Abstract
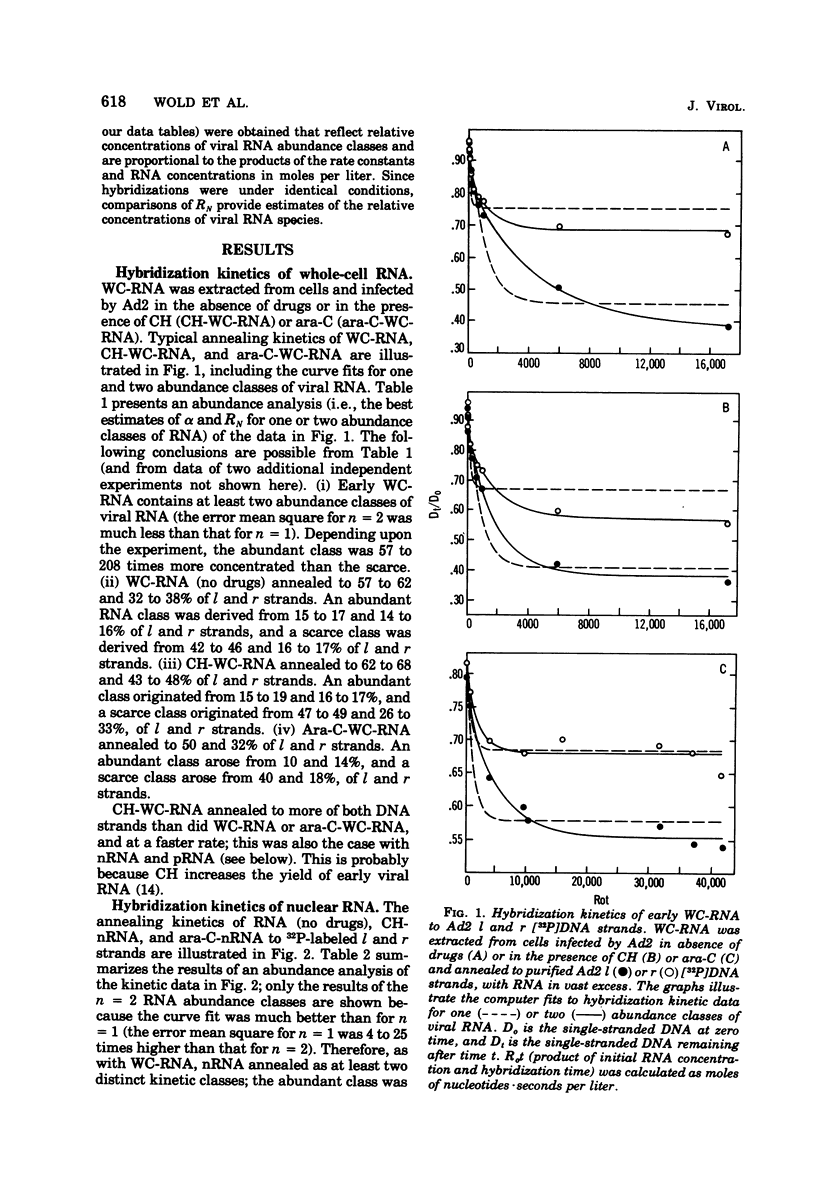
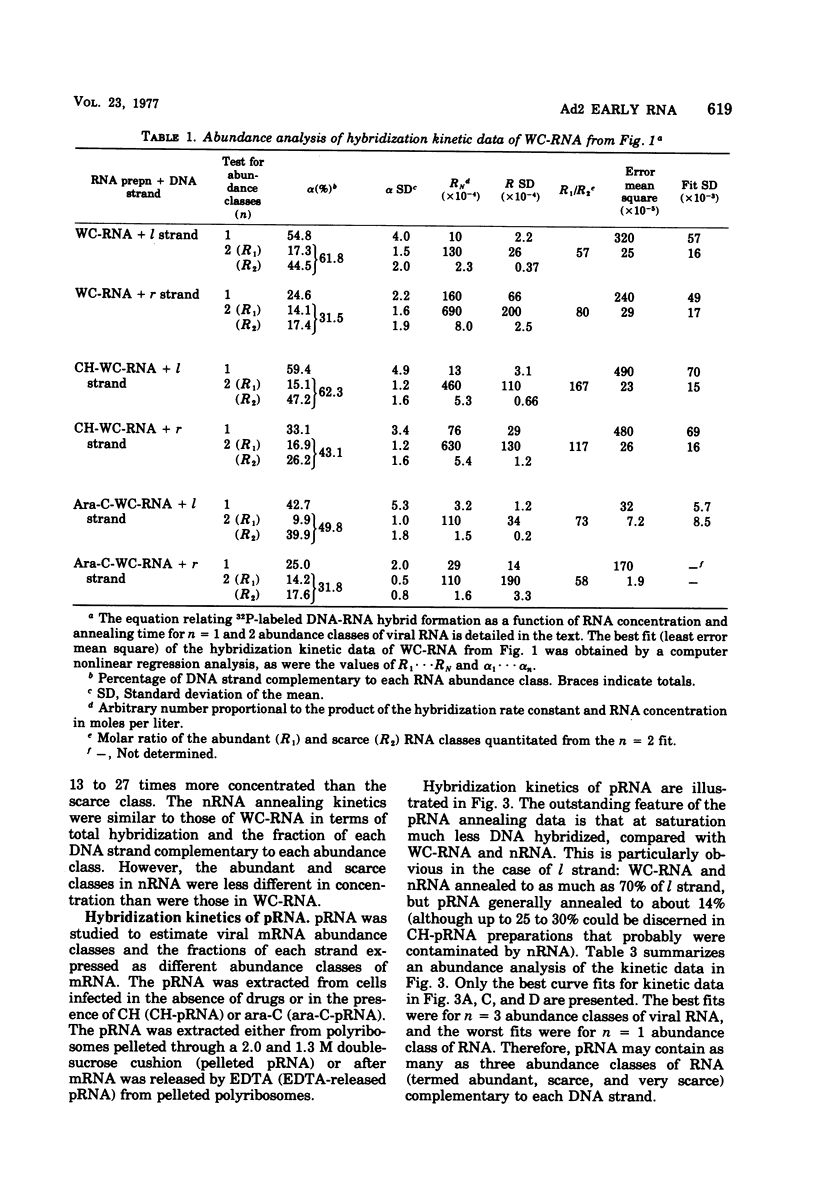
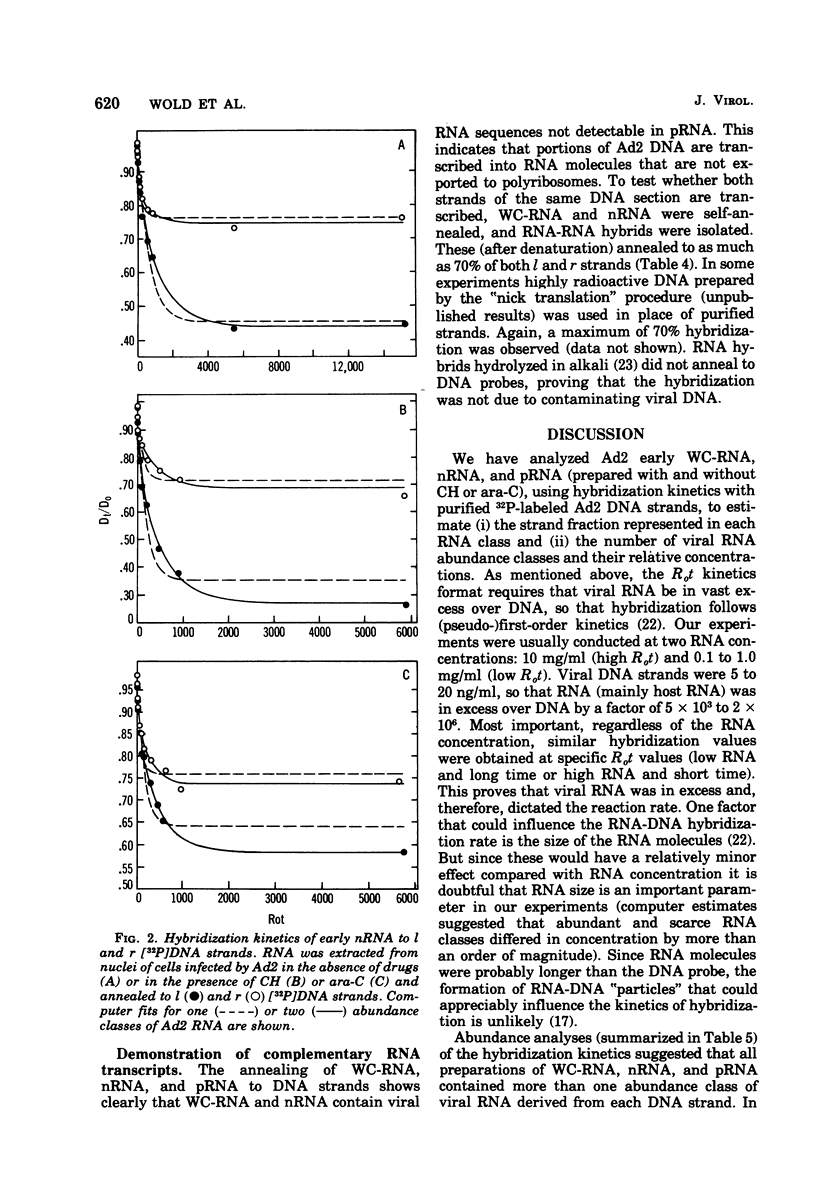
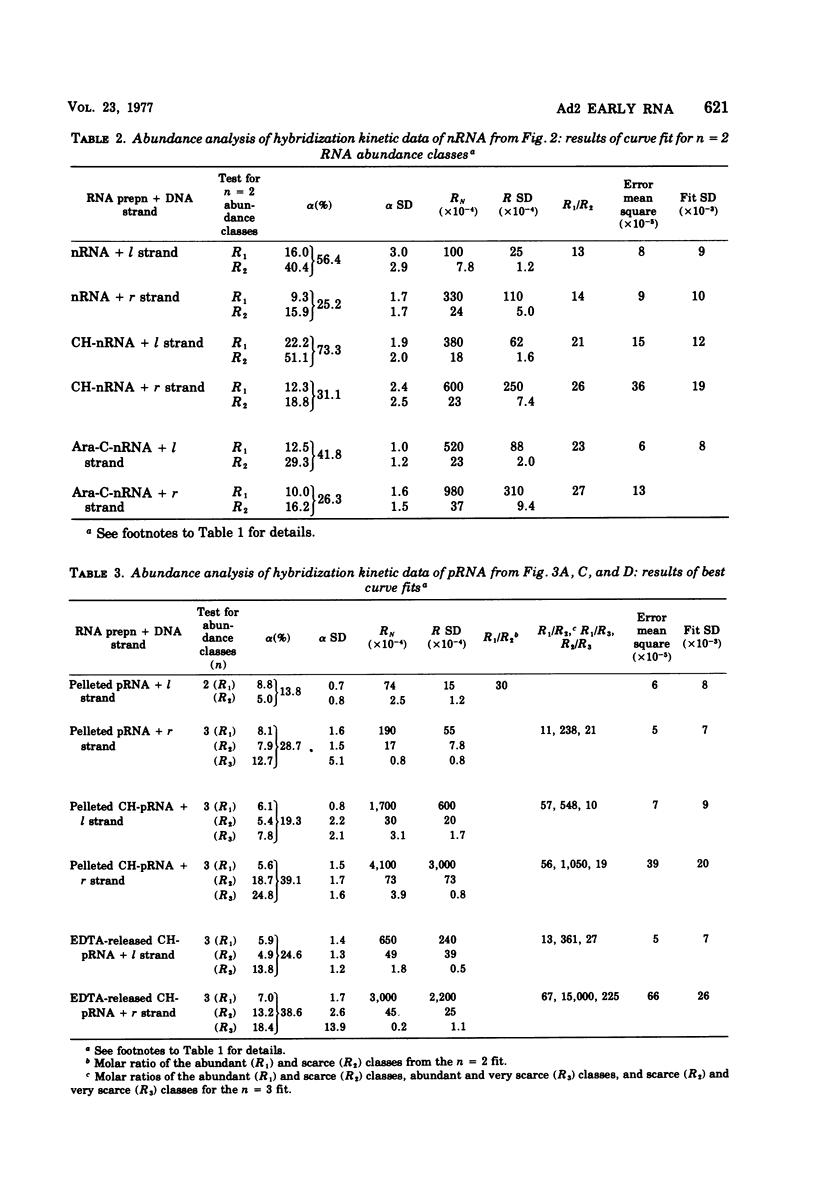
RNA from unfractionated cells, nuclei, and polyribosomes was extracted from adenovirus 2 (Ad2)-infected KB cells early in infection and annealed in vast excess in liquid to purified Ad2 l (heavy) and r (light) [32P]DNA strands (specific activity, 3 × 106 to 1.5 × 107 cpm/μg). The number of abundance classes of Ad2 RNA, their relative concentrations, and the strand fraction from which they arose were determined by a computer-assisted nonlinear regression analysis of hybridization kinetic data. Whole-cell RNA and nuclear RNA annealed to 60 and 40%, respectively, of l and r strands. Well-defined abundance (kinetic) classes were identified: abundant and scarce classes were complementary to 15 to 17 and 40 to 45%, respectively, of l strand, and to 11 to 16 and 17 to 23%, respectively, of r strand. In whole-cell RNA and nuclear RNA the abundant classes were 57 to 208 and 13 to 27 times more concentrated, respectively, than scarce classes. RNA-RNA hybrids were isolated that annealed to about 70% of both strands, indicating that whole-cell RNA and nuclear RNA hybridization values were minimal. Polyribosomal RNA appeared to anneal as three abundance classes to each DNA strand; abundant, scarce, and very scarce classes, respectively, hybridized to 6, 5, and about 10% of l strand and 7 (6 to 8), 10 (8 to 13), and about 19% of r strand. The abundant classes were 41 (11 to 67) times more concentrated than the