspect. 2011;119(8):1182–8. doi: 10.1289/ehp.1003183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Eskenazi B, Marks AR, Bradman A, Harley K, Barr DB, Johnson C, et al. Organophosphate pesticide exposure and neurodevelopment in young Mexican-American children. Environ Health Perspect. 2007;115(5):792–8. doi: 10.1289/ehp.9828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Rauh VA, Garfinkel R, Perera FP, Andrews HF, Hoepner L, Barr DB, et al. Impact of prenatal chlorpyrifos exposure on neurodevelopment in the first 3 years of life among inner-city children. Pediatrics. 2006;118(6):e1845–59. doi: 10.1542/peds.2006-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Rauh V, Arunajadai S, Horton M, Perera F, Hoepner L, Barr DB, et al. Seven-year neurodevelopmental scores and prenatal exposure to chlorpyrifos, a common agricultural pesticide. Environ Health Perspect. 2011;119(8):1196–201. doi: 10.1289/ehp.1003160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Cohn BA, Cirillo PM, Christianson RE. Prenatal DDT exposure and testicular cancer: a nested case-control study. Arch Environ Occup Health. 65(3):127–34. doi: 10.1080/19338241003730887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Wigle DT, Turner MC, Krewski D. A systematic review and meta-analysis of childhood leukemia and parental occupational pesticide exposure. Environ Health Perspect. 2009;117(10):1505–13. doi: 10.1289/ehp.0900582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Wigle DT, Arbuckle TE, Turner MC, Bérubé A, Yang Q, Liu S, et al. Epidemiologic evidence of relationships between reproductive and child health outcomes and environmental chemical contaminants. J Toxicol Environ Health B Crit Rev. 2008;11(5–6):373–517. doi: 10.1080/10937400801921320. [DOI] [PubMed] [Google Scholar]
- 173.Van Maele-Fabry G, Lantin A-C, Hoet P, Lison D. Childhood leukaemia and parental occupational exposure to pesticides: a systematic review and meta-analysis. Cancer Causes Control. 2010;21(6):787–809. doi: 10.1007/s10552-010-9516-7. [DOI] [PubMed] [Google Scholar]
- 174.Van Maele-Fabry G, Hoet P, Lison D. Parental occupational exposure to pesticides as risk factor for brain tumors in children and young adults: a systematic review and meta-analysis. Environ Int. 2013;56:19–31. doi: 10.1016/j.envint.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 175.Doyle P, Roman E, Beral V, Brookes M. Spontaneous abortion in dry cleaning workers potentially exposed to perchloroethylene. Occup Environ Med. 1997;54(12):848–53. doi: 10.1136/oem.54.12.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Sharara FI, Seifer DB, Flaws JA. Environmental toxicants and female reproduction. Fertil Steril. 1998;70(4):613–22. doi: 10.1016/s0015-0282(98)00253-2. [DOI] [PubMed] [Google Scholar]
- 177.Schettler T, Solomon G, Valenti M, Huddle A. Generations at Risk: reproductive health and the environment. Cambridge, MA: MIT Press; 1999. [Google Scholar]
- 178.Kyyrönen P, Taskinen H, Lindbohm ML, Hemminki K, Heinonen OP. Spontaneous abortions and congenital malformations among women exposed to tetrachloroethylene in dry cleaning. J Epidemiol Community Health. 1989;43(4):346–51. doi: 10.1136/jech.43.4.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Hruska KS, Furth PA, Seifer DB, Sharara FI, Flaws JA. Environmental factors in infertility. Clin Obstet Gynecol. 2000;43(4):821–9. doi: 10.1097/00003081-200012000-00014. [DOI] [PubMed] [Google Scholar]
- 180.McMartin KI, Chu M, Kopecky E, Einarson TR, Koren G. Pregnancy outcome following maternal organic solvent exposure: a meta-analysis of epidemiologic studies. Am J Ind Med. 1998;34(3):288–92. doi: 10.1002/(sici)1097-0274(199809)34:3<288::aid-ajim12>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 181.Ahmed P, Jaakkola JJK. Exposure to organic solvents and adverse pregnancy outcomes. Hum Reprod. 2007;22(10):2751–7. doi: 10.1093/humrep/dem200. [DOI] [PubMed] [Google Scholar]
- 182.Hannigan JH, Bowen SE. Reproductive toxicology and teratology of abused toluene. Syst Biol Reprod Med. 2010;56(2):184–200. doi: 10.3109/19396360903377195. [DOI] [PubMed] [Google Scholar]
- 183.Jones HE, Balster RL. Inhalant abuse in pregnancy. Obstet Gynecol Clin North Am. 1998;25(1):153–67. doi: 10.1016/s0889-8545(05)70363-6. [DOI] [PubMed] [Google Scholar]
- 184.Wilkins-Haug L. Teratogen update: toluene. Teratology. 1997;55(2):145–51. doi: 10.1002/(SICI)1096-9926(199702)55:2<145::AID-TERA5>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 185.Stillerman KP, Mattison DR, Giudice LC, Woodruff TJ. Environmental exposures and adverse pregnancy outcomes: a review of the science. Reprod Sci. 2008;15(7):631–50. doi: 10.1177/1933719108322436. [DOI] [PubMed] [Google Scholar]
- 186.Council NR. Assessing the Human Health Risks of Trichloroethylene. Washington, D.C: National Academies Press; 2006. [Accessed May 1, 2015]. Available from: http://www.nap.edu/catalog/11707/assessing-the-human-health-risks-of-trichloroethylene-key-scientific-issues. [Google Scholar]
