Abstract
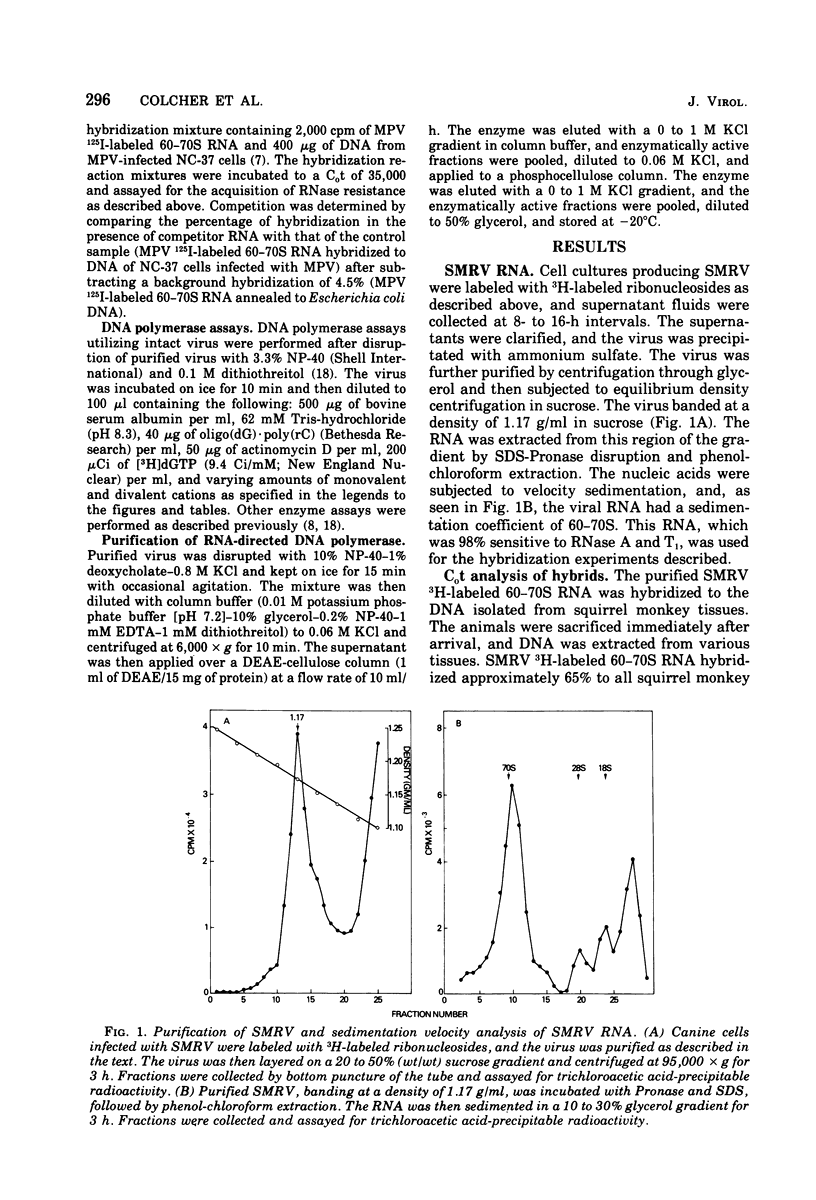
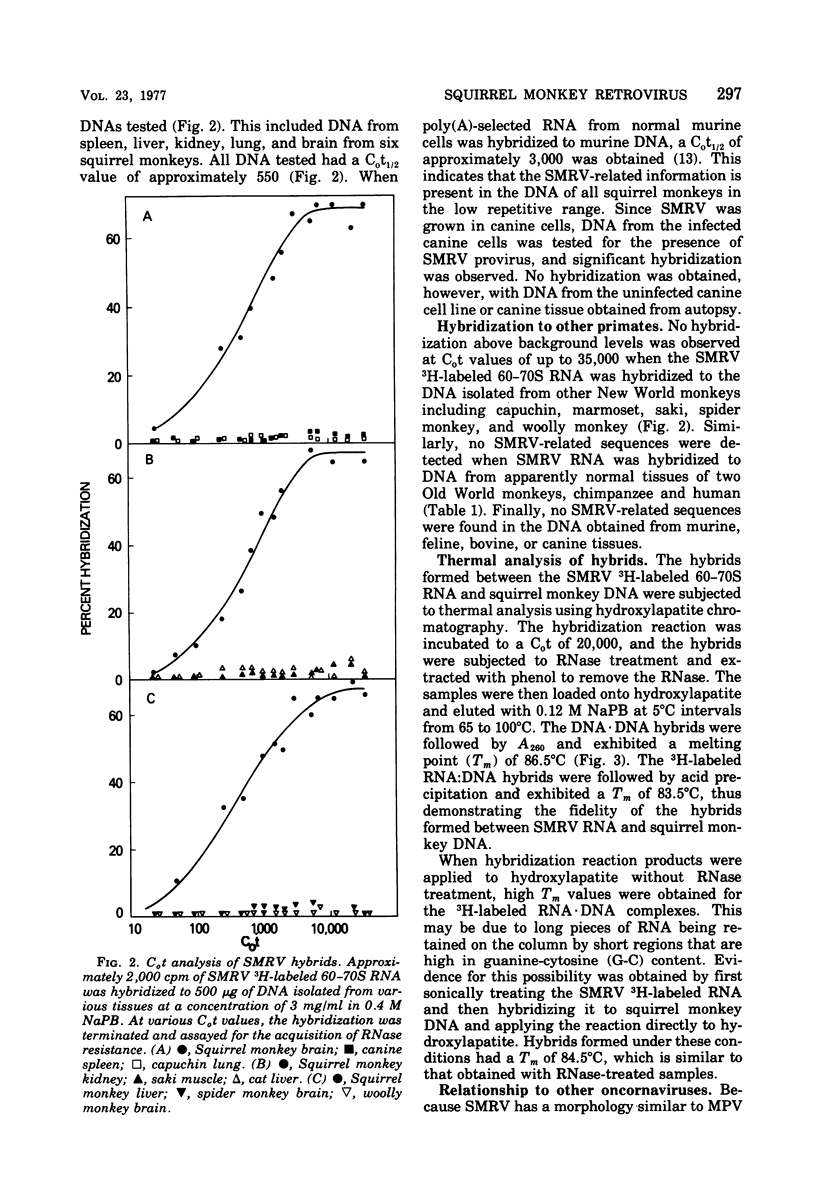
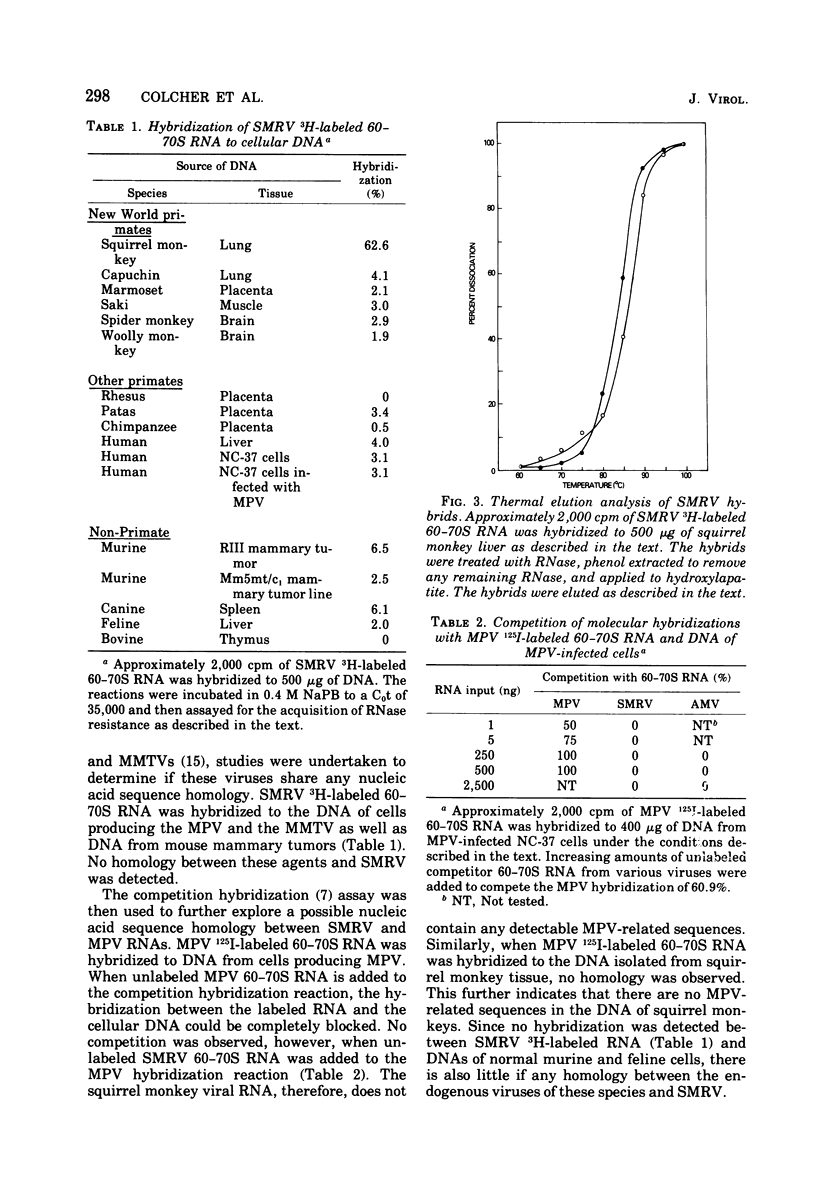
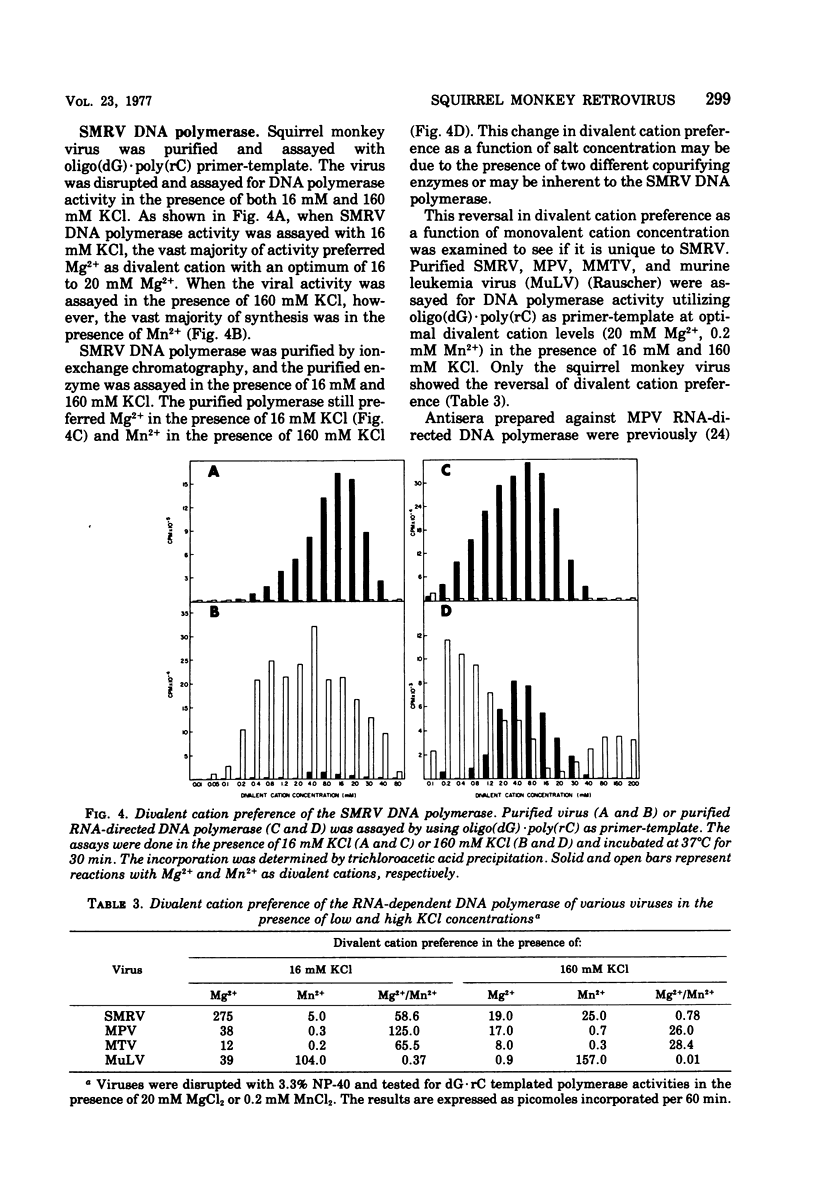
Squirrel monkey retrovirus (SMRV) was isolated by cocultivation of squirrel monkey lung cells with canine cells. 3H-labeled 60-70S SMRV RNA, isolated from virus grown in canine cells, hybridized to the same extent and to the same Cot1/2 value to the DNA of all tissues of all squirrel monkeys tested; Cot1/2 values show that SMRV proviral sequences are present in the low repetitive range. No SMRV proviral sequences were detected in tissues from a variety of other New World monkeys, Old World monkeys, or apes. Murine, feline, bovine, and canine cells also contain no detectable SMRV proviral sequences. Competitive molecular hybridization studies revealed no detectable sequence homology between the 60-70S RNAs of SMRV and Mason-Pfizer virus (MPV). The virion-associated DNA polymerase of SMRV is similar to that of MPV in that it has a molecular weight of approximately 80,000 and prefers magnesium as a divalent cation using oligo(dG)-poly(rC) as primer-template. The virion-associated DNA polymerase of SMRV can be clearly distinguished from those of MPV and the mouse mammary tumor viruses, however, by its preference for manganese as a divalent cation in the presence of high salt.