scarce classes and 103 times more concentrated than the very scarce classes. Although the biological significance of these classes is not known, the very scarce classes probably represent nuclear RNA contaminants of polyribosomal RNA. The abundant and scarce classes may comprise mRNA, because together they are complementary to about the same fraction of each DNA strand (11% [10 to 12%] and 17% [14 to 20%] of l and r strands) known to be expressed as early mRNA. Thus, nuclear RNA contains Ad2 RNA sequences not found on polyribosomes; most or all of both DNA strands are transcribed, but only certain transcripts are processed into mRNA. It is not known whether “non-mRNA” transcripts are intermediates in the pathway of early mRNA production.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adenovirus strand nomenclature: a proposal. J Virol. 1977 Jun;22(3):830–831. doi: 10.1128/jvi.22.3.830-831.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büttner W., Veres-Molnár Z., Green M. Isolation of DNA Strand-specific early messenger RNA species in cells infected by human adenovirus 2. Proc Natl Acad Sci U S A. 1974 Aug;71(8):2951–2955. doi: 10.1073/pnas.71.8.2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büttner W., Veres-Molnár Z., Green M. Preparative isolation and mapping of adenovirus 2 early messenger RNA species. J Mol Biol. 1976 Oct 25;107(2):93–114. doi: 10.1016/s0022-2836(76)80020-4. [DOI] [PubMed] [Google Scholar]