- 187.McGlynn KA, Guo X, Graubard BI, Brock JW, Klebanoff MA, Longnecker MP. Maternal pregnancy levels of polychlorinated biphenyls and risk of hypospadias and cryptorchidism in male offspring. Environ Health Perspect. 2009;117(9):1472–6. doi: 10.1289/ehp.0800389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Cohn BA, Cirillo PM, Sholtz RI, Ferrara A, Park J-S, Schwingl PJ. Polychlorinated biphenyl (PCB) exposure in mothers and time to pregnancy in daughters. Reprod Toxicol. 2011;31(3):290–6. doi: 10.1016/j.reprotox.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.James RA, Hertz-Picciotto I, Willman E, Keller JA, Charles MJ. Determinants of serum polychlorinated biphenyls and organochlorine pesticides measured in women from the child health and development study cohort, 1963–1967. Environ Health Perspect. 2002;110(7):617–24. doi: 10.1289/ehp.02110617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.McGraw JE, Waller DP. Fish ingestion and congener specific polychlorinated biphenyl and p,p′-dichlorodiphenyldichloroethylene serum concentrations in a great lakes cohort of pregnant African American women. Environ Int. 2009;35(3):557–65. doi: 10.1016/j.envint.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 191.Takser L, Mergler D, Baldwin M, de Grosbois S, Smargiassi A, Lafond J. Thyroid hormones in pregnancy in relation to environmental exposure to organochlorine compounds and mercury. Environ Health Perspect. 2005;113(8):1039–45. doi: 10.1289/ehp.7685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Bloom MS, Buck Louis GM, Schisterman EF, Liu A, Kostyniak PJ. Maternal serum polychlorinated biphenyl concentrations across critical windows of human development. Environ Health Perspect. 2007;115(9):1320–4. doi: 10.1289/ehp.10086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Bradman A, Fenster L, Sjödin A, Jones RS, Patterson DG, Eskenazi B. Polybrominated diphenyl ether levels in the blood of pregnant women living in an agricultural community in California. Environ Health Perspect. 2007;115(1):71–4. doi: 10.1289/ehp.8899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Braun JM, Kalkbrenner AE, Just AC, Yolton K, Calafat AM, Sjödin A, et al. Gestational exposure to endocrine-disrupting chemicals and reciprocal social, repetitive, and stereotypic behaviors in 4- and 5-year-old children: the HOME study. Environ Health Perspect. 2014;122(5):513–20. doi: 10.1289/ehp.1307261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Serme-Gbedo YK, Abdelouahab N, Pasquier J-C, Cohen AA, Takser L. Maternal levels of endocrine disruptors, polybrominated diphenyl ethers, in early pregnancy are not associated with lower birth weight in the Canadian birth cohort GESTE. Environ Heal. 2016;15(1):1. doi: 10.1186/s12940-016-0134-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Casas M, Nieuwenhuijsen M, Martinez D, Ballester F, Basagaña X, Basterrechea M, et al. Prenatal exposure to PCB-153, p,p′-DDE and birth outcomes in 9000 mother-child pairs: exposure-response relationship and effect modifiers. Environ Int. 2015;74:23–31. doi: 10.1016/j.envint.2014.09.013. [DOI] [PubMed] [Google Scholar]
- 197.Fängström B, Hovander L, Bignert A, Athanassiadis I, Linderholm L, Grandjean P, et al. Concentrations of Polybrominated Diphenyl Ethers, Polychlorinated Biphenyls, and Polychlorobiphenylols in Serum from Pregnant Faroese Women and Their Children 7 Years Later. Environ Sci Technol. 2005;39(24):9457–63. doi: 10.1021/es0513032. [DOI] [PubMed] [Google Scholar]
- 198.Berghuis SA, Soechitram SD, Hitzert MM, Sauer PJJ, Bos AF. Prenatal exposure to polychlorinated biphenyls and their hydroxylated metabolites is associated with motor development of three-month-old infants. Neurotoxicology. 2013;38:124–30. doi: 10.1016/j.neuro.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 199.Jusko TA, Sonneborn D, Palkovicova L, Kocan A, Drobna B, Trnovec T, et al. Pre- and postnatal polychlorinated biphenyl concentrations and longitudinal measures of thymus volume in infants. Environ Health Perspect. 2012;120(4):595–600. doi: 10.1289/ehp.1104229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Hernik A, Góralczyk K, Struciński P, Czaja K, Korcz W, Minorczyk M, et al. Polybrominated diphenyl ethers and polychlorinated biphenyls in cord blood from women in Poland. Chemosphere. 2013;93(3):526–31. doi: 10.1016/j.chemosphere.2013.06.045. [DOI] [PubMed] [Google Scholar]
- 201.Lopez-Espinosa M-J, Vizcaino E, Murcia M, Llop S, Espada M, Seco V, et al. Association between thyroid hormone levels and 4,4′-DDE concentrations in pregnant women (Valencia, Spain) Environ Res. 2009;109(4):479–85. doi: 10.1016/j.envres.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 202.Bergonzi R, Specchia C, Dinolfo M, Tomasi C, De Palma G, Frusca T, et al. Distribution of persistent organochlorine pollutants in maternal and foetal tissues: data from an Italian polluted urban area. Chemosphere. 2009;76(6):747–54. doi: 10.1016/j.chemosphere.2009.05.026. [DOI] [PubMed] [Google Scholar]
