Abstract
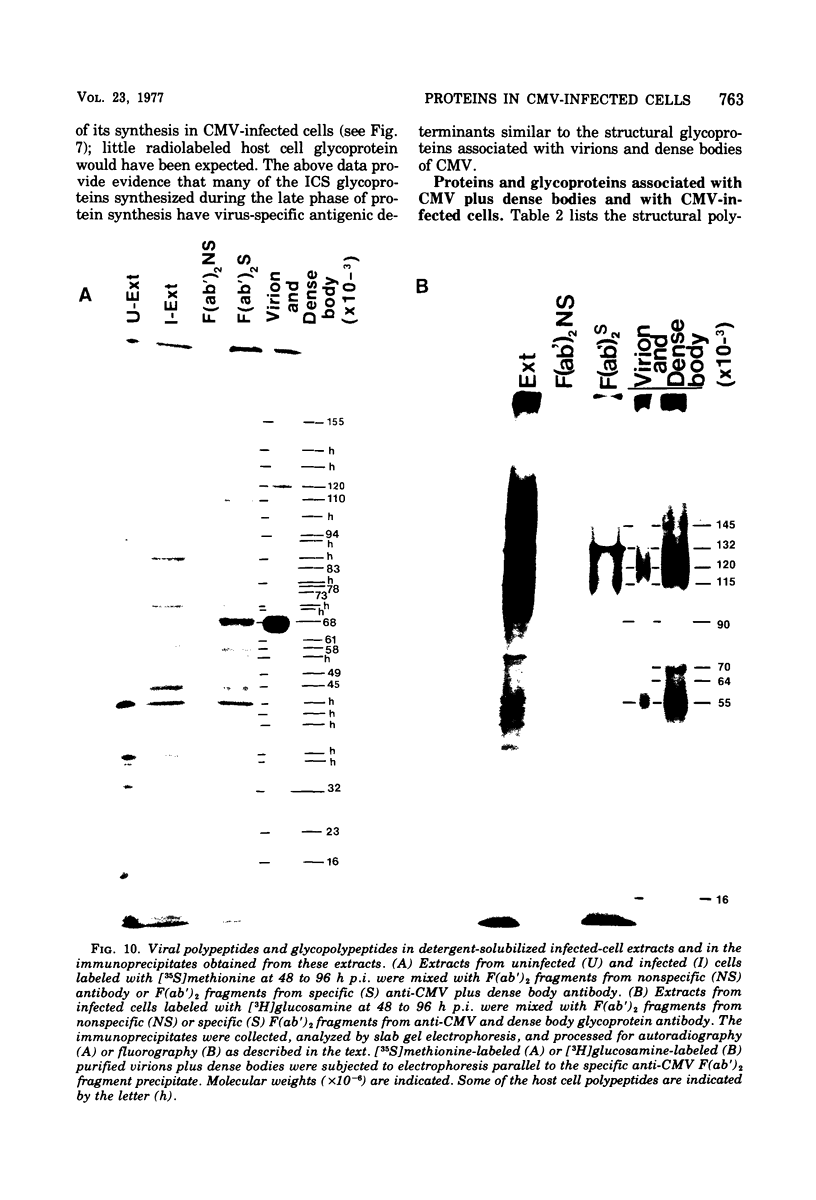
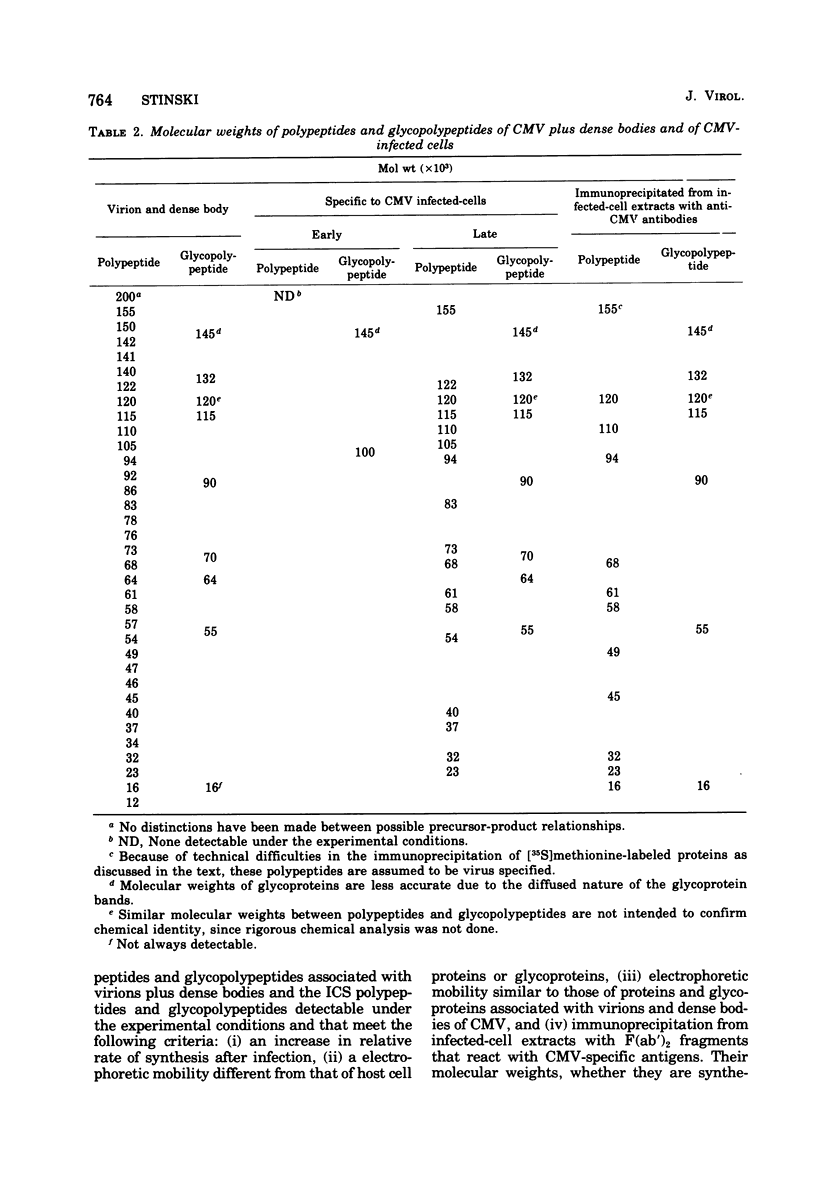
In cytomegalovirus-infected cells, the rate of protein synthesis was detected as two peaks. One occurred during the early phase of infection, 0 to 36 h postinfection, and the other occurred during the late phase, after the initiation of viral DNA synthesis. Double-isotopic-label difference analysis demonstrated that host and viral proteins were synthesized simultaneously during both phases. In the early phase, approximately 70 to 90% of the total proteins synthesized were host proteins, whereas approximately 10 to 30% were viral, even at a multiplicity of infection of 20 PFU/cell. Virus-related proteins or glycoproteins were referred to as infected-cell specific (ICS). Two ICS glycoproteins (gp145 and 100) were clearly detectable and were synthesized preferentially in the early phase of infection. Their synthesis was concomitant with stimulation of the protein synthesis rate. In the late phase of infection, approximately 50 to 60% of the total protein synthesis was viral and approximately 40 to 50% was host. The ICS proteins and glycoproteins detected during the late phase of infection were viral structural proteins. Infectious virus was not detectable until 48 to 72 h postinfection. An inhibitor of viral DNA synthesis, phosphonoacetic acid, prevented the appearance of the late-phase ICS proteins and glycoproteins, but there was little or no effect on early ICS glycoprotein synthesis. Radiolabeled ICS proteins and glycoproteins were identified by their relative rates of synthesis, by their different electrophoretic mobilities compared with those of host proteins and host glycoproteins, and by their similar electrophoretic mobilities compared to those of proteins and glycoproteins associated with virions and dense bodies of cytomegalovirus. Structural viral antigens in the infected-cell extracts were removed by immunoprecipitation, using F(ab′)2 fragments of cytomegalovirus-specific antibodies, and identified as described above. The last two criteria were used to identify viral structural ICS proteins and glycoproteins. Although approximately 35 structural proteins were found to be associated with purified virions and dense bodies, the continued synthesis of host cell proteins complicated their identification in infected cells. Nevertheless, seven of the nine structural glycoproteins were identified as ICS glycoproteins.
