Abstract
The regeneration of the 11-cis-retinyl imine chromophore of rhodopsin during the visual cycle and mechanisms that control this process are central questions in the field of vision research. The retinal pigment epithelium (RPE)-specific protein RPE65 is centrally involved in the isomerization and hydrolysis of alltrans-retinyl esters. In this study, we investigated RPE65 cleavage and potential regulatory mechanisms under oxidative stress conditions. The D407 RPE cell cultures were exposed to H2O2 (100–1000 µM). Changes in the levels of RPE65 and proteins related to apoptosis were investigated using gel electrophoresis and western blotting. Mass spectrometry was used to confirm the identity of RPE65. C57BL/6J (M450) and C3HeB/FeJ (L450) mice were used for in vivo experiments. We found that a novel 45 kDa truncated fragment of the RPE65 protein, designated RPE45, appears in RPE cells upon light exposure or oxidative stress. RPE45 is generated in vitro by recombinant caspases via an ubiquitination-dependent mechanism. Collectively, our results indicate that oxidative stress during the visual cycle results in cleavage of RPE65.
Keywords: Visual cycle, RPE65, Retinal pigment epithelium, Oxidative stress, Light
1. Introduction
The biochemical resynthesis of the 11-cis-retinyl imine chromophore of rhodopsin and controlling mechanisms of this process are still central questions in visual signaling [1–4]. Light-induced cis-trans retinoid photoisomerization activates the G-protein-coupled receptor pathway, converting light energy into an electrical signal by hyperpolarization of photoreceptor cells. For continued vertebrate vision, 11-cis-retinyl Schiff base must be regenerated in the retinal pigment epithelium (RPE) and photoreceptor cells by specific enzymes that include retinol dehydrogenases (RDHs), lecithin retinol acyltransferase (LRAT) and RPE65 [3,4]. Steady-state of retinoid concentrations and photosensitivity are controlled by the biochemical reactions that lead to 11-cis-retinal regeneration, known as the visual cycle.
Active functioning of the visual cycle seems to be involved in light-induced retinal degeneration in a mouse model [5]. Light damage only occurs when the retina is supplied with 11-cis-retinal, whereas inhibition of retinoid regeneration causes resistance to light damage [6]. RPE65 L450M mutant mice, which exhibit slow rhodopsin regeneration, are more resistant to light damage than are wild-type mice, indicating that induction of light damage requires continuous isomerization/hydrolysis of all-trans retinyl ester and consecutive bleaching of rhodopsin [7]. The RPE is susceptible to oxidative stress due to its high oxygen consumption, the generation of reactive oxygen species (ROS), the presence of a high percentage of unsaturated fatty acids, and exposure to light [8,9]. Bright light may generate free radicals and increase ROS production, which further induces oxidative stress in RPE cells.
Although the overall pathway of retinoid resynthesis in the eye is generally well characterized, the mechanisms that regulate it under oxidative stress conditions remain elusive. For example, it is unknown how light and oxygen imbalances influence proteins related to retinoid metabolism in the RPE [10,11]. Recently, we found that levels of RPE65 and RPE45, as truncated form of RPE65, were increased after short-term exposure to intense light in the human RPE cultures [12]. In the present study, we report that the level of RPE45 is present in cells exposed to high oxidative stress in vitro and in vivo. We further suggest that the RPE45 fragment may be generated via an ubiquitination mechanism involving interaction of specific proteases with RPE65.
2. Methods
2.1. D407 RPE cell cultures and exposure to H2O2
D407 cells were maintained in DMEM supplemented with 10% FBS and 2 mM glutamine at 37 °C in a humidified 5% CO2 atmosphere. After reaching 75–80% confluence, cells grown in a 6-well tissue culture plate were treated with 200 µM H2O2 and the control was treated with PBS for 1 h. Medium containing H2O2 was removed and cells were maintained in conditioning media for 6 h.
2.2. In vivo animal model
Detailed information was described previously in Ref. [12]. Female C57BL/6J (M450) and C3HeB/FeJ (L450) mice were purchased from Jackson Laboratory (Bar Harbor, ME) at 4–5 weeks of age and acclimatized in our laboratory. Mice, housed four per cage, were provided food and water ad libitum at a constant temperature with a 12/12 h light/dark regimen (300 l× at cage level). At 15 weeks of age, mice were euthanized every 4 h over a 24 h period, and left and right eye cups were harvested. Eye cup samples were snap frozen in liquid nitrogen and stored at −80 °C for molecular studies. Red lights were used during the dark periods to facilitate human activities. All animals were handled in compliance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
2.3. Bovine RPE cell cultures
Fresh bovine eyes were obtained from a local abattoir (Brown Packing Company, Gaffney, SC) immediately after excision from the animal. The post-mortem stability and procedures for preparing bovine RPE cells have been described in detail elsewhere [13,14]. Briefly, eyes were opened 5 mm posterior to the limbus, and vitreous and retina were removed. After washing with phosphate-buffered saline (PBS), eye cups were incubated in 0.25% trypsin in Dulbecco’s minimum essential medium (DMEM; Gibco, Grand Island, NY) for 60 min at 37 °C. RPE cells were collected under a dissecting microscope using a Pasteur pipette. After adding culture medium (DMEM/F12; Gibco, Grand Island, NY) containing 10% fetal bovine serum (FBS), cells were centrifuged and resuspended in culture medium, and plated into 6-well plates (Nunc). Second passage cells were used for experiments. Before exposure to light or H2O2, the medium was removed and cells were washed three times with serum-free DMEM. Cells were washed with ice-cold PBS and lysed with buffer containing 20 mM Tris–Cl (pH 7.4), 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100, 2.5 mM sodium pyrophosphate, 1 mM Na3 VO4, 1 mg/mL leupeptin and 1 mM phenylmethylsulfonyl fluoride, and collected by centrifugation.