- 203.Veyhe AS, Hofoss D, Hansen S, Thomassen Y, Sandanger TM, Odland JØ, et al. The Northern Norway Mother-and-Child Contaminant Cohort (MISA) Study: PCA analyses of environmental contaminants in maternal sera and dietary intake in early pregnancy. Int J Hyg Environ Health. 2015;218(2):254–64. doi: 10.1016/j.ijheh.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 204.Long M, Knudsen A-KS, Pedersen HS, Bonefeld-Jørgensen EC. Food intake and serum persistent organic pollutants in the Greenlandic pregnant women: The ACCEPT sub-study. Sci Total Environ. 2015;529:198–212. doi: 10.1016/j.scitotenv.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 205.de Cock M, de Boer MR, Lamoree M, Legler J, van de Bor M. Prenatal exposure to endocrine disrupting chemicals in relation to thyroid hormone levels in infants - a Dutch prospective cohort study. Environ Health. 2014;13:106. doi: 10.1186/1476-069X-13-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Adlard B, Davis K, Liang CL, Curren SM, Rodríguez-Dozal S, Riojas-Rodríguez H, et al. Persistent organic pollutants (POPs) and metals in primiparous women: a comparison from Canada and Mexico. Sci Total Environ. 2014;500–501:302–13. doi: 10.1016/j.scitotenv.2014.08.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Forde MS, Dewailly E, Robertson L, Laouan Sidi EA, Côté S, Dumas P, et al. Prenatal exposure to persistent organic pollutants and polybrominated diphenyl ethers in 10 Caribbean countries. Environ Res. 2014;133:211–9. doi: 10.1016/j.envres.2014.05.021. [DOI] [PubMed] [Google Scholar]
- 208.Donauer S, Chen A, Xu Y, Calafat AM, Sjodin A, Yolton K. Prenatal exposure to polybrominated diphenyl ethers and polyfluoroalkyl chemicals and infant neurobehavior. J Pediatr. 2015;166(3):736–42. doi: 10.1016/j.jpeds.2014.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Apelberg BJ, Witter FR, Herbstman JB, Calafat AM, Halden RU, Needham LL, et al. Cord serum concentrations of perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) in relation to weight and size at birth. Environ Health Perspect. 2007;115(11):1670–6. doi: 10.1289/ehp.10334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Hamm MP, Cherry NM, Chan E, Martin JW, Burstyn I. Maternal exposure to perfluorinated acids and fetal growth. J Expo Sci Environ Epidemiol. 2010;20(7):589–97. doi: 10.1038/jes.2009.57. [DOI] [PubMed] [Google Scholar]
- 211.Arbuckle TE, Kubwabo C, Walker M, Davis K, Lalonde K, Kosarac I, et al. Umbilical cord blood levels of perfluoroalkyl acids and polybrominated flame retardants. Int J Hyg Environ Health. 2013;216(2):184–94. doi: 10.1016/j.ijheh.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 212.Vélez MP, Arbuckle TE, Fraser WD. Female exposure to phenols and phthalates and time to pregnancy: the Maternal-Infant Research on Environmental Chemicals (MIREC) Study. Fertil Steril. 2015;103(4):1011–20. e2. doi: 10.1016/j.fertnstert.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 213.Maisonet M, Terrell ML, McGeehin MA, Christensen KY, Holmes A, Calafat AM, et al. Maternal concentrations of polyfluoroalkyl compounds during pregnancy and fetal and postnatal growth in British girls. Environ Health Perspect. 2012;120(10):1432–7. doi: 10.1289/ehp.1003096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Chen M-H, Ha E-H, Wen T-W, Su Y-N, Lien G-W, Chen C-Y, et al. Perfluorinated compounds in umbilical cord blood and adverse birth outcomes. PLoS One. 2012;7(8):e42474. doi: 10.1371/journal.pone.0042474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Wu K, Xu X, Peng L, Liu J, Guo Y, Huo X. Association between maternal exposure to perfluorooctanoic acid (PFOA) from electronic waste recycling and neonatal health outcomes. Environ Int. 2012;48:1–8. doi: 10.1016/j.envint.2012.06.018. [DOI] [PubMed] [Google Scholar]
- 216.Castorina R, Bradman A, Sjödin A, Fenster L, Jones RS, Harley KG, et al. Determinants of serum polybrominated diphenyl ether (PBDE) levels among pregnant women in the CHAMACOS cohort. Environ Sci Technol. 2011;45(15):6553–60. doi: 10.1021/es104295m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Mazdai A, Dodder NG, Abernathy MP, Hites RA, Bigsby RM. Polybrominated diphenyl ethers in maternal and fetal blood samples. Environ Health Perspect. 2003;111(9):1249–52. doi: 10.1289/ehp.6146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Foster WG, Gregorovich S, Morrison KM, Atkinson SA, Kubwabo C, Stewart B, et al. Human maternal and umbilical cord blood concentrations of polybrominated diphenyl ethers. Chemosphere. 2011;84(10):1301–9. doi: 10.1016/j.chemosphere.2011.05.028. [DOI] [PubMed] [Google Scholar]