- Craig E. A. Analysis of early adenovirus 2 RNA using Eco R-R1 viral DNA fragments. J Virol. 1975 May;15(5):1202–1213. doi: 10.1128/jvi.15.5.1202-1213.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig E. A., Raskas H. J. Effect of cycloheximide on RNA metabolism early in productive infection with adenovirus 2. J Virol. 1974 Jul;14(1):26–32. doi: 10.1128/jvi.14.1.26-32.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig E. A., Raskas H. J. Nuclear transcripts larger than the cytoplasmic mRNAs are specified by segments of the adenovirus genome coding for early functions. Cell. 1976 Jun;8(2):205–213. doi: 10.1016/0092-8674(76)90004-0. [DOI] [PubMed] [Google Scholar]
- Flint S. J., Gallimore P. H., Sharp P. A. Comparison of viral RNA sequences in adenovirus 2-transformed and lytically infected cells. J Mol Biol. 1975 Jul 25;96(1):47–68. doi: 10.1016/0022-2836(75)90181-3. [DOI] [PubMed] [Google Scholar]
- Flint S. J., Sharp P. A. Adenovirus transcription. V. Quantitation of viral RNA sequences in adenovirus 2-infected and transformed cells. J Mol Biol. 1976 Sep 25;106(3):749–774. doi: 10.1016/0022-2836(76)90263-1. [DOI] [PubMed] [Google Scholar]
- Frenkel N., Roizman B. Ribonucleic acid synthesis in cells infected with herpes simplex virus: controls of transcription and of RNA abundance. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2654–2658. doi: 10.1073/pnas.69.9.2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M. R., Green M., Mackey J. K. Evidence for post-transcriptional selection of viral mRNA in cells transformed by human adenovirus 12. Nature. 1976 May 27;261(5558):340–342. doi: 10.1038/261340a0. [DOI] [PubMed] [Google Scholar]