2.4. Caspase treatment
Total bovine RPE extracts in assay buffer (25 mM HEPES, pH 7.5, 0.1% CHAPS, 10 mM dithiothreitol) were incubated with recombinant human caspases-1, -3, -6, -7 and -8 (R&D system) for 1 h at 37 °C. Proteins, extracted from RPE cells derived from fresh bovine eyes, were incubated with recombinant caspase-3 at concentrations of 0, 100, 300, 500 and 1000 ng/100 µl RPE. The caspase inhibitor ZAD, was used as a negative control.
2.5. 1D, 2D-SDS-PAGE, western blot, and mass spectrometry analysis
RPE65 degradation and ubiquitination were identified by western blotting as described previously using an anti-RPE65 peptide antibody (Genemed, San Antonio, TX), monoclonal anti-RPE65 protein antibody (Abcam, Cambridge, MA and Affinity BioReagents, Rockford, IL) and an anti-ubiquitination antibody (Abcam, Cambridge, MA) [15–17]. Protein concentration was semi-quantitatively determined by densitometric analysis of band intensity. Each experiment was repeated three times. Mass spectrometry analysis was described previously in Refs. [15–17].
3. Results
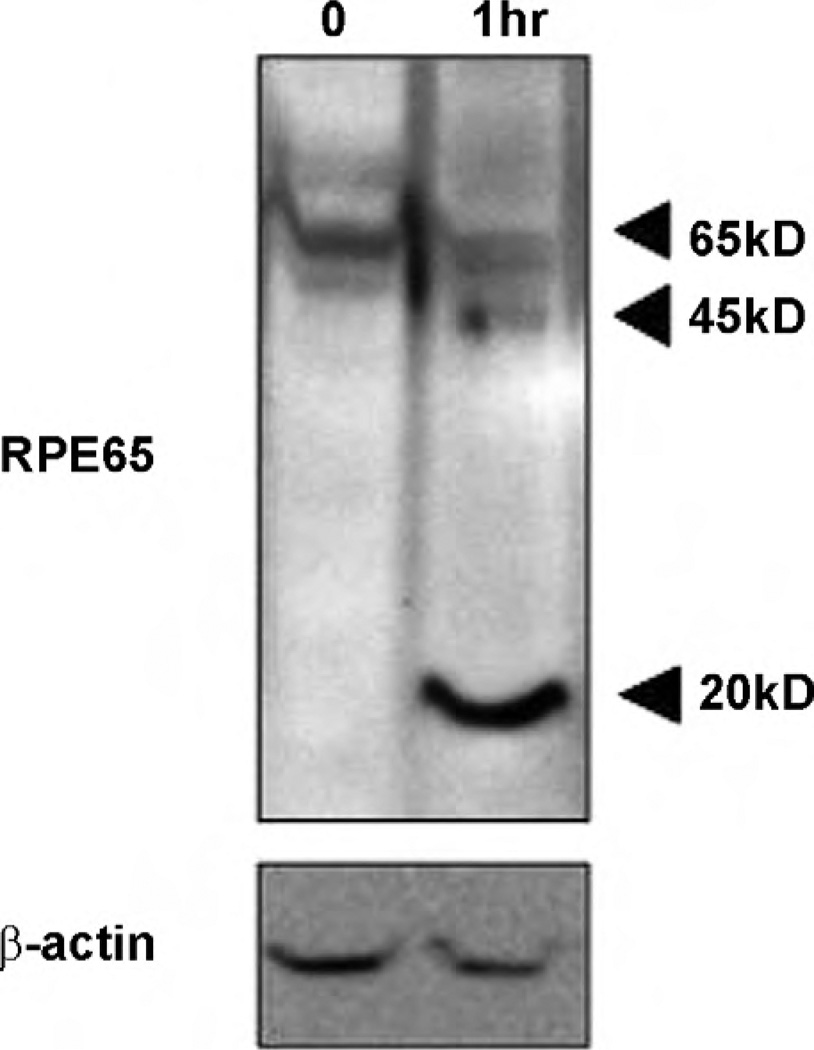
To determine whether oxidative stress could induce RPE65 truncation, we treated RPE D407 cells with H2O2 and analyzed whole-cell extracts by western blotting. As shown in Fig. 1, H2O2 induced an increase in the levels of two fragments of RPE65, one approximately 45 kDa (RPE45) and one of 20 kDa (RPE20). Similar results were obtained using early passage of human RPE cells and bovine RPE cells in an H2O2 dose-dependent manner. The identities of RPE65 and RPE45 were confirmed by mass spectrometry analysis using MALDI-TOF-TOF and the electrospray tandem method, respectively. The RPE45 band was identified as an N-terminal fragment of RPE65 based on the sequences of peptides corresponding to amino acids 15–33 (LFETVEELSSPLTAHVTGR [MH+] = 2087.3373) and 34–44 (IPLWLTGSLLR [MH+] = 1269.5730).