- 219.Stapleton HM, Eagle S, Anthopolos R, Wolkin A, Miranda ML. Associations between polybrominated diphenyl ether (PBDE) flame retardants, phenolic metabolites, and thyroid hormones during pregnancy. Environ Health Perspect. 2011;119(10):1454–9. doi: 10.1289/ehp.1003235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Horton MK, Bousleiman S, Jones R, Sjodin A, Liu X, Whyatt R, et al. Predictors of serum concentrations of polybrominated flame retardants among healthy pregnant women in an urban environment: a cross-sectional study. Environ Health. 2013;12:23. doi: 10.1186/1476-069X-12-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Chen A, Park J-S, Linderholm L, Rhee A, Petreas M, DeFranco EA, et al. Hydroxylated polybrominated diphenyl ethers in paired maternal and cord sera. Environ Sci Technol. 2013;47(8):3902–8. doi: 10.1021/es3046839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Guvenius DM, Aronsson A, Ekman-Ordeberg G, Bergman A, Norén K. Human prenatal and postnatal exposure to polybrominated diphenyl ethers, polychlorinated biphenyls, polychlorobiphenylols, and pentachlorophenol. Environ Health Perspect. 2003;111(9):1235–41. doi: 10.1289/ehp.5946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Vizcaino E, Grimalt JO, Lopez-Espinosa M-J, Llop S, Rebagliato M, Ballester F. Polybromodiphenyl ethers in mothers and their newborns from a non-occupationally exposed population (Valencia, Spain) Environ Int. 2011;37(1):152–7. doi: 10.1016/j.envint.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 224.Lopez-Espinosa M-J, Costa O, Vizcaino E, Murcia M, Fernandez-Somoano A, Iñiguez C, et al. Prenatal Exposure to Polybrominated Flame Retardants and Fetal Growth in the INMA Cohort (Spain) Environ Sci Technol. 2015;49(16):10108–16. doi: 10.1021/acs.est.5b01793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Antignac J-P, Cariou R, Zalko D, Berrebi A, Cravedi J-P, Maume D, et al. Exposure assessment of French women and their newborn to brominated flame retardants: determination of tri- to deca- polybromodiphenylethers (PBDE) in maternal adipose tissue, serum, breast milk and cord serum. Environ Pollut. 2009;157(1):164–73. doi: 10.1016/j.envpol.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 226.Jakobsson K, Fång J, Athanasiadou M, Rignell-Hydbom A, Bergman A. Polybrominated diphenyl ethers in maternal serum, umbilical cord serum, colostrum and mature breast milk. Insights from a pilot study and the literature. Environ Int. 2012;47:121–30. doi: 10.1016/j.envint.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 227.Grimalt JO, Howsam M, Carrizo D, Otero R, de Marchi MRR, Vizcaino E. Integrated analysis of halogenated organic pollutants in sub-millilitre volumes of venous and umbilical cord blood sera. Anal Bioanal Chem. 2010;396(6):2265–72. doi: 10.1007/s00216-010-3460-y. [DOI] [PubMed] [Google Scholar]
- 228.Frederiksen M, Vorkamp K, Mathiesen L, Mose T, Knudsen LE. Placental transfer of the polybrominated diphenyl ethers BDE-47, BDE-99 and BDE-209 in a human placenta perfusion system: an experimental study. Environ Health. 2010;9:32. doi: 10.1186/1476-069X-9-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Xu X, Yekeen TA, Xiao Q, Wang Y, Lu F, Huo X. Placental IGF-1 and IGFBP-3 expression correlate with umbilical cord blood PAH and PBDE levels from prenatal exposure to electronic waste. Environ Pollut. 2013;182:63–9. doi: 10.1016/j.envpol.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 230.Kim TH, Lee YJ, Lee E, Patra N, Lee J, Kwack SJ, et al. Exposure assessment of polybrominated diphenyl ethers (PBDE) in umbilical cord blood of Korean infants. J Toxicol Environ Health A. 2009;72(21–22):1318–26. doi: 10.1080/15287390903212436. [DOI] [PubMed] [Google Scholar]
- 231.Shy C-G, Huang H-L, Chang-Chien G-P, Chao H-R, Tsou T-C. Neurodevelopment of infants with prenatal exposure to polybrominated diphenyl ethers. Bull Environ Contam Toxicol. 2011;87(6):643–8. doi: 10.1007/s00128-011-0422-9. [DOI] [PubMed] [Google Scholar]
- 232.Kim TH, Bang DY, Lim HJ, Won AJ, Ahn MY, Patra N, et al. Comparisons of polybrominated diphenyl ethers levels in paired South Korean cord blood, maternal blood, and breast milk samples. Chemosphere. 2012;87(1):97–104. doi: 10.1016/j.chemosphere.2011.11.074. [DOI] [PubMed] [Google Scholar]
- 233.Kim U-J, Lee I-S, Kim HS, Oh J-E. Monitoring of PBDEs concentration in umbilical cord blood and breast milk from Korean population and estimating the effects of various parameters on accumulation in humans. Chemosphere. 2011;85(3):487–93. doi: 10.1016/j.chemosphere.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 234.Stasinska A, Heyworth J, Reid A, Callan A, Odland JØ, Trong Duong P, et al. Polybrominated diphenyl ether (PBDE) concentrations in plasma of pregnant women from Western Australia. Sci Total Environ. 2014;493:554–61. doi: 10.1016/j.scitotenv.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 235.Ding G, Yu J, Cui C, Chen L, Gao Y, Wang C, et al. Association between prenatal exposure to polybrominated diphenyl ethers and young children’s neurodevelopment in China. Environ Res. 2015;142:104–11. doi: 10.1016/j.envres.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 236.Choi G, Kim S, Kim S, Kim S, Choi Y, Kim H-J, et al. Occurrences of major polybrominated diphenyl ethers (PBDEs) in maternal and fetal cord blood sera in Korea. Sci Total Environ. 2014;491–492:219–26. doi: 10.1016/j.scitotenv.2014.02.071. [DOI] [PubMed] [Google Scholar]
