Abstract
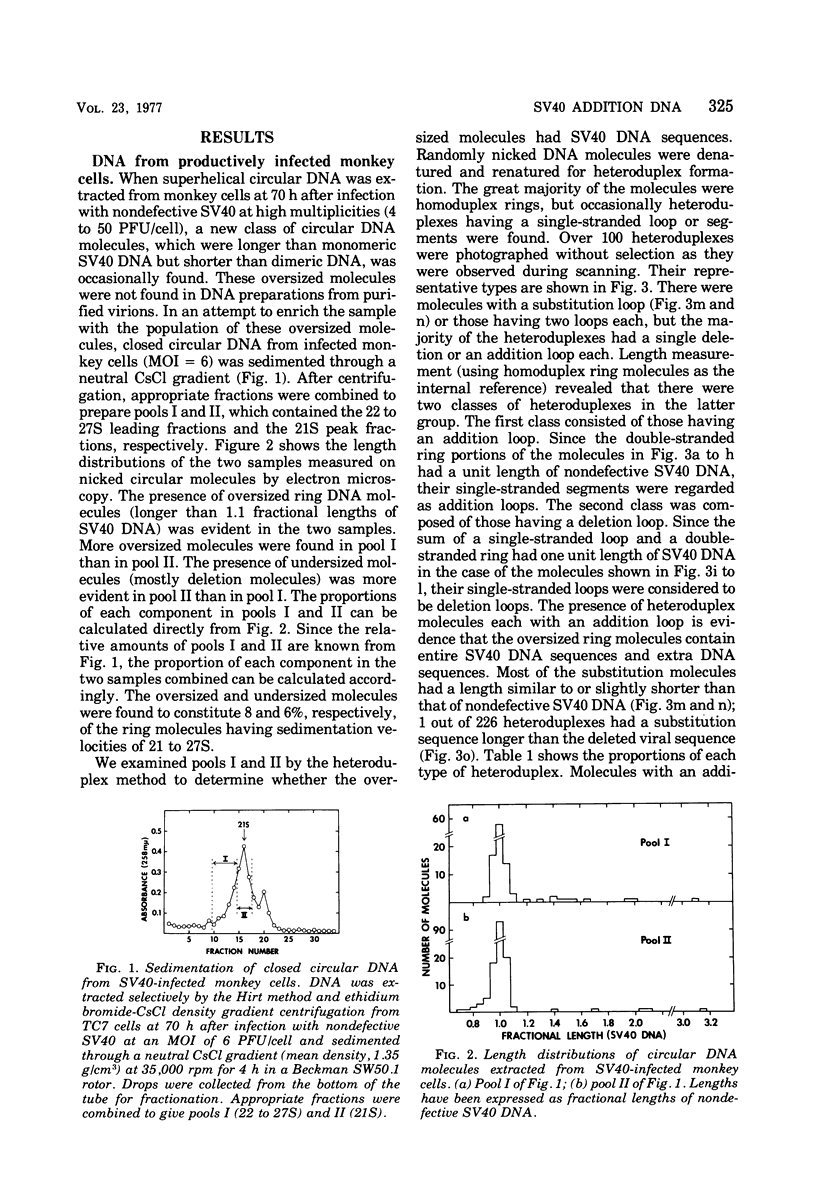
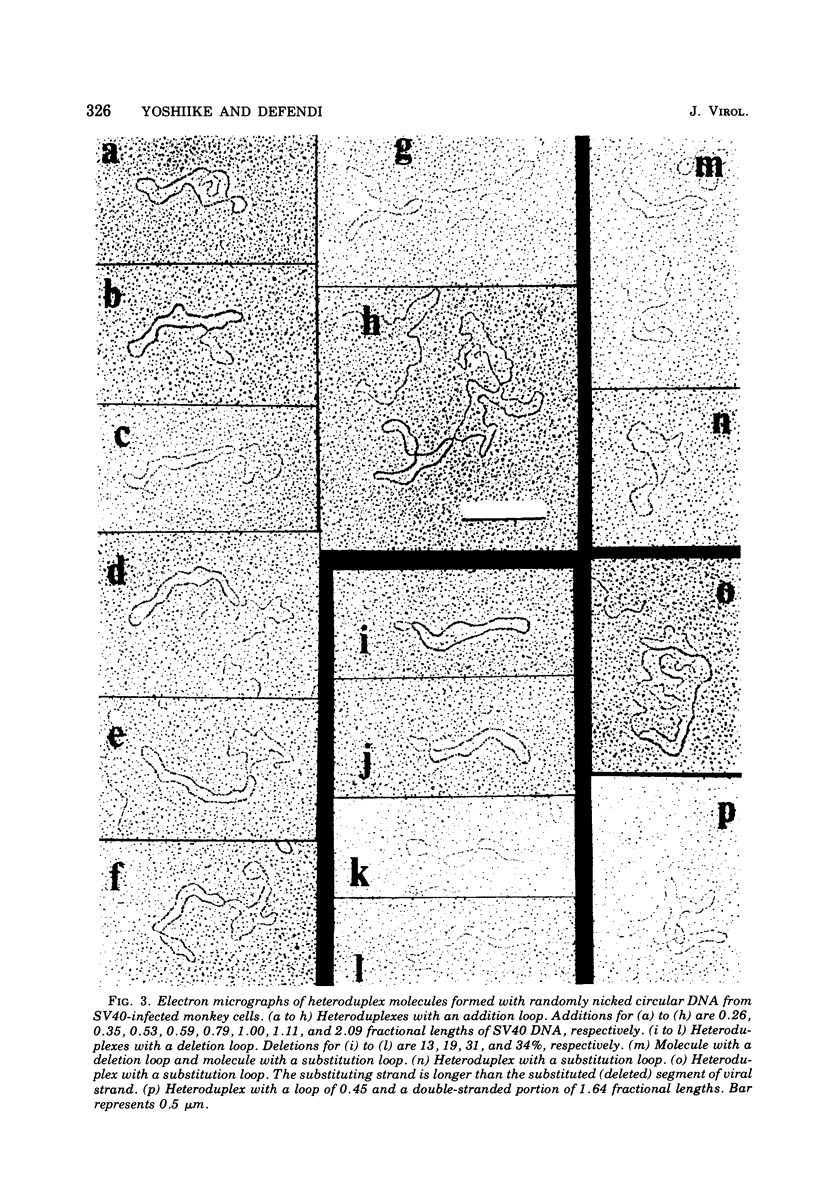
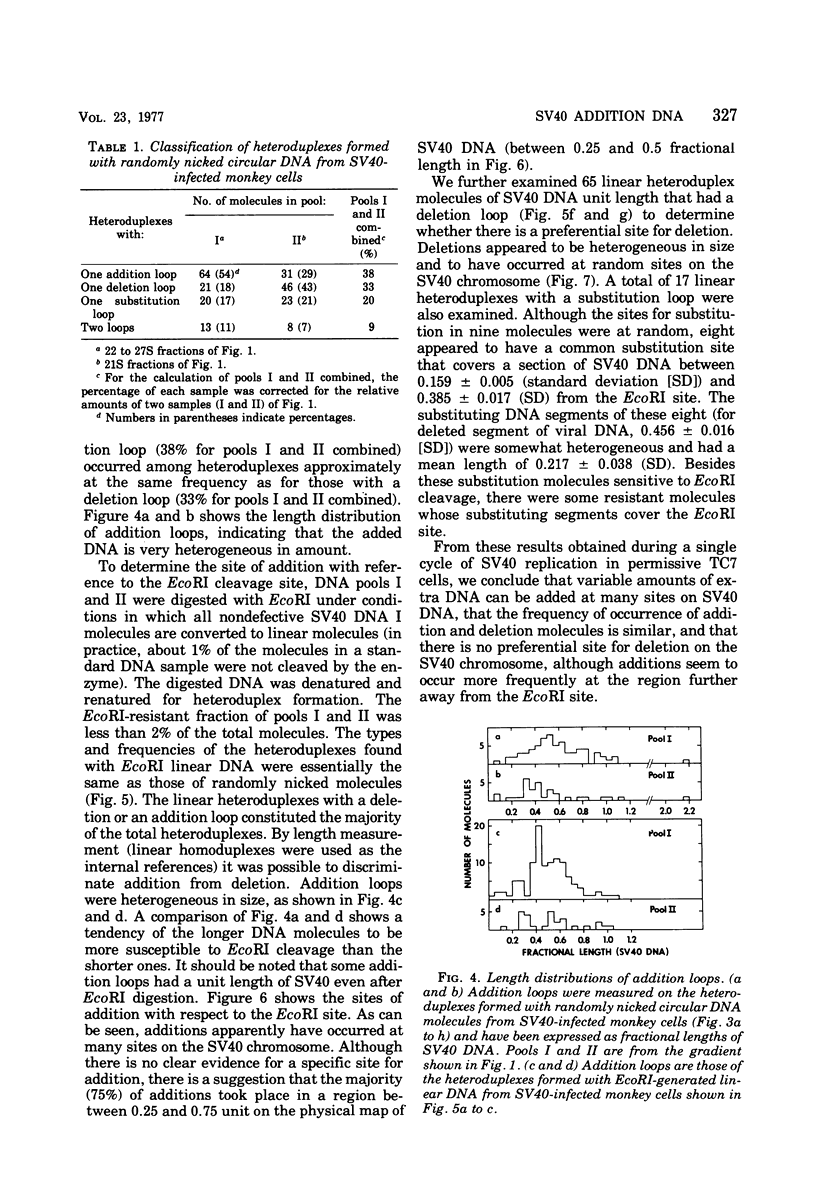
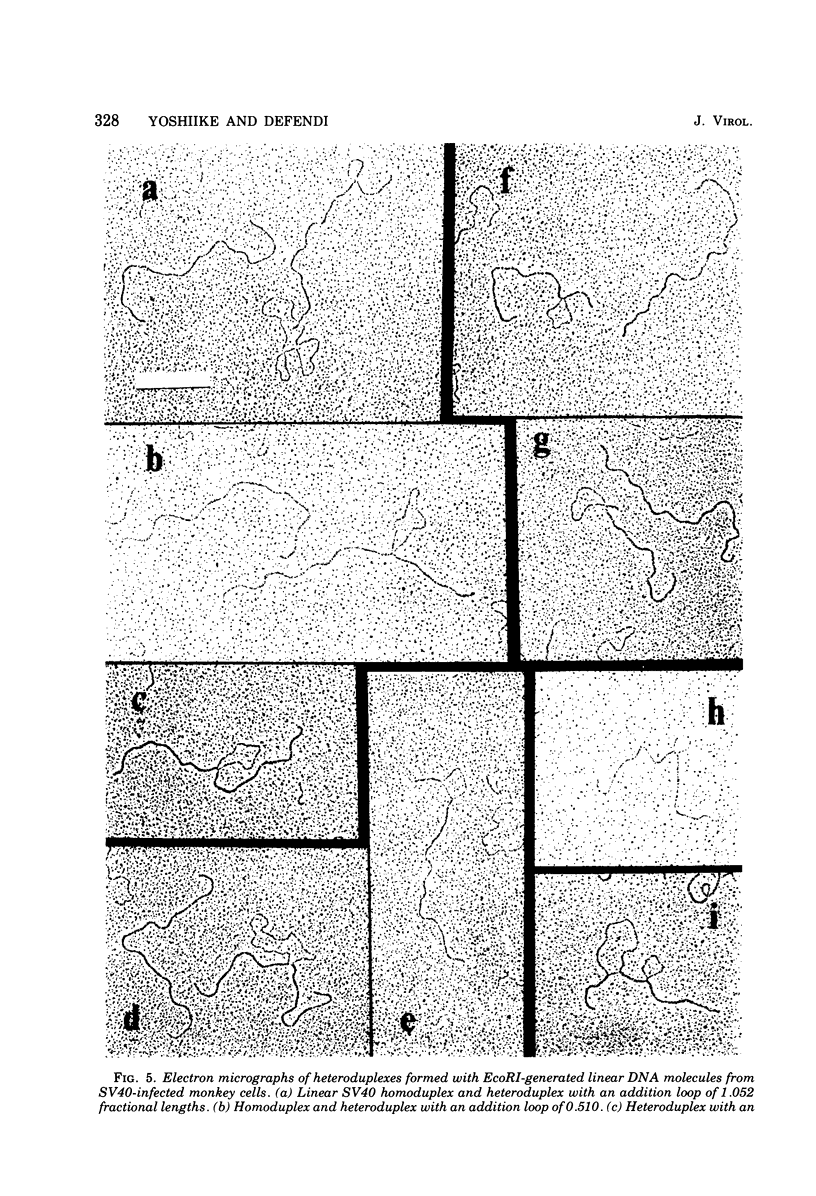
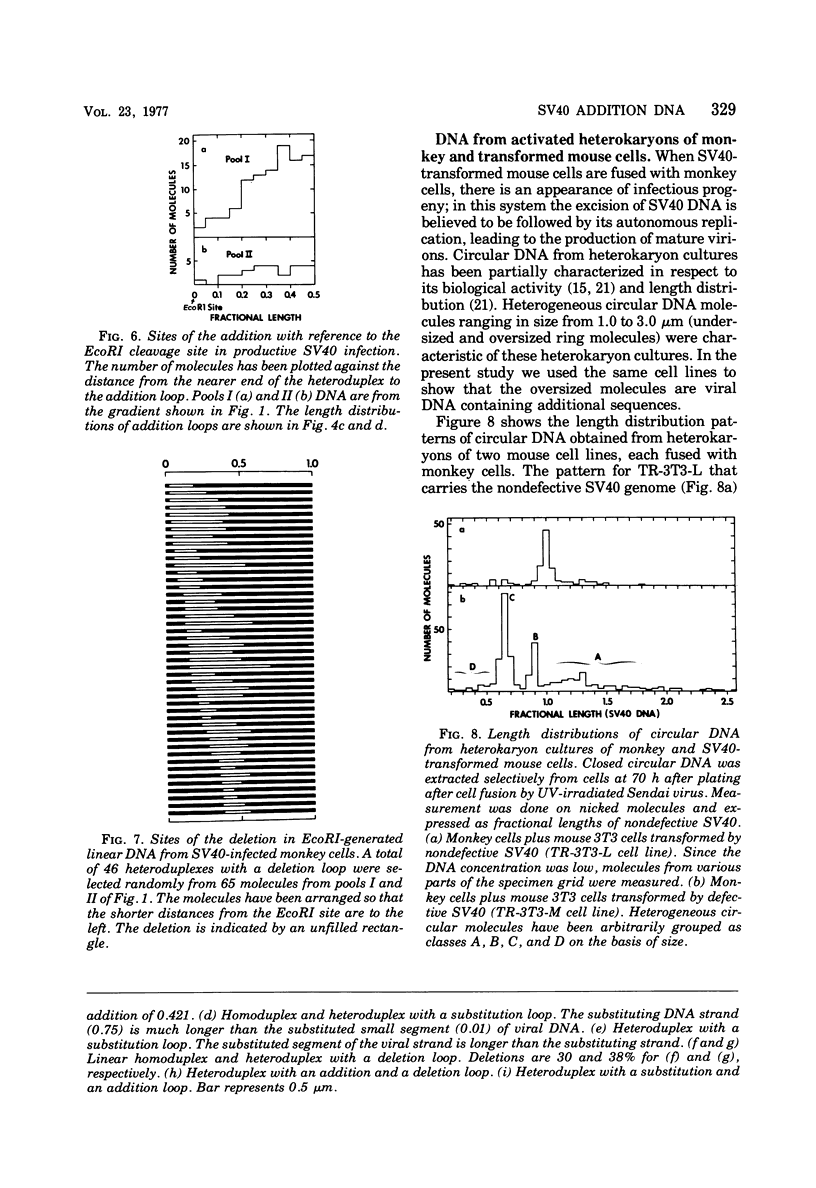
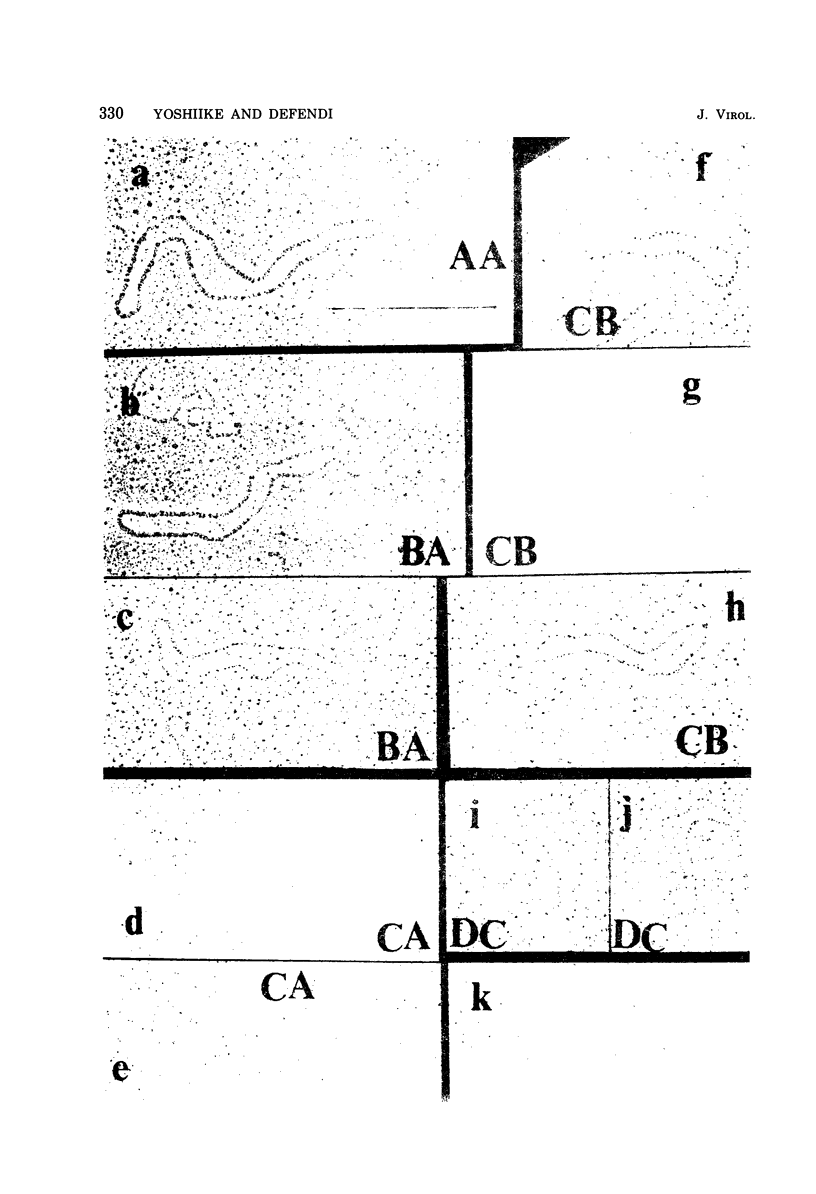
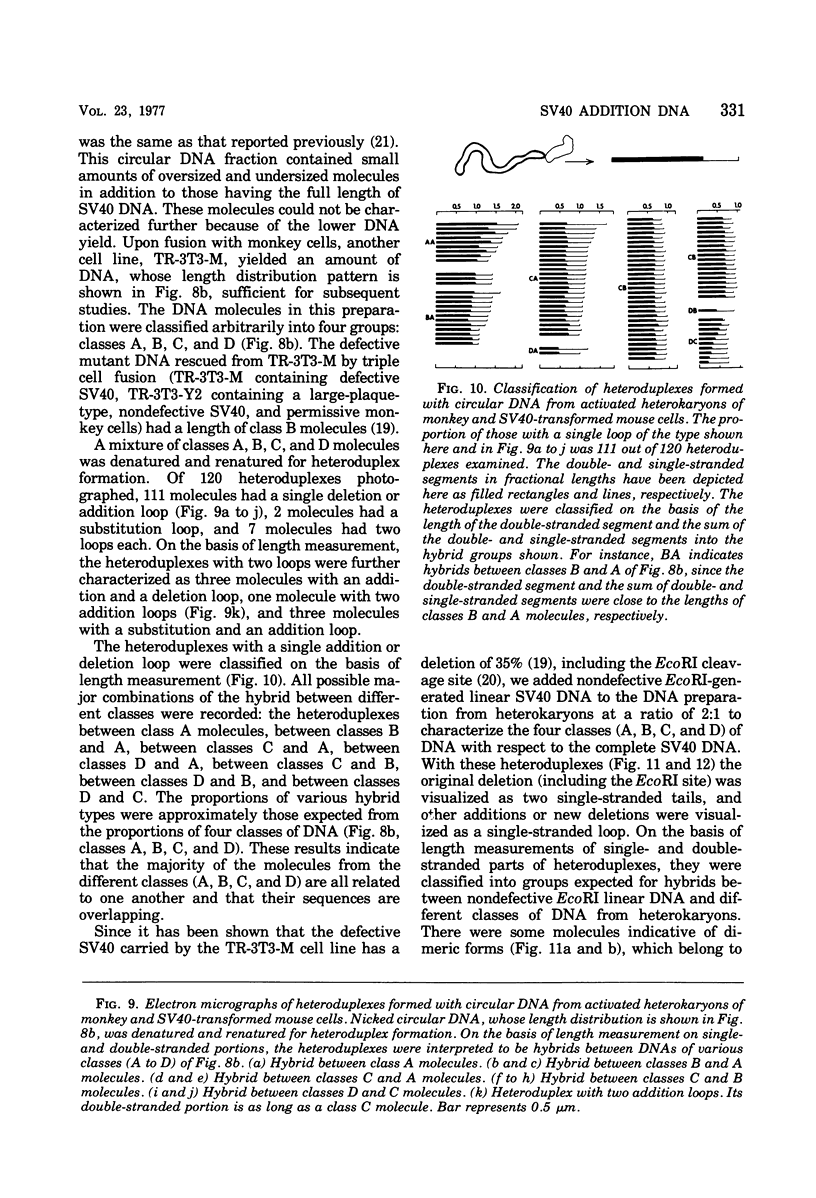
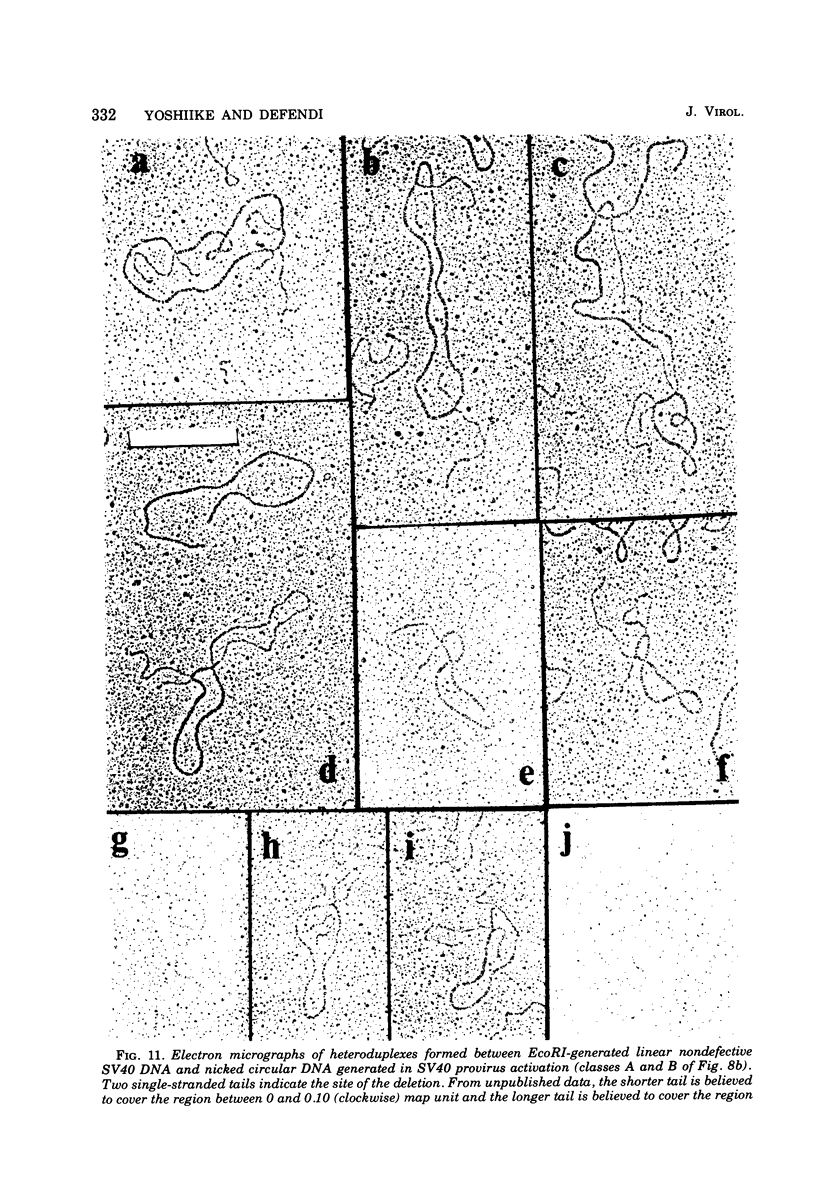
The possible addition of extra sequences to simian virus 40 (SV40) DNA was analyzed by electron microscopy in two different cell systems, productively infected monkey cells and activated heterokaryons on monkey and transformed mouse 3T3 cells. We found that the closed circular DNA fraction, extracted from monkey cells at 70 h after infection with nondefective SV40 at a multiplicity of infection of 6 PFU/cell, contained oversized molesules (1.1 to 