Fig. 1.
RPE45 and RPE20 under oxidative stress in vitro. After 1 h in oxidative stress, RPE65 was cleaved to 45 and 20 kDa fragments (Lane 2), compared to control (Lane 1). RPE D407 cells were treated with 200 µM H2O2 and the control was treated with PBS for 1 h. H2O2 was removed and then cells were maintained in conditioning media for another 6 h before harvest. Proteins were separated by SDS-PAGE (10 µg/Lane), then RPE65 was analyzed by western blot using monoclonal anti-RPE65 protein antibody. RPE cells were maintained in DMEM supplemented with 10% FBS and 2 mM glutamine at 37 °C and 5% CO2.
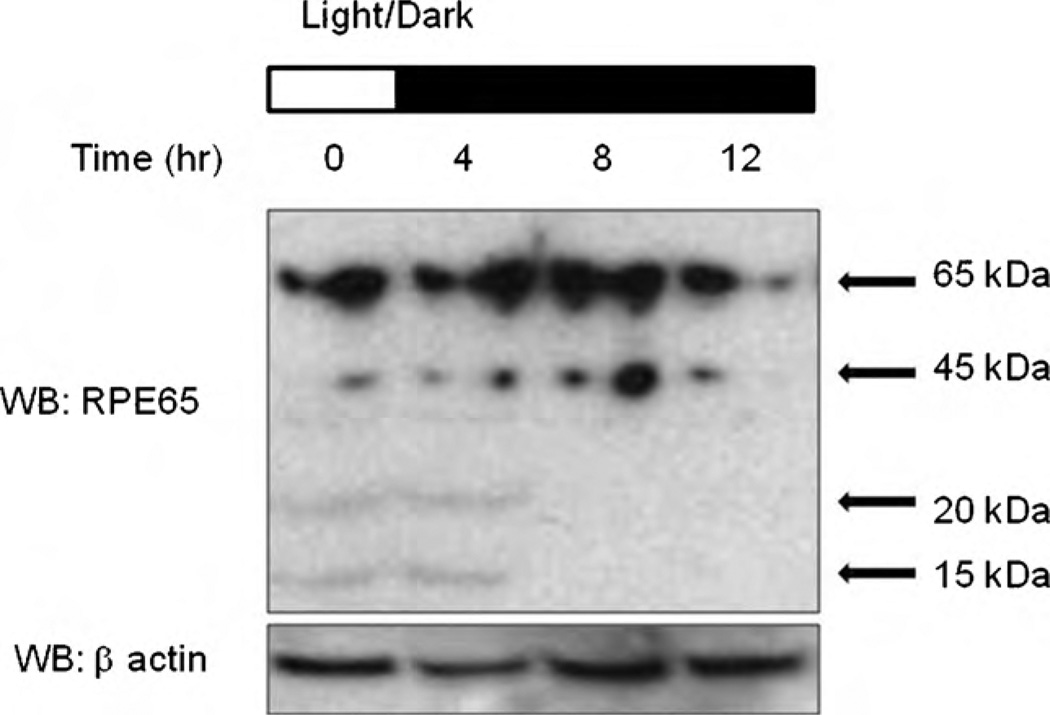
To investigate RPE65 regulation in vivo, RPE65 from mice subjected to normal 12 h light (300 l×) and 12 h dark cycles was accessed at six time points in a 24 h period. Western blot analysis revealed that RPE65 was cleaved to 45 kDa, and further to 20 and 15 kDa depending on certain time points (Fig. 2). Previous studies demonstrated that the visual cycle might be circadian coordinated as a means of effectively protecting the retina and the RPE from the detrimental effects of light-induced damage at the times of day when light is more intense [12].
Fig. 2.
Changes in RPE65 expression in a mouse model in vivo. C57BL/6J mice were exposed to cycles of 12 h light and 12 h dark. They were sacrificed over a period of 24 h at 4 h intervals. Eyes were enucleated, RPE/retinal cell proteins extracted and the levels of RPE65 determined by western blotting. Proteins were separated by SDS-PAGE and visualized by western blot using anti-RPE65 antibody.
We examined the appearance of RPE45 and RPE20 fragments in vitro with different amounts of H2O2 (100, 300, 500 and 1000 µM) in order to mimic different levels of oxidative stress on bovine RPE cells (Fig. 3). As expected, RPE45 and RPE20 were produced in a dose-dependent manner after H2O2 treatment.
Fig. 3.
Cleavage of RPE65 in vitro. 100–1000 µM of H2O2 cleaved RPE65 in vitro. Bovine RPE cells were treated with various amounts of H2O2 in 1 h. Proteins were separated by SDS-PAGE and RPE65 was visualized by western blotting using anti-RPE65 peptide antibody. RPE45 and 20 kDa bands appeared in a dose-dependent manner. 100 µMof H2O2 showed clear 45 and 20 kDa bands, whereas 300, 500 and 1000 µM showed two additional bands around 15 kDa.