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A., Stephenson J. R. Endogenous type-C RNA viruses of mammalian cells. Biochim Biophys Acta. 1976 Dec 23;458(4):323–354. doi: 10.1016/0304-419x(76)90006-8. [DOI] [PubMed] [Google Scholar]
- Aaronson S. A., Tronick S. R., Stephenson J. R. Endogenous type C RNA virus of Odocoileus hemionus, a mammalian species of New World origin. Cell. 1976 Nov;9(3):489–494. doi: 10.1016/0092-8674(76)90094-5. [DOI] [PubMed] [Google Scholar]
- BERNHARD W. The detection and study of tumor viruses with the electron microscope. Cancer Res. 1960 Jun;20:712–727. [PubMed] [Google Scholar]
- Benveniste R. E., Heinemann R., Wilson G. L., Callahan R., Todaro G. J. Detection of baboon type C viral sequences in various primate tissues by molecular hybridization. J Virol. 1974 Jul;14(1):56–67. doi: 10.1128/jvi.14.1.56-67.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan R., Benveniste R. E., Sherr C. J., Schidlovsky G., Todaro G. J. A new class of genetically transmitted retravirus isolated from Mus cervicolor. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3579–3583. doi: 10.1073/pnas.73.10.3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colcher D., Drohan W., Schlom Mason-Pfizer virus RNA genome: relationship to the RNA of morphologically similar isolates and other oncornaviruses. J Virol. 