- 237.Chen Z-J, Liu H-Y, Cheng Z, Man Y-B, Zhang K-S, Wei W, et al. Polybrominated diphenyl ethers (PBDEs) in human samples of mother-newborn pairs in South China and their placental transfer characteristics. Environ Int. 2014;73:77–84. doi: 10.1016/j.envint.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 238.Xu L, Huo X, Zhang Y, Li W, Zhang J, Xu X. Polybrominated diphenyl ethers in human placenta associated with neonatal physiological development at a typical e-waste recycling area in China. Environ Pollut. 2015;196:414–22. doi: 10.1016/j.envpol.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 239.Wolff MS, Engel SM, Berkowitz GS, Ye X, Silva MJ, Zhu C, et al. Prenatal phenol and phthalate exposures and birth outcomes. Environ Health Perspect. 2008;116(8):1092–7. doi: 10.1289/ehp.11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Donohue KM, Miller RL, Perzanowski MS, Just AC, Hoepner LA, Arunajadai S, et al. Prenatal and postnatal bisphenol A exposure and asthma development among inner-city children. J Allergy Clin Immunol. 2013;131(3):736–42. doi: 10.1016/j.jaci.2012.12.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Padmanabhan V, Siefert K, Ransom S, Johnson T, Pinkerton J, Anderson L, et al. Maternal bisphenol-A levels at delivery: a looming problem? J Perinatol. 2008;28(4):258–63. doi: 10.1038/sj.jp.7211913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Pycke BFG, Geer LA, Dalloul M, Abulafia O, Jenck AM, Halden RU. Human fetal exposure to triclosan and triclocarban in an urban population from Brooklyn, New York. Environ Sci Technol. 2014;48(15):8831–8. doi: 10.1021/es501100w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Arbuckle TE, Davis K, Marro L, Fisher M, Legrand M, LeBlanc A, et al. Phthalate and bisphenol A exposure among pregnant women in Canada--results from the MIREC study. Environ Int. 2014;68:55–65. doi: 10.1016/j.envint.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 244.Arbuckle TE, Weiss L, Fisher M, Hauser R, Dumas P, Bérubé R, et al. Maternal and infant exposure to environmental phenols as measured in multiple biological matrices. Sci Total Environ. 2015;508:575–84. doi: 10.1016/j.scitotenv.2014.10.107. [DOI] [PubMed] [Google Scholar]
- 245.Mortensen ME, Calafat AM, Ye X, Wong L-Y, Wright DJ, Pirkle JL, et al. Urinary concentrations of environmental phenols in pregnant women in a pilot study of the National Children’s Study. Environ Res. 2014;129:32–8. doi: 10.1016/j.envres.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Meeker JD, Cantonwine DE, Rivera-González LO, Ferguson KK, Mukherjee B, Calafat AM, et al. Distribution, variability, and predictors of urinary concentrations of phenols and parabens among pregnant women in Puerto Rico. Environ Sci Technol. 2013;47(7):3439–47. doi: 10.1021/es400510g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Philippat C, Botton J, Calafat AM, Ye X, Charles M-A, Slama R. Prenatal exposure to phenols and growth in boys. Epidemiology. 2014;25(5):625–35. doi: 10.1097/EDE.0000000000000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Casas L, Fernández MF, Llop S, Guxens M, Ballester F, Olea N, et al. Urinary concentrations of phthalates and phenols in a population of Spanish pregnant women and children. Environ Int. 2011;37(5):858–66. doi: 10.1016/j.envint.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 249.Myridakis A, Fthenou E, Balaska E, Vakinti M, Kogevinas M, Stephanou EG. Phthalate esters, parabens and bisphenol-A exposure among mothers and their children in Greece (Rhea cohort) Environ Int. 2015;83:1–10. doi: 10.1016/j.envint.2015.05.014. [DOI] [PubMed] [Google Scholar]
- 250.Tefre de Renzy-Martin K, Frederiksen H, Christensen JS, Boye Kyhl H, Andersson A-M, Husby S, et al. Current exposure of 200 pregnant Danish women to phthalates, parabens and phenols. Reproduction. 2014;147(4):443–53. doi: 10.1530/REP-13-0461. [DOI] [PubMed] [Google Scholar]
- 251.Cantonwine D, Meeker JD, Hu H, Sánchez BN, Lamadrid-Figueroa H, Mercado-García A, et al. Bisphenol a exposure in Mexico City and risk of prematurity: a pilot nested case control study. Environ Health. 2010;9:62. doi: 10.1186/1476-069X-9-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Lee B-E, Park H, Hong Y-C, Ha M, Kim Y, Chang N, et al. Prenatal bisphenol A and birth outcomes: MOCEH (Mothers and Children’s Environmental Health) study. Int J Hyg Environ Health. 2014;217(2–3):328–34. doi: 10.1016/j.ijheh.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 253.Callan AC, Hinwood AL, Heffernan A, Eaglesham G, Mueller J, Odland JØ. Urinary bisphenol A concentrations in pregnant women. Int J Hyg Environ Health. 2013;216(6):641–4. doi: 10.1016/j.ijheh.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 254.Tang R, Chen M-J, Ding G-D, Chen X-J, Han X-M, Zhou K, et al. Associations of prenatal exposure to phenols with birth outcomes. Environ Pollut. 2013;178:115–20. doi: 10.1016/j.envpol.2013.03.023. [DOI] [PubMed] [Google Scholar]