3.1. Are RPE45 and RPE20 caspase-generated fragments of RPE65?
We speculated that RPE45 may result from degradation of RPE65 by ROS-induced proteases. It is likely that RPE cells have a defense mechanism against oxidative stress and we therefore investigated the expression of anti-apoptotic factors in oxidative stress. Previously, we found that the anti-apoptotic factor Bcl-XL, was increased in the light; moreover, c-Fos, another death signal factor, was up-regulated in the light also [12]. The positive correlation between the expression of these anti-apoptotic factors and RPE65 under oxidative stress suggests that RPE65 might be up-or down-stream regulator related to an apoptotic signaling pathway. To test our hypothesis that RPE65 participates in apoptosis in the RPE cells under oxidative stress, we investigated the role of caspases in RPE65 regulation.
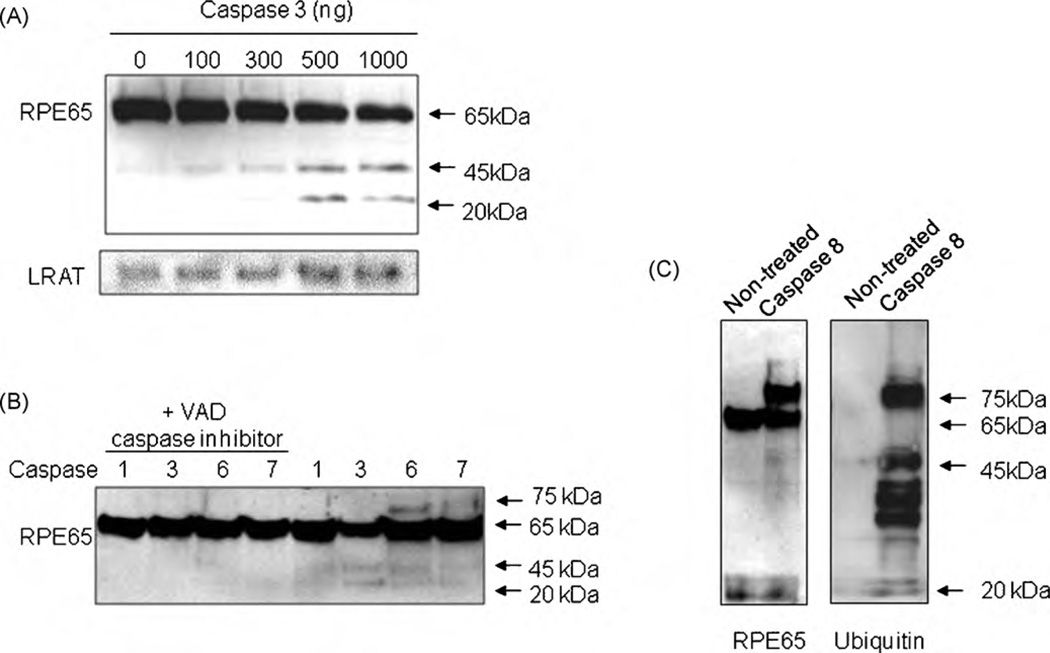
Proteins extracted from bovine RPE cells were treated with caspase-3 (10, 100, 500, 1000 ng) in vitro. In a manner similar to H2O2, caspase-3-induced the expression of RPE45 and RPE20 in a dose-dependent manner, suggesting that caspases may be activated when ROS levels are increased (Fig. 4A). To determine the specificity of caspase-3 on the proteolysis of RPE65, another essential visual cycle enzyme, lecithin retinol acyltransferase (LRAT) was visualized under the same conditions [15]. No caspase-3-induced proteolysis of LRAT was detected (Fig. 4A). Other caspases were also investigated to study substrate specificity and it was found that caspase-6 and -7 also induced the appearance of a protein of 45 kDa (Fig. 4B). Caspase-6 showed a 75 kDa band implying a putative ubiquitination (ubiquitin is ~8.5 kDa) mechanism possibly due to a slower truncation reaction. When caspase inhibitor (Fig. 4B, Lanes 1–4) was incubated with caspases, reactions were inhibited to imply that the truncation reaction of RPE65 is mediated by specific caspases. Western blot analysis was performed to determine whether the ubiquitin pathway participates in caspase-mediated proteolysis of RPE65. After caspase-8 treatment, a new 75 kDa band appeared along with RPE65 (Fig. 4B). This new protein was confirmed as Ub-RPE65 using an anti-ubiquitin antibody (Fig. 4C). Western blot with anti-RPE65 peptide antibody (left panel) showed that caspase-8 induced proteins at 75, 45 and 20 kDa. Western blot using anti-ubiquitin showed that RPE65 at 75 kDa is ubiquitinated.
Fig. 4.
