Abstract
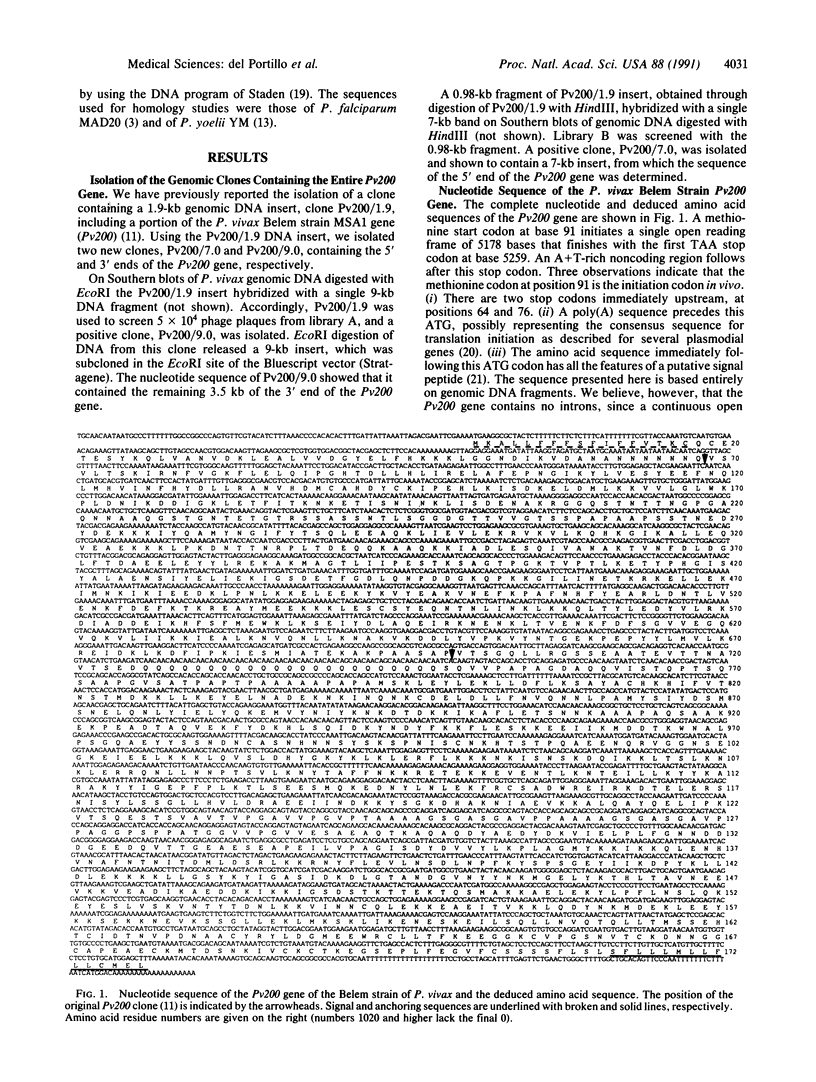
Merozoite surface antigen 1 (MSA1) of several species of plasmodia has been shown to be a promising candidate for a vaccine directed against the asexual blood stages of malaria. We report the cloning and characterization of the MSA1 gene of the human malaria parasite Plasmodium vivax. This gene, which we call Pv200, encodes a polypeptide of 1726 amino acids and displays features described for MSA1 genes of other species, such as signal peptide and anchoring sequences, conserved cysteine residues, number of potential N-glycosylation sites, and repeats consisting here of 23 glutamine residues in a row. When the nucleotide and deduced amino acid sequences of the MSA1 of P. vivax are compared to those of another human malaria parasite, Plasmodium falciparum, and to those of the rodent parasite Plasmodium yoelii, 10 regions of high amino acid similarity are observed despite the very different dG + dC contents of the corresponding genes. All of the interspecies conserved regions reside within the conserved or semiconserved blocks delimited by the sequences of different alleles of the MSA1 gene of P. falciparum.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burns J. M., Jr, Majarian W. R., Young J. F., Daly T. M., Long C. A. A protective monoclonal antibody recognizes an epitope in the carboxyl-terminal cysteine-rich domain in the precursor of the major merozoite surface antigen of the rodent malarial parasite, Plasmodium yoelii. J Immunol. 1989 Oct 15;143(8):2670–2676. [PubMed] [Google Scholar]
- Certa U., Rotmann D., Matile H., Reber-Liske R. A naturally occurring gene encoding the major surface antigen precursor p190 of Plasmodium falciparum lacks tripeptide repeats. EMBO J. 1987 Dec 20;6(13):4137–4142. doi: 10.1002/j.1460-2075.1987.tb02759.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Holder A. A., Freeman R. R. Biosynthesis and processing of a Plasmodium falciparum schizont antigen recognized by immune serum and a monoclonal antibody. J Exp Med. 1982 Nov 1;156(5):1528–1538. doi: 10.1084/jem.156.5.1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holder A. A., Lockyer M. J., Odink K. G., Sandhu J. S., Riveros-Moreno V., Nicholls S. C., Hillman Y., Davey L. S., Tizard M. L., Schwarz R. T. Primary structure of the precursor to the three major surface antigens of Plasmodium falciparum merozoites. Nature. 1985 Sep 19;317(6034):270–273. doi: 10.1038/317270a0. [DOI] [PubMed] [Google Scholar]
- Holder A. A. The precursor to major merozoite surface antigens: structure and role in immunity. Prog Allergy. 1988;41:72–97. [PubMed] [Google Scholar]
- Kimura E., Mattei D., di Santi S. M., Scherf A. Genetic diversity in the major merozoite surface antigen of Plasmodium falciparum: high prevalence of a third polymorphic form detected in strains derived from malaria patients. Gene. 1990 Jul 2;91(1):57–62. doi: 10.1016/0378-1119(90)90162-k. [DOI] [PubMed] [Google Scholar]
- Lewis A. P. Cloning and analysis of the gene encoding the 230-kilodalton merozoite surface antigen of Plasmodium yoelii. Mol Biochem Parasitol. 1989 Oct;36(3):271–282. doi: 10.1016/0166-6851(89)90175-8. [DOI] [PubMed] [Google Scholar]