- 255.Braun JM, Just AC, Williams PL, Smith KW, Calafat AM, Hauser R. Personal care product use and urinary phthalate metabolite and paraben concentrations during pregnancy among women from a fertility clinic. J Expo Sci Environ Epidemiol. 2014;24(5):459–66. doi: 10.1038/jes.2013.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Miodovnik A, Engel SM, Zhu C, Ye X, Soorya LV, Silva MJ, et al. Endocrine disruptors and childhood social impairment. Neurotoxicology. 2011;32(2):261–7. doi: 10.1016/j.neuro.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Sathyanarayana S, Barrett E, Butts S, Wang C, Swan SH. Phthalate exposure and reproductive hormone concentrations in pregnancy. Reproduction. 2014;147(4):401–9. doi: 10.1530/REP-13-0415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Buckley JP, Palmieri RT, Matuszewski JM, Herring AH, Baird DD, Hartmann KE, et al. Consumer product exposures associated with urinary phthalate levels in pregnant women. J Expo Sci Environ Epidemiol. 2012;22(5):468–75. doi: 10.1038/jes.2012.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Ferguson KK, McElrath TF, Meeker JD. Environmental phthalate exposure and preterm birth. JAMA Pediatr. 2014;168(1):61–7. doi: 10.1001/jamapediatrics.2013.3699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Cantonwine DE, Cordero JF, Rivera-González LO, Anzalota Del Toro LV, Ferguson KK, Mukherjee B, et al. Urinary phthalate metabolite concentrations among pregnant women in Northern Puerto Rico: distribution, temporal variability, and predictors. Environ Int. 2014;62:1–11. doi: 10.1016/j.envint.2013.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Philippat C, Mortamais M, Chevrier C, Petit C, Calafat AM, Ye X, et al. Exposure to phthalates and phenols during pregnancy and offspring size at birth. Environ Health Perspect. 2012;120(3):464–70. doi: 10.1289/ehp.1103634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Casas M, Valvi D, Ballesteros-Gomez A, Gascon M, Fernández MF, Garcia-Esteban R, et al. Exposure to Bisphenol A and Phthalates during Pregnancy and Ultrasound Measures of Fetal Growth in the INMA-Sabadell Cohort. Environ Health Perspect. 2016;124(4):521–8. doi: 10.1289/ehp.1409190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Polanska K, Ligocka D, Sobala W, Hanke W. Phthalate exposure and child development: the Polish Mother and Child Cohort Study. Early Hum Dev. 2014;90(9):477–85. doi: 10.1016/j.earlhumdev.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 264.Cutanda F, Koch HM, Esteban M, Sánchez J, Angerer J, Castaño A. Urinary levels of eight phthalate metabolites and bisphenol A in mother-child pairs from two Spanish locations. Int J Hyg Environ Health. 2015;218(1):47–57. doi: 10.1016/j.ijheh.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 265.Téllez-Rojo MM, Cantoral A, Cantonwine DE, Schnaas L, Peterson K, Hu H, et al. Prenatal urinary phthalate metabolites levels and neurodevelopment in children at two and three years of age. Sci Total Environ. 2013;461–462:386–90. doi: 10.1016/j.scitotenv.2013.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Ku HY, Su PH, Wen HJ, Sun HL, Wang CJ, Chen HY, et al. Prenatal and postnatal exposure to phthalate esters and asthma: a 9-year follow-up study of a taiwanese birth cohort. PLoS One. 2015;10(4):e0123309. doi: 10.1371/journal.pone.0123309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Irvin EA, Calafat AM, Silva MJ, Aguilar-Villalobos M, Needham LL, Hall DB, et al. An estimate of phthalate exposure among pregnant women living in Trujillo, Peru. Chemosphere. 2010;80(11):1301–7. doi: 10.1016/j.chemosphere.2010.06.048. [DOI] [PubMed] [Google Scholar]
- 268.Suzuki Y, Niwa M, Yoshinaga J, Mizumoto Y, Serizawa S, Shiraishi H. Prenatal exposure to phthalate esters and PAHs and birth outcomes. Environ Int. 2010;36(7):699–704. doi: 10.1016/j.envint.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 269.Razzaghi H, Tinker SC, Crider K. Blood mercury concentrations in pregnant and nonpregnant women in the United States: National Health and Nutrition Examination Survey 1999–2006. Am J Obstet Gynecol. 2014;210(4):357e1–9. doi: 10.1016/j.ajog.2013.10.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Wells EM, Navas-Acien A, Herbstman JB, Apelberg BJ, Silbergeld EK, Caldwell KL, et al. Low-level lead exposure and elevations in blood pressure during pregnancy. Environ Health Perspect. 2011;119(5):664–9. doi: 10.1289/ehp.1002666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Geer LA, Persad MD, Palmer CD, Steuerwald AJ, Dalloul M, Abulafia O, et al. Assessment of prenatal mercury exposure in a predominately Caribbean immigrant community in Brooklyn, NY. J Environ Monit. 2012;14(3):1035–43. doi: 10.1039/c2em10835f. [DOI] [PubMed] [Google Scholar]
- 272.Ashley-Martin J, Dodds L, Arbuckle TE, Ettinger AS, Shapiro GD, Fisher M, et al. Maternal blood metal levels and fetal markers of metabolic function. Environ Res. 2015;136:27–34. doi: 10.1016/j.envres.2014.10.024. [DOI] [PubMed] [Google Scholar]