- Landgraf-Leurs M., Green M. Adenovirus DNA. 3. Separation of the complementary strands of adenovirus types 2, 7 and 12 DNA molecules. J Mol Biol. 1971 Aug 28;60(1):185–202. doi: 10.1016/0022-2836(71)90457-8. [DOI] [PubMed] [Google Scholar]
- Landgraf-Leurs M., Green M. DNA strand selection during the transcription of the adenovirus 2 genome in infected and transformed cells. Biochim Biophys Acta. 1973 Jul 27;312(4):667–673. doi: 10.1016/0005-2787(73)90070-1. [DOI] [PubMed] [Google Scholar]
- Parsons J. T., Green M. Biochemical studies on adenovirus multiplication. 18. Resolution of early virus-specific RNA species in Ad 2 infected and transformed cells. Virology. 1971 Jul;45(1):154–162. doi: 10.1016/0042-6822(71)90122-x. [DOI] [PubMed] [Google Scholar]
- Pettersson U., Tibbetts C., Philipson L. Hybridization maps of early and late messenger RNA sequences on the adenovirus type 2 genome. J Mol Biol. 1976 Mar 15;101(4):479–501. doi: 10.1016/0022-2836(76)90241-2. [DOI] [PubMed] [Google Scholar]
- Sharp P. A., Gallimore P. H., Flint S. J. Mapping of adenovirus 2 RNA sequences in lytically infected cells and transformed cell lines. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):457–474. doi: 10.1101/sqb.1974.039.01.058. [DOI] [PubMed] [Google Scholar]
- Smith M. J., Britten R. J., Davidson E. H. Studies on nucleic acid reassociation kinetics: reactivity of single-stranded tails in DNA-DNA renaturation. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4805–4809. doi: 10.1073/pnas.72.12.4805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D. C., Green M. Biochemical studies on adenovirus multiplication. XV. Transcription of the adenovirus type II genome during productive infection. Virology. 1969 Oct;39(2):205–210. doi: 10.1016/0042-6822(69)90040-3. [DOI] [PubMed] [Google Scholar]