Cleavage of RPE65 by caspases. (A) Cleavage of RPE65 by caspase-3 in vitro. RPE proteins from bovine RPE cells were incubated with various concentrations of recombinant caspase-3 (0, 100, 300, 500, 1000 ng/100 µl RPE). Caspase-3 cleaves RPE65 into specific 45 and 20 kDa fragments. Caspase-3 has no effect on LRAT. (B) Cleavage of RPE65 by caspases-1, -3, -6 and -7. RPE proteins were incubated with caspase-1, -3, -6 and -7 with (Lanes 1–4) or without (Lanes 5–8) caspase inhibitor carbobenzoxyvalyl-alanyl-aspartyl-[O-methyl]-fluoromethylketone (Z-VAD-FMK). Caspases-3 and -6 cleave RPE65 into specific 45 and 20 kDa fragments. Caspases with caspase inhibitor added did not cleave RPE65 (Lanes 1–4). (C) Ubiquitination and cleavage of RPE65 by caspase-8. RPE proteins were incubated with caspase-8 and separated by SDS-PAGE.
RPE65 has two caspase-specific fragmentation sites, DKED (AA215–218) and DKAD (AA375–378) that are conserved. Our results suggest that RPE45 produced after exposure to light or oxidative stress is the result of caspase-mediated fragmentation of RPE65 protein at the N-terminus as shown by mass spectrometry analysis.
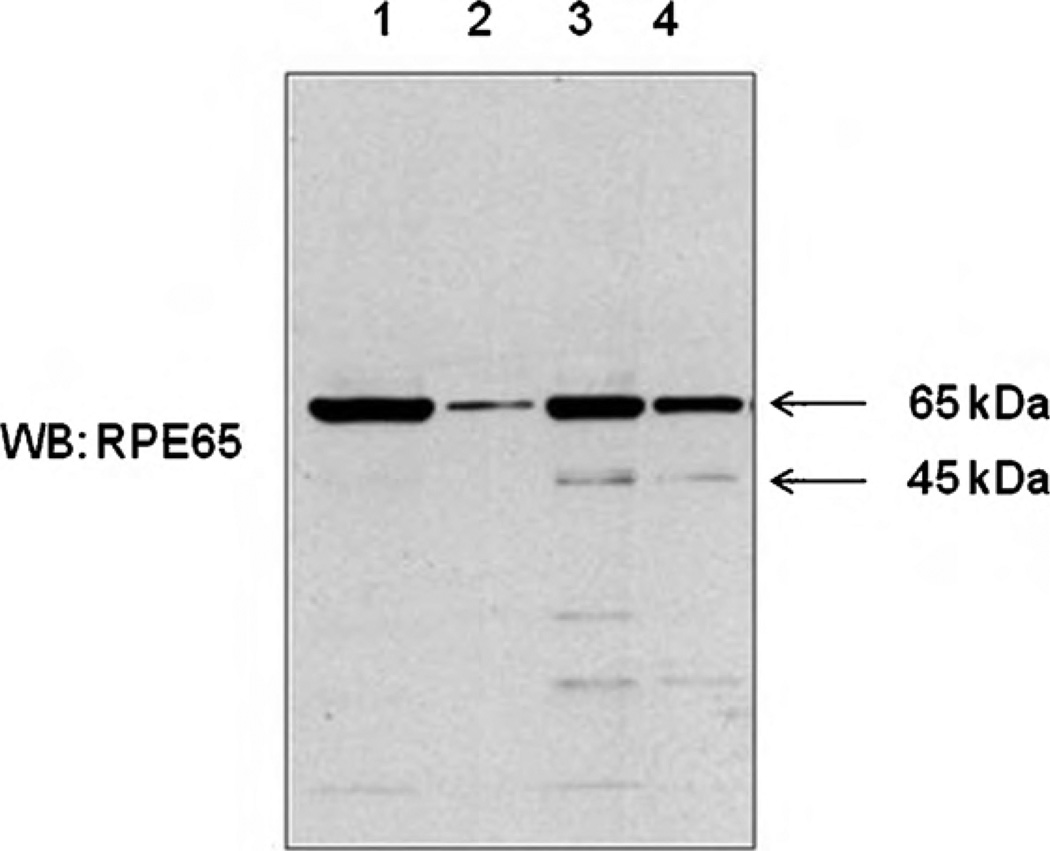
To investigate the function of RPE45 and post-translational modification, RPE65 was incubated at pH= 11 or 7.5 with hydroxylamine. In both cases, 40–60% of RPE65 lost their membrane association capability in 1 h of incubation at room temperature, and this process increased the concentration of RPE45 (Fig. 5). This implies RPE65 has a reversible modification cleaved by hydroxylamine or pH= 11, and this modification influences membrane association and cleavage of RPE65.
Fig. 5.
Membrane association of RPE65 and RPE45. Proteins from bovine RPE cells was incubated pH= 7.5 PBS buffer (Lanes 1 and 2) or hydroxylamine (Lanes 3 and 4). Samples were centrifuged (175,000 × g) and proteins were separated by SDS-PAGE and proteins were visualized by western blot using RPE65 antibody. Lane 1, pH= 7.5, pellet; Lane 2, pH= 7.5, supernatant; Lane 3, NH2OH, pellet and Lane 4, NH2OH, supernatant.