- 273.Sanders AP, Flood K, Chiang S, Herring AH, Wolf L, Fry RC. Towards prenatal biomonitoring in North Carolina: assessing arsenic, cadmium, mercury, and lead levels in pregnant women. PLoS One. 2012;7(3):e31354. doi: 10.1371/journal.pone.0031354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Rabito FA, Kocak M, Werthmann DW, Tylavsky FA, Palmer CD, Parsons PJ. Changes in low levels of lead over the course of pregnancy and the association with birth outcomes. Reprod Toxicol. 2014;50:138–44. doi: 10.1016/j.reprotox.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 275.Soon R, Dye TD, Ralston NV, Berry MJ, Sauvage LM. Seafood consumption and umbilical cord blood mercury concentrations in a multiethnic maternal and child health cohort. BMC Pregnancy Childbirth. 2014;14(1):209. doi: 10.1186/1471-2393-14-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Kahn LG, Liu X, Rajovic B, Popovac D, Oberfield S, Graziano JH, et al. Blood lead concentration and thyroid function during pregnancy: results from the Yugoslavia Prospective Study of Environmental Lead Exposure. Environ Health Perspect. 2014;122(10):1134–40. doi: 10.1289/ehp.1307669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Neugebauer J, Wittsiepe J, Kasper-Sonnenberg M, Schöneck N, Schölmerich A, Wilhelm M. The influence of low level pre- and perinatal exposure to PCDD/Fs, PCBs, and lead on attention performance and attention-related behavior among German school-aged children: results from the Duisburg Birth Cohort Study. Int J Hyg Environ Health. 2015;218(1):153–62. doi: 10.1016/j.ijheh.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 278.Jedrychowski WA, Perera FP, Majewska R, Mrozek-Budzyn D, Mroz E, Roen EL, et al. Depressed height gain of children associated with intrauterine exposure to polycyclic aromatic hydrocarbons (PAH) and heavy metals: the cohort prospective study. Environ Res. 2015;136:141–7. doi: 10.1016/j.envres.2014.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Llop S, Aguinagalde X, Vioque J, Ibarluzea J, Guxens M, Casas M, et al. Prenatal exposure to lead in Spain: cord blood levels and associated factors. Sci Total Environ. 2011;409(11):2298–305. doi: 10.1016/j.scitotenv.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 280.Llop S, Lopez-Espinosa M-J, Murcia M, Alvarez-Pedrerol M, Vioque J, Aguinagalde X, et al. Synergism between exposure to mercury and use of iodine supplements on thyroid hormones in pregnant women. Environ Res. 2015;138:298–305. doi: 10.1016/j.envres.2015.02.026. [DOI] [PubMed] [Google Scholar]
- 281.Valent F, Mariuz M, Bin M, Little D, Mazej D, Tognin V, et al. Associations of prenatal mercury exposure from maternal fish consumption and polyunsaturated fatty acids with child neurodevelopment: a prospective cohort study in Italy. J Epidemiol. 2013;23(5):360–70. doi: 10.2188/jea.JE20120168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Polańska K, Hanke W, Sobala W, Trzcinka-Ochocka M, Ligocka D, Strugała-Stawik H, et al. Predictors of environmental lead exposure among pregnant women - a prospective cohort study in Poland. Ann Agric Environ Med. 2014;21(1):49–54. [PubMed] [Google Scholar]
- 283.Miklavčič A, Cuderman P, Mazej D, Snoj Tratnik J, Krsnik M, Planinšek P, et al. Biomarkers of low-level mercury exposure through fish consumption in pregnant and lactating Slovenian women. Environ Res. 2011;111(8):1201–7. doi: 10.1016/j.envres.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 284.Kozikowska I, Binkowski ŁJ, Szczepańska K, Sławska H, Miszczuk K, Œliwińska M, et al. Mercury concentrations in human placenta, umbilical cord, cord blood and amniotic fluid and their relations with body parameters of newborns. Environ Pollut. 2013;182:256–62. doi: 10.1016/j.envpol.2013.07.030. [DOI] [PubMed] [Google Scholar]
- 285.Kayaalti Z, Kaya-Akyüzlü D, Söylemez E, Söylemezoğlu T. Maternal hemochromatosis gene H63D single-nucleotide polymorphism and lead levels of placental tissue, maternal and umbilical cord blood. Environ Res. 2015;140:456–61. doi: 10.1016/j.envres.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 286.Ursinyova M, Uhnakova I, Serbin R, Masanova V, Husekova Z, Wsolova L. The relation between human exposure to mercury and thyroid hormone status. Biol Trace Elem Res. 2012;148(3):281–91. doi: 10.1007/s12011-012-9382-0. [DOI] [PubMed] [Google Scholar]
- 287.Zhang A, Hu H, Sánchez BN, Ettinger AS, Park SK, Cantonwine D, et al. Association between prenatal lead exposure and blood pressure in children. Environ Health Perspect. 2012;120(3):445–50. doi: 10.1289/ehp.1103736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Lin C-C, Chen Y-C, Su F-C, Lin C-M, Liao H-F, Hwang Y-H, et al. In utero exposure to environmental lead and manganese and neurodevelopment at 2 years of age. Environ Res. 2013;123:52–7. doi: 10.1016/j.envres.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 289.Al-Saleh I, Al-Rouqi R, Obsum CA, Shinwari N, Mashhour A, Billedo G, et al. Mercury (Hg) and oxidative stress status in healthy mothers and its effect on birth anthropometric measures. Int J Hyg Environ Health. 217(4–5):567–85. doi: 10.1016/j.ijheh.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 290.Jin L, Liu J, Ye B, Ren A. Concentrations of selected heavy metals in maternal blood and associated factors in rural areas in Shanxi Province, China. Environ Int. 2014;66:157–64. doi: 10.1016/j.envint.2014.01.016. [DOI] [PubMed] [Google Scholar]