- Mackay M., Goman M., Bone N., Hyde J. E., Scaife J., Certa U., Stunnenberg H., Bujard H. Polymorphism of the precursor for the major surface antigens of Plasmodium falciparum merozoites: studies at the genetic level. EMBO J. 1985 Dec 30;4(13B):3823–3829. doi: 10.1002/j.1460-2075.1985.tb04154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutchan T. F., Dame J. B., Miller L. H., Barnwell J. Evolutionary relatedness of Plasmodium species as determined by the structure of DNA. Science. 1984 Aug 24;225(4664):808–811. doi: 10.1126/science.6382604. [DOI] [PubMed] [Google Scholar]
- Patarroyo M. E., Amador R., Clavijo P., Moreno A., Guzman F., Romero P., Tascon R., Franco A., Murillo L. A., Ponton G. A synthetic vaccine protects humans against challenge with asexual blood stages of Plasmodium falciparum malaria. Nature. 1988 Mar 10;332(6160):158–161. doi: 10.1038/332158a0. [DOI] [PubMed] [Google Scholar]
- Perkins M. E., Rocco L. J. Sialic acid-dependent binding of Plasmodium falciparum merozoite surface antigen, Pf200, to human erythrocytes. J Immunol. 1988 Nov 1;141(9):3190–3196. [PubMed] [Google Scholar]
- Pirson P. J., Perkins M. E. Characterization with monoclonal antibodies of a surface antigen of Plasmodium falciparum merozoites. J Immunol. 1985 Mar;134(3):1946–1951. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saul A., Battistutta D. Analysis of the sequences flanking the translational start sites of Plasmodium falciparum. Mol Biochem Parasitol. 1990 Aug;42(1):55–62. doi: 10.1016/0166-6851(90)90112-y. [DOI] [PubMed] [Google Scholar]
- Staden R. An interactive graphics program for comparing and aligning nucleic acid and amino acid sequences. Nucleic Acids Res. 1982 May 11;10(9):2951–2961. doi: 10.1093/nar/10.9.2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe K., Mackay M., Goman M., Scaife J. G. Allelic dimorphism in a surface antigen gene of the malaria parasite Plasmodium falciparum. J Mol Biol. 1987 May 20;195(2):273–287. doi: 10.1016/0022-2836(87)90649-8. [DOI] [PubMed] [Google Scholar]
- Udagama P. V., David P. H., Peiris J. S., Ariyaratne Y. G., Perera K. L., Mendis K. N. Demonstration of antigenic polymorphism in Plasmodium vivax malaria with a panel of 30 monoclonal antibodies. Infect Immun. 1987 Nov;55(11):2604–2611. doi: 10.1128/iai.55.11.2604-2611.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udagama P. V., Gamage-Mendis A. C., David P. H., Peiris J. S., Perera K. L., Mendis K. N., Carter R. Genetic complexity of Plasmodium vivax parasites in individual human infections analyzed with monoclonal antibodies against variant epitopes on a single parasite protein. Am J Trop Med Hyg. 1990 Feb;42(2):104–110. doi: 10.4269/ajtmh.1990.42.104. [DOI] [PubMed] [Google Scholar]
- Weber J. L., Leininger W. M., Lyon J. A. Variation in the gene encoding a major merozoite surface antigen of the human malaria parasite Plasmodium falciparum. Nucleic Acids Res. 1986 Apr 25;14(8):3311–3323. doi: 10.1093/nar/14.8.3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Portillo H. A., Gysin J., Mattei D. M., Khouri E., Udagama P. V., Mendis K. N., David P. H. Plasmodium vivax: cloning and expression of a major blood-stage surface antigen. Exp Parasitol. 1988 Dec;67(2):346–353. doi: 10.1016/0014-4894(88)90081-1. [DOI] [PubMed] [Google Scholar]