- Tibbetts C., Pettersson U. Complementary strand-specific sequences from unique fragments of adenovirus type 2 DNA for hybridization-mapping experiments. J Mol Biol. 1974 Oct 5;88(4):767–784. doi: 10.1016/0022-2836(74)90398-2. [DOI] [PubMed] [Google Scholar]
- Tibbetts C., Pettersson U., Johansson K., Philpson L. Relationship of mRNA from productively infected cells to the complementary strands of adenovirus type 2 DNA. J Virol. 1974 Feb;13(2):370–377. doi: 10.1128/jvi.13.2.370-377.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall R., Philipson L., Darnell J. E. Processing of adenovirus specific nuclear RNA during virus replication. Virology. 1972 Oct;50(1):27–34. doi: 10.1016/0042-6822(72)90342-x. [DOI] [PubMed] [Google Scholar]
- Wetmur J. G. Hybridization and renaturation kinetics of nucleic acids. Annu Rev Biophys Bioeng. 1976;5:337–361. doi: 10.1146/annurev.bb.05.060176.002005. [DOI] [PubMed] [Google Scholar]
- Wold W. S., Green M., Brackmann K. H., Cartas M. A., Devine C. Genome expression and mRNA maturation at late stages of productive adenovirus type 2 infection. J Virol. 1976 Nov;20(2):465–477. doi: 10.1128/jvi.20.2.465-477.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer S. G., Raskas H. J. Synthesis of complementary viral transcripts early in productive infection with adenovirus 2. Virology. 1976 Mar;70(1):118–126. doi: 10.1016/0042-6822(76)90241-5. [DOI] [PubMed] [Google Scholar]