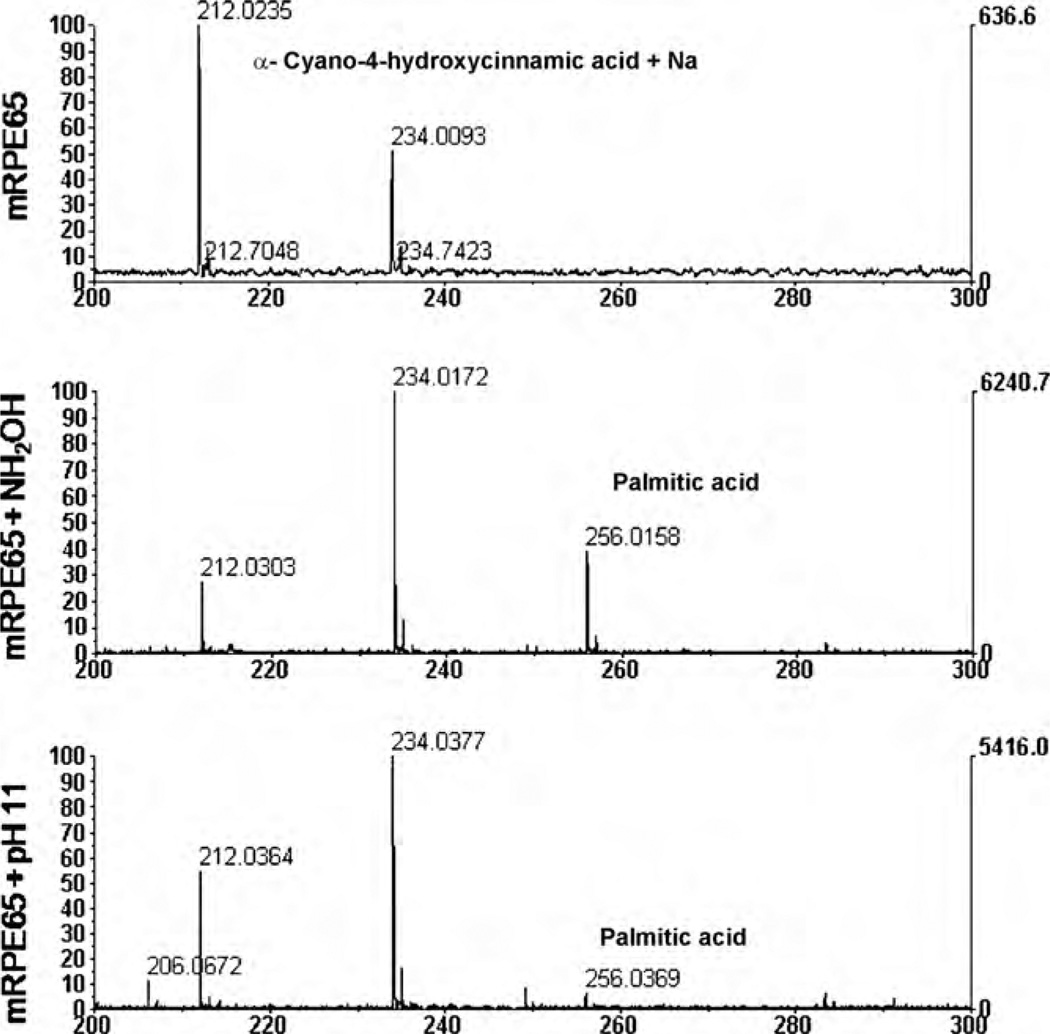
After basic or hydroxylamine incubation, RPE65 was analyzed by MALDI-TOF mass spectrometry to identify any reversible molecule(s) separated from RPE65 protein. As shown in Fig. 6, palmitic acid (MW= 256) was detected to confirm that membrane association is related to RPE65 palmitoylation, providing the affinity of RPE65 for the membrane.
Fig. 6.
Palmitoylation of RPE65. Microsomal membrane associated RPE65 (mRPE65) was analyzed by MALDI-TOF mass spectrometry after incubation with hydroxylamine or pH= 11. Palmitic acid (MW= 256) was detected in both hydroxylamine and pH= 11 incubation. Matrix peak is shown as MW= 212.
4. Discussion
Reactions in the visual cycle include major chemical conversions of retinoids, such as isomerization of cis-trans retinoid by rhodopsin and RPE65, oxidation/reduction by retinol dehydrogenases, and ester formation/hydrolysis by LRAT and RPE65. However, it is not known how light regulates retinoid flux in the RPE.
RPE65 is reported to be an all-trans-retinyl ester isomerohydrolase necessary for the synthesis of 11-cis-retinol [18–20], and is clearly important for 11-cis-retinal biosynthesis in vivo [21]. Ablation of RPE65 expression in a mouse knock-out model severely reduces 11-cis-retinal biosynthesis and results in the accumulation of all-trans-retinyl esters as oil droplets in the RPE, indicating that RPE65 may be involved in retinyl ester processing. Several mutations in the RPE65 gene have been shown to be associated with eye diseases, including autosomal recessive childhood-onset severe retinal dystrophy, Leber’s congenital amaurosis (LCA) and retinitis pigmentosa [22,23].