2.0 fractional lengths of SV40 DNA) constituting about 8% of the molecules having lengths equal to or shorter than SV40 dinner DNA. The oversized molecules had the entired SV40 sequences. The added DNA was heterogeneous in length. The sites of addition were not specific with reference to the EcoRi site. These results suggest that recombination between monkey and SV40 DNAs or partial duplication of SV40 DNA occurs at many sites on the SV40 chromosome. The integrated SV40 DNA is excised and replicates in activated heterokaryons. In this system, besides SV40 DNA we found heterogeneous undersized and oversized molecules containing SV40 sequences in the closed circular DNA population. Additions differeing in size appeared to be overlapping and to have occurred at a preferential site on the SV40 chromosome. These results support the hypothesis that host DNA can be added to SV40 DNA at the site of integration at the time of excision.
Full text
PDF


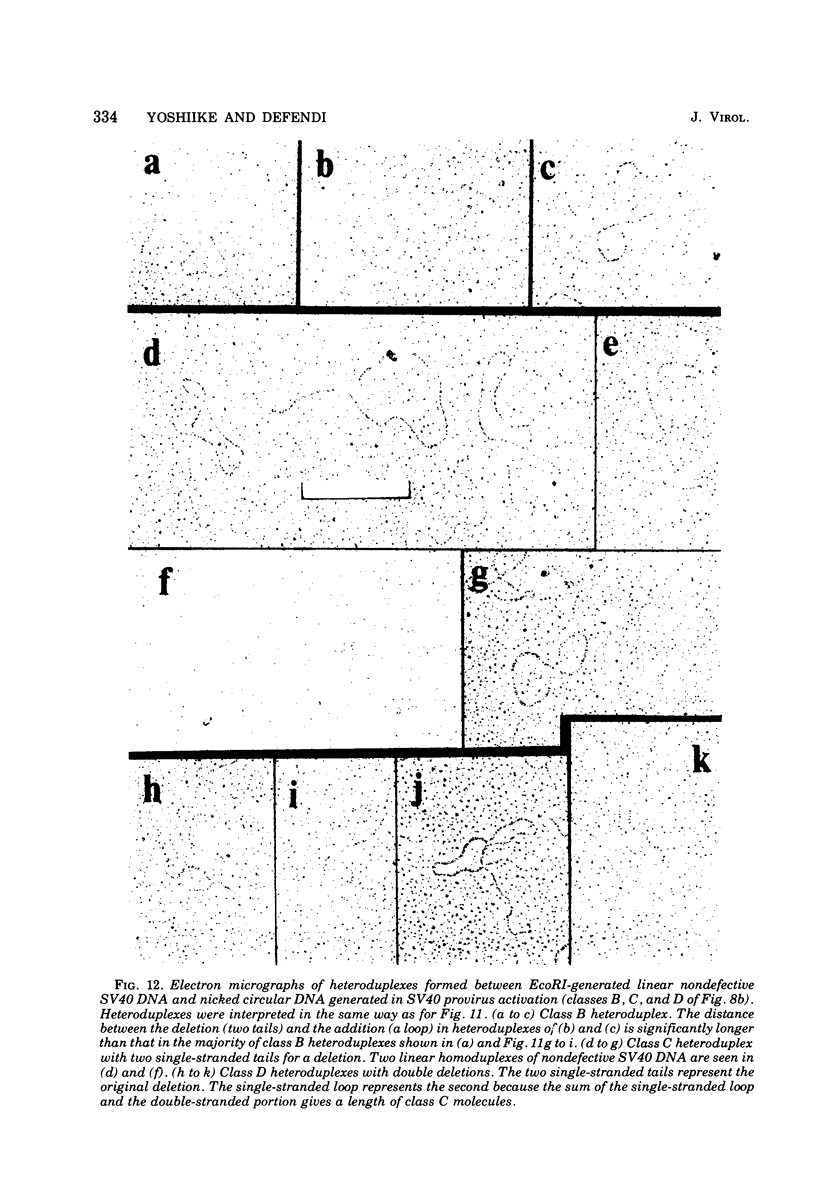
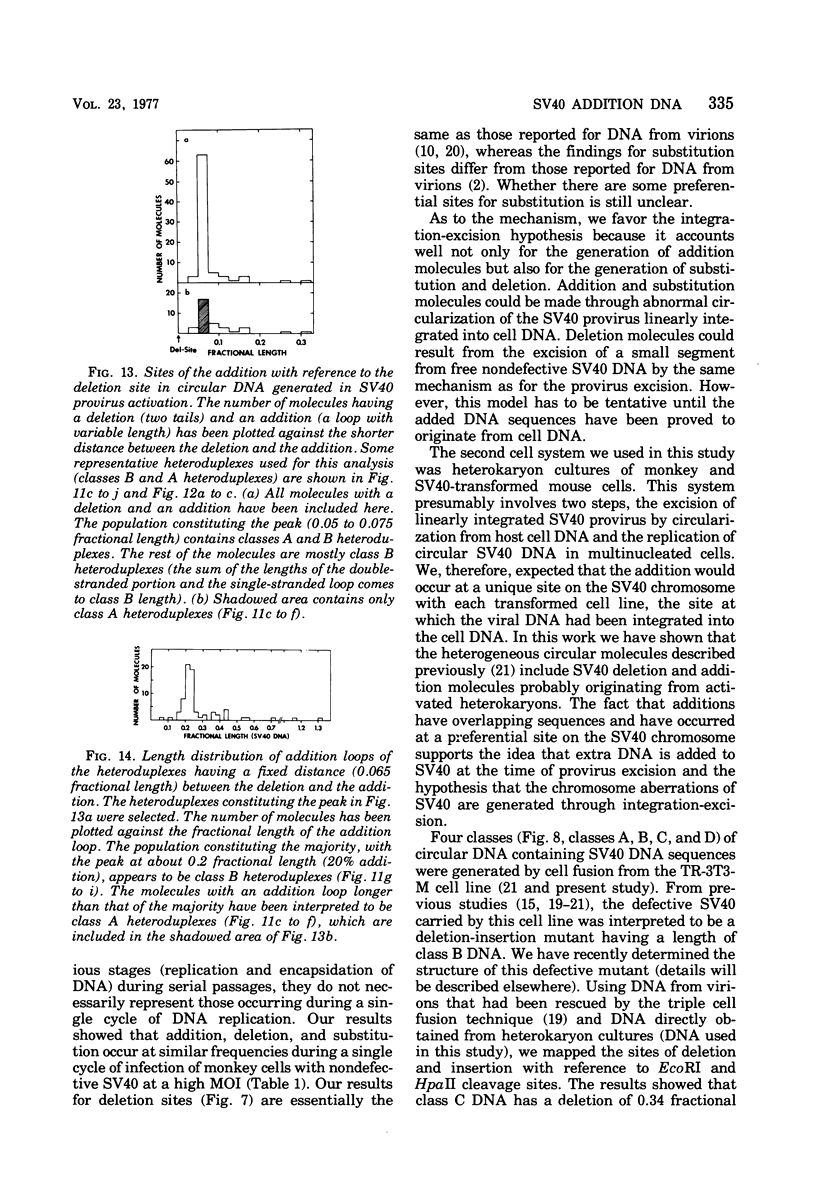


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chow L. T., Boyer H. W., Tischer E. G., Goodman H. M. Electron microscopic mapping of the attachment sites on SV40 DNA during lytic infection. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):109–117. doi: 10.1101/sqb.1974.039.01.016. [DOI] [PubMed] [Google Scholar]
- Hirai K., Defendi V. Integration of simian virus 40 deoxyribonucleic acid into the deoxyribonucleic acid of permissive monkey kidney cells. J Virol. 1972 Apr;9(4):705–707. doi: 10.1128/jvi.9.4.705-707.