1976 Mar;17(3):705–712. doi: 10.1128/jvi.17.3.705-712.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colcher D., Spiegelman S., Schlom J. Sequence homology between the RNA of Mason-Pfizer monkey virus and the RNA of human malignant breast tumors. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4975–4979. doi: 10.1073/pnas.71.12.4975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commerford S. L. Iodination of nucleic acids in vitro. Biochemistry. 1971 May 25;10(11):1993–2000. doi: 10.1021/bi00787a005. [DOI] [PubMed] [Google Scholar]
- Dahlberg J. E., Perk K., Dalton A. J. Virus-like particles induced in guinea pig cells by 5-bromo-2'-deoxyuridine are morphologically similar to murine B-type virus. Nature. 1974 Jun 28;249(460):828–830. doi: 10.1038/249828a0. [DOI] [PubMed] [Google Scholar]
- Dalton A. J., Melnick J. L., Bauer H., Beaudreau G., Bentvelzen P., Bolognesi D., Gallo R., Graffi A., Haguenau F., Heston W. The case for a family of reverse transcriptase viruses: Retraviridae. Intervirology. 1974;4(4):201–206. doi: 10.1159/000149963. [DOI] [PubMed] [Google Scholar]
- Drohan W., Colcher D., Schochetman G., Schlom J. Distribution of Mason-Pfizer virus-specific sequences in the DNA of primates. J Virol. 1977 Jul;23(1):36–43. doi: 10.1128/jvi.23.1.36-43.