Here, we investigated the effects of oxidative stress on RPE65 regulation. The current study and the previous study show that treatment with H2O2 or light exposure promotes RPE65 cleavage in vitro [12]. H2O2 was used as an oxidative stress model in vitro considering relatively high concentration of H2O2 in the eye [8,9]. Light exposed in vitro and in vivo model already implied molecular mechanism that might be connected to oxidative stress with RPE65 turnover [12]. Changes in RPE65 expression were also seen in vivo during cycles of light and dark conditions, indicating a possible relationship between light cues and RPE65 regulation. The dose-dependent induction of RPE45, a fragment of RPE65 generated by caspase-mediated cleavage in response to H2O2 exposure, indicates that RPE45 is a potential biomarker of oxidative stress- and light-induced apoptosis. Stress-induced proteolysis is an important signal transduction pathway mediated by caspases, presenilin and amyloid precursor protein that can lead to neurodegenerative disorders and inflammation [24]. Interestingly, C57BL/6 mice have a gene polymorphism (M450) associated with lower level of RPE65 expression and reduced light damage sensitivity. We observed a higher threshold for RPE45 appearance when the pigment epithelium was exposed to light and oxidative stress simultaneously compared to that seen when the cells were exposed to oxidative stress alone, suggesting that a protective mechanism may exist that inhibits RPE65 protein degradation by anti-apoptotic molecules under light-induced oxidative stress.
Caspase-3, a common mediator of different apoptotic signaling pathways, also generates the same fragmentation pattern as H2O2, suggesting that both caspase-3 and H2O2 promote proteolysis of RPE65 at similar sites. Both mitochondria-mediated and cell death receptor-mediated apoptotic pathways converge on caspase-3. RPE65 is a highly uveitogenic and antigenic protein that may be among those involved in certain autoimmune diseases of the eye [25]. Thus, adequate turnover of this specific and abundant protein in the RPE would have a significant role in various pathologic contexts, including oxidative stress or intense light exposure. Although each retinoid reaction in the visual cycle has been studied in detail, the mechanisms that control this cycle have not been clearly defined. 11-cis-retinol is known to inhibit all-trans-retinyl ester isomerohydrolase, whereas addition of retinol binding proteins stimulates this process.
Palmitoylation switch hypothesis in the visual cycle was recently proposed [11]. However, the control mechanism and the presence of lipid modification of RPE65 require further investigation. Our data showed that cleavage of RPE65 is related to palmitoylation and membrane association. In basic or nucleophilic conditions to break thioester bond, increased cleavage of RPE65 and dissociation from the membrane were observed.
In this study, we found that cleavage of RPE65 is affected by oxidative stress. A process involving an ubiquitin-dependent, protease-mediated truncation of RPE65 represents a possible turnover mechanism for controlling this abundant, yet essential, protein in the visual cycle under high oxidative stress conditions on RPE.
Acknowledgments
We thank Ruonan Zhang, Eun Young Oh and Dr. Xiaoming Yang for their excellent technical assistance. We thank Dr. Eun-Kyoung Choi and Dr. D. Margaret Hunt for insightful discussions on caspase reactions and RT-PCR experiments. The authors thank Dr. Elizabeth Hager and Dr. Steven Bailey for their critical reading and suggestions. This study was supported by the Centenary Award from the University of South Carolina; a start-up fund from the Department of Ophthalmology, University of South Carolina; the New Investigator Award from the International Foundation; the Century II Equipment fund, a start-up package and the Research Excellent Fund from Michigan Technological University.
Abbreviations
- AMD
age-related macular degeneration
- LRAT
lecithin retinol acyltransferase
- RDH
retinol dehydrogenase
- ROS
reactive oxygen species
- RPE
retinal pigment epithelium.
References
- 1.Wald G. Carotenoids and the vitamin A cycle in vision. Nature. 1934;134:65. [Google Scholar]
- 2.Dowling JE. Chemistry of visual adaptation in the rat. Nature. 1960;188:114–118. doi: 10.1038/188114a0. [DOI] [PubMed] [Google Scholar]
- 3.Saari JC. Biochemistry of visual pigment regeneration. Invest. Ophthalmol. Vis. Sci. 2000;41:337–348. [PubMed] [Google Scholar]
- 4.Rando RR. The biochemistry of the visual cycle. Chem. Rev. 2001;101:1881–1896. doi: 10.1021/cr960141c. [DOI] [PubMed] [Google Scholar]
- 5.Grimm C, Wenzel A, Hafezi F, Yu S, Redmond TM, Remé CE. Protection of RPE65-deficient mice identifies rhodopsin as a mediator of light-induced retinal degeneration. Nat. Genet. 2000;25:63–66. doi: 10.1038/75614. [DOI] [PubMed] [Google Scholar]