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai K., Lehman J., Defendi V. Integration of simian virus 40 deoxyribonucleic acid into the deoxyribonucleic acid of primary infected Chinese hamster cells. J Virol. 1971 Nov;8(5):708–715. doi: 10.1128/jvi.8.5.708-715.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Hudson B., Upholt W. B., Devinny J., Vinograd J. The use of an ethidium analogue in the dye-buoyant density procedure for the isolation of closed circular DNA: the variation of the superhelix density of mitochondrial DNA. Proc Natl Acad Sci U S A. 1969 Mar;62(3):813–820. doi: 10.1073/pnas.62.3.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koprowski H., Jensen F. C., Steplewski Z. Activation of production of infectious tumor virus SV40 in heterokaryon cultures. Proc Natl Acad Sci U S A. 1967 Jul;58(1):127–133. doi: 10.1073/pnas.58.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavi S., Winocour E. Acquisition of sequences homologous to host deoxyribonucleic acid by closed circular simian virus 40 deoxyribonucleic acid. J Virol. 1972 Feb;9(2):309–316. doi: 10.1128/jvi.9.2.309-316.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertz J. E., Carbon J., Herzberg M., Davis R. W., Berg P. Isolation and characterization of individual clones of simian virus 40 mutants containing deletions duplications and insertions in their DNA. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):69–84. doi: 10.1101/sqb.1974.039.01.012. [DOI] [PubMed] [Google Scholar]