- 291.Hong Y-C, Kulkarni SS, Lim Y-H, Kim E, Ha M, Park H, et al. Postnatal growth following prenatal lead exposure and calcium intake. Pediatrics. 2014;134(6):1151–9. doi: 10.1542/peds.2014-1658. [DOI] [PubMed] [Google Scholar]
- 292.Kim Y, Ha E-H, Park H, Ha M, Kim Y, Hong Y-C, et al. Prenatal lead and cadmium co-exposure and infant neurodevelopment at 6 months of age: the Mothers and Children’s Environmental Health (MOCEH) study. Neurotoxicology. 2013;35:15–22. doi: 10.1016/j.neuro.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 293.Vigeh M, Yokoyama K, Matsukawa T, Shinohara A, Ohtani K. Low level prenatal blood lead adversely affects early childhood mental development. J Child Neurol. 2014;29(10):1305–11. doi: 10.1177/0883073813516999. [DOI] [PubMed] [Google Scholar]
- 294.Jiang Y, Wang H, Chen J, Zhang G, Chen L, Dai W, et al. Blood lead levels during different trimesters of pregnancy and the possible influencing factors in Chengdu, China. Biol Trace Elem Res. 2011;144(1–3):27–35. doi: 10.1007/s12011-011-9020-2. [DOI] [PubMed] [Google Scholar]
- 295.La-Llave-León O, Lugo-Soto R, Aguilar-Durán M, Estrada-Martínez S, Salas-Pacheco J-M, Sandoval-Carrillo A, et al. Relationship between blood lead levels and hematological indices in pregnant women. Women Health. 2015;55(1):90–102. doi: 10.1080/03630242.2014.972019. [DOI] [PubMed] [Google Scholar]
- 296.Wu M, Yan C, Xu J, Wu W, Li H, Zhou X. Umbilical cord blood mercury levels in China. J Environ Sci (China) 2013;25(2):386–92. doi: 10.1016/s1001-0742(12)60061-8. [DOI] [PubMed] [Google Scholar]
- 297.Channa K, Odland JØ, Kootbodien T, Theodorou P, Naik I, Sandanger TM, et al. Differences in prenatal exposure to mercury in South African communities residing along the Indian Ocean. Sci Total Environ. 2013;463–464:11–9. doi: 10.1016/j.scitotenv.2013.05.055. [DOI] [PubMed] [Google Scholar]
- 298.Forde MS, Dewailly E, Robertson L, Laouan Sidi EA, Côté S, Sandy L, et al. Mercury and lead blood concentrations in pregnant women from 10 caribbean countries. Environ Sci Process Impacts. 2014;16(9):2184–90. doi: 10.1039/c4em00239c. [DOI] [PubMed] [Google Scholar]
- 299.Hinwood AL, Callan AC, Ramalingam M, Boyce M, Heyworth J, McCafferty P, et al. Cadmium, lead and mercury exposure in non smoking pregnant women. Environ Res. 2013;126:118–24. doi: 10.1016/j.envres.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 300.Guo B-Q, Cai S-Z, Guo J-L, Xu J, Wu W, Li H, et al. Levels of prenatal mercury exposure and their relationships to neonatal anthropometry in Wujiang City, China. Environ Pollut. 2013;182:184–9. doi: 10.1016/j.envpol.2013.07.023. [DOI] [PubMed] [Google Scholar]
- 301.Kazi TG, Shah F, Shaikh HR, Afridi HI, Shah A, Naeemullah, et al. Exposure of lead to mothers and their new born infants, residents of industrial and domestic areas of Pakistan. Environ Sci Pollut Res Int. 2014;21(4):3021–30. doi: 10.1007/s11356-013-2223-7. [DOI] [PubMed] [Google Scholar]
- 302.Ding G, Cui C, Chen L, Gao Y, Zhou Y, Shi R, et al. Prenatal low-level mercury exposure and neonatal anthropometry in rural northern China. Chemosphere. 2013;92(9):1085–9. doi: 10.1016/j.chemosphere.2013.01.045. [DOI] [PubMed] [Google Scholar]
- 303.Xie X, Ding G, Cui C, Chen L, Gao Y, Zhou Y, et al. The effects of low-level prenatal lead exposure on birth outcomes. Environ Pollut. 2013;175:30–4. doi: 10.1016/j.envpol.2012.12.013. [DOI] [PubMed] [Google Scholar]
- 304.Ou L, Chen C, Chen L, Wang H, Yang T, Xie H, et al. Low-level prenatal mercury exposure in north China: an exploratory study of anthropometric effects. Environ Sci Technol. 2015;49(11):6899–908. doi: 10.1021/es5055868. [DOI] [PubMed] [Google Scholar]
- 305.Obi E, Orisakwe OE, Okafor C, Igwebe A, Ebenebe J, Afonne OJ, et al. Towards prenatal biomonitoring in eastern Nigeria: assessing lead levels and anthropometric parameters of newborns. J UOEH. 2014;36(3):159–70. doi: 10.7888/juoeh.36.159. [DOI] [PubMed] [Google Scholar]
- 306.Shen W, Zhang B, Liu S, Wu H, Gu X, Qin L, et al. Association of blood lead levels with methylenetetrahydrofolate reductase polymorphisms among Chinese pregnant women in Wuhan city. PLoS One. 2015;10(2):e0117366. doi: 10.1371/journal.pone.0117366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Reddy YS, YA, Ramalaksmi BA, Kumar BD. Lead and trace element levels in placenta, maternal and cord blood: a cross-sectional pilot study. J Obstet Gynaecol Res. 2014;40(12):2184–90. doi: 10.1111/jog.12469. [DOI] [PubMed] [Google Scholar]
- 308.Rahman A, Al-Rashidi HAG, Khan A-R. Association of maternal blood lead level during pregnancy with child blood lead level and pregnancy outcome in Kuwait. Ecol Food Nutr. 2012;51(1):40–57. doi: 10.1080/03670244.2012.635571. [DOI] [PubMed] [Google Scholar]
- 309.Ugwuja EI, Ibiam UA, Ejikeme BN, Obuna JA, Agbafor KN. Blood Pb Levels in pregnant Nigerian women in Abakaliki, South-Eastern Nigeria. Environ Monit Assess. 2013;185(5):3795–801. doi: 10.1007/s10661-012-2828-1. [DOI] [PubMed] [Google Scholar]