- 6.Sieving PA, Chaudhry P, Kondo M, Provenzano M, Wu D, Carlson TJ, Bush RA, Thompson DA. Inhibition of the visual cycle in vivo by 13-cis retinoic acid protects from light damage and provides a mechanism for night blindness in isotretinoin therapy. Proc. Natl. Acad. Sci. U.S.A. 2001;13:1835–1840. doi: 10.1073/pnas.041606498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wenzel A, Reme CE, Williams TP, Hafezi F, Grimm C. The Rpe65 Leu450Met variation increases retinal resistance against light-induced degeneration by slowing rhodopsin regeneration. J. Neurosci. 2001;21:53–58. doi: 10.1523/JNEUROSCI.21-01-00053.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bok D. The retinal pigment epithelium: a versatile partner in vision. J. Cell Sci. 1993;17(Suppl):189–195. doi: 10.1242/jcs.1993.supplement_17.27. [DOI] [PubMed] [Google Scholar]
- 9.Strauss O. The retinal pigment epithelium in visual function. Physiol. Rev. 2005;85:845–881. doi: 10.1152/physrev.00021.2004. [DOI] [PubMed] [Google Scholar]
- 10.Bernstein PS, Law WC, Rando RR. Isomerization of all-trans-retinoids to 11-cis-retinoids in vitro. Proc. Natl. Acad. Sci. U.S.A. 1987;87:1849–1853. doi: 10.1073/pnas.84.7.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xue L, Gollapalli DR, Maiti P, Jahng WJ, Rando RR. A palmitoylation switch mechanism in the regulation of the visual cycle. Cell. 2004;117:761–771. doi: 10.1016/j.cell.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 12.Chung H, Lee H, Lamoke F, Hrushesky WJ, Wood PA, Jahng WJ. Neuroprotective role of erythropoietin by antiapoptosis in the retina. J. Neurosci. Res. 2009;87:2365–2374. doi: 10.1002/jnr.22046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frohlich E, Klessen C. Regional differences and post-mortem stability of enzymatic activities in the retinal pigment epithelium. Graefes Arch. Clin. Exp. Ophthalmol. 2003;241:385–393. doi: 10.1007/s00417-003-0640-x. [DOI] [PubMed] [Google Scholar]
- 14.Yanagihara N, Moriwaki M, Shiraki K, Miki T, Otani S. The involvement of polyamines in the proliferation of cultured retinal pigment epithelial cells. Invest. Ophthalmol. Vis. Sci. 1996;37:1975–1983. [PubMed] [Google Scholar]
- 15.Jahng WJ, Xue L, Rando RR. Lecithin retinol acyltransferase is a founder member of a novel family of enzymes. Biochemistry. 2003;42:12805–12812. doi: 10.1021/bi035370p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jahng WJ, David C, Nesnas N, Nakanishi K, Rando RR. A cleavable affinity biotinylating agent reveals a retinoid binding role for RPE65. Biochemistry. 2003;42:6159–6168. doi: 10.1021/bi034002i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamirally S, Kamil JP, Ndassa-Colday YM, Lin AJ, Jahng WJ, Baek MC, Noton S, Silva LA, Simpson-Holley M, Knipe DM, Golan DE, Marto JA, Coen DM. Viral mimicry of Cdc2/cyclin-dependent kinase 1 mediates disruption of nuclear lamina during human cytomegalovirus nuclear egress. PLoS Pathog. 2009 Jan 1;5:e1000275. doi: 10.1371/journal.ppat.1000275. Epub 2009 January 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moiseyev G, Chen Y, Takahashi Y, Wu BX, Ma JX. RPE65 is the isomerohydrolase in the retinoid visual cycle. Proc. Natl. Acad. Sci. U.S.A. 2005;102:12413–12418. doi: 10.1073/pnas.0503460102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jin M, Li S, Moghrabi WN, Sun H, Travis GH. Rpe65 is the retinoid isomerase in bovine retinal pigment epithelium. Cell. 2005;122:449–459. doi: 10.1016/j.cell.2005.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Redmond TM, Poliakov E, Yu S, Tsai JY, Lu Z, Gentleman S. Mutation of key residues of RPE65 abolishes its enzymatic role as isomerohydrolase in the visual cycle. Proc. Natl. Acad. Sci. U.S.A. 2005;102:13658–13663. doi: 10.1073/pnas.0504167102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Organisciak DT, Darrow RM, Barsalou L, Kutty RK, Wiggert B. Susceptibility to retinal light damage in transgenic rats with rhodopsin mutations. Invest. Ophthalmol. Vis. Sci. 2003;44:486–492. doi: 10.1167/iovs.02-0708. [DOI] [PubMed] [Google Scholar]
- 22.Gu SM, Thompson DA, Srikumari CR, Lorenz B, Finckh U, Nicoletti A, Murthy KR, Rathmann M, Kumaramanickavel G, Denton MJ, Gal A. Mutations in RPE65 cause autosomal recessive childhood-onset severe retinal dystrophy. Nat. Genet. 1997;17:194–197. doi: 10.1038/ng1097-194. [DOI] [PubMed] [Google Scholar]
- 23.Morimura H, Fishman GA, Grover SA, Fulton AB, Berson EL, Dryja TP. Mutations in the RPE65 gene in patients with autosomal recessive retinitis pig-mentosa or Leber congenital amaurosis. Proc. Natl. Acad. Sci. U.S.A. 1998;95:3088–3093. doi: 10.1073/pnas.95.6.3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martinon F, Tschopp J. Inflammatory caspases: linking an intracellular innate immune system to autoinflammatory diseases. Cell. 2004;117:561–574. doi: 10.1016/j.cell.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 25.Ham DI, Gentleman S, Chan CC, McDowell JH, Redmond TM, Gery I. RPE65 is highly uveitogenic in rats. Invest. Ophthalmol. Vis. Sci. 2002;43:2258–2263. [PubMed] [Google Scholar]