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drohan W., Kettmann R., Colcher D., Schlom J. Isolation of the mouse mammary tumor virus sequences not transmitted as germinal provirus in the C3H and RIII mouse strains. J Virol. 1977 Mar;21(3):986–995. doi: 10.1128/jvi.21.3.986-995.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenner F. The classification and nomenclature of viruses. Summary of results of meetings of the International Committee on Taxonomy of Viruses in Madrid, September 1975. Intervirology. 1975;6(1):1–12. doi: 10.1159/000149448. [DOI] [PubMed] [Google Scholar]
- Heberling R. L., Barker S. T., Kalter S. S., Smith G. C., Helmke R. J. Oncornavirus: isolation from a squirrel monkey (Saimiri sciureus) lung culture. Science. 1977 Jan 21;195(4275):289–292. doi: 10.1126/science.63993. [DOI] [PubMed] [Google Scholar]
- Kalter S. S., Helmke R. J., Panigel M., Heberling R. L., Felsburg P. J., Axelrod L. R. Observations of apparent C-type particles in baboon (Papio cynocephalus) placentas. Science. 1973 Mar 30;179(4080):1332–1333. doi: 10.1126/science.179.4080.1332. [DOI] [PubMed] [Google Scholar]
- Kettmann R., Portetelle D., Mammerickx M., Cleuter Y., Dekegel D., Galoux M., Ghysdael J., Burny A., Chantrenne H. Bovine leukemia virus: an exogenous RNA oncogenic virus. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1014–1018. doi: 10.1073/pnas.73.4.