- Sambrook J., Westphal H., Srinivasan P. R., Dulbecco R. The integrated state of viral DNA in SV40-transformed cells. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1288–1295. doi: 10.1073/pnas.60.4.1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai H. T., Smith C. A., Sharp P. A., Vinograd J. Sequence heterogeneity in closed simian virus 40 deoxyribonucleic acid. J Virol. 1972 Feb;9(2):317–325. doi: 10.1128/jvi.9.2.317-325.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida S., Watanabe S. Transformation of mouse 3T3 cells by T antigen-forming defective SV40 virions (T particles). Virology. 1969 Dec;39(4):721–728. doi: 10.1016/0042-6822(69)90009-9. [DOI] [PubMed] [Google Scholar]
- Watanabe S. Virus DNA synthesizing ability of T antigen-forming defective SV40 produced by successive undiluted passages. J Gen Virol. 1975 Jan;26(1):49–57. doi: 10.1099/0022-1317-26-1-49. [DOI] [PubMed] [Google Scholar]
- Watkins J. F., Dulbecco R. Production of SV40 virus in heterokaryons of transformed and susceptible cells. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1396–1403. doi: 10.1073/pnas.58.4.1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshiike K., Furuno A., Uchida S. Rescue of defective SV40 from a transformed mouse 3T3 cell line: selection of a specific defective. Virology. 1974 Aug;60(2):342–352. doi: 10.1016/0042-6822(74)90329-8. [DOI] [PubMed] [Google Scholar]
- Yoshiike K. Studies on DNA from low-density particles of SV40. I. Heterogeneous defective virions produced by successive undiluted passages. Virology. 1968 Mar;34(3):391–401. doi: 10.1016/0042-6822(68)90059-7. [DOI] [PubMed] [Google Scholar]
- Yoshiike K., Watanabe S., Suzuki K., Uchida S. Circular DNA from heterokaryons of SV40-transformed mouse and African green monkey cells. Jpn J Microbiol. 1975 Jun;19(3):237–240. doi: 10.1111/j.1348-0421.1975.tb00876.x. [DOI] [PubMed] [Google Scholar]
- Yoshike K., Furuno A., Watanabe S., Uchida S., Matsubara K. Characterization of defective simian virus 40 DNA: comparison between large-plaque and small-plaque types. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):85–93. doi: 10.1101/sqb.1974.039.01.013. [DOI] [PubMed] [Google Scholar]
- Yoshike K. Studies on DNA from low-density particles of SV40. II. Noninfectious virions associated with a large-plaque variant. Virology. 1968 Mar;34(3):402–409. doi: 10.1016/0042-6822(68)90060-3. [DOI] [PubMed] [Google Scholar]