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimball P. C., Michalides R., Colcher D., Schlom J. Characterization of mouse mammary tumor viruses from primary tumor cell cultures. II. Biochemical and biophysical studies. J Natl Cancer Inst. 1976 Jan;56(1):119–124. doi: 10.1093/jnci/56.1.119. [DOI] [PubMed] [Google Scholar]
- Michalides R., Schlom J., Dahlberg J., Perk K. Biochemical properties of the bromodeoxyuridine-induced guinea pig virus. J Virol. 1975 Oct;16(4):1039–1050. doi: 10.1128/jvi.16.4.1039-1050.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson-Rees W. A., Owens R. B., Arnstein P., Kniazeff A. J. Source, alterations, characteristics and use of a new dog cell line (Cf2Th). In Vitro. 1976 Oct;12(10):665–669. doi: 10.1007/BF02797468. [DOI] [PubMed] [Google Scholar]
- Schlom J., Spiegelman S. DNA polymerase activities and nucleic acid components of virions isolated from a spontaneous mammary carcinoma from a rhesus monkey. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1613–1617. doi: 10.1073/pnas.68.7.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaniv A., Ono T., Kacian D., Colcher D., Witkin S., Schlom J., Spiegelman S. Serological analysis of reverse transcriptase of the Mason-Pfizer monkey virus. Virology. 1974 May;59(1):335–338. doi: 10.1016/0042-6822(74)90233-5. [DOI] [PubMed] [Google Scholar]