Full text
PDF














Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albrecht T., Nachtigal M., St Jeor S. C., Rapp F. Induction of cellular DNA synthesis and increased mitotic activity in syrian hamster embryo cells abortively infected with human cytomegalovirus. J Gen Virol. 1976 Feb;30(2):167–177. doi: 10.1099/0022-1317-30-2-167. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- DeMarchi J. M., Kaplan A. S. Replication of human cytomegalovirus DNA: lack of dependence on cell DNA synthesis. J Virol. 1976 Jun;18(3):1063–1070. doi: 10.1128/jvi.18.3.1063-1070.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Fiala M., Honess R. W., Heiner D. C., Heine J. W., Jr, Murnane J., Wallace R., Guze L. B. Cytomegalovirus proteins. I. Polypeptides of virions and dense bodies. J Virol. 1976 Jul;19(1):243–254. doi: 10.1128/jvi.19.1.243-254.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa T., Fioretti A., Plotkin S. Growth characteristics of cytomegalovirus in human fibroblasts with demonstration of protein synthesis early in viral replication. J Virol. 1973 Jun;11(6):991–997. doi: 10.1128/jvi.11.6.991-997.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa T., Sakuma S., Plotkin S. A. Human cytomegalovirus infection of WI-38 cells stimulates mitochondrial DNA synthesis. Nature. 1976 Jul 29;262(5567):414–416. doi: 10.1038/262414a0. [DOI] [PubMed] [Google Scholar]
- Furukawa T., Tanaka S., Plotkin S. A. Stimulation of macromolecular synethesis in guinea pig cells by human CMV. Proc Soc Exp Biol Med. 1975 Jan;148(1):211–214. doi: 10.3181/00379727-148-38508. [DOI] [PubMed] [Google Scholar]
- Geder L. Evidence for early nuclear antigens in cytomegalovirus-infected cells. J Gen Virol. 1976 Aug;32(2):315–319. doi: 10.1099/0022-1317-32-2-315. [DOI] [PubMed] [Google Scholar]
- Hightower L. E., Bratt M. A. Protein synthesis in Newcastle disease virus-infected chicken embryo cells. J Virol. 1974 Apr;13(4):788–800. doi: 10.1128/jvi.13.4.788-800.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honess R. W., Roizman B. Proteins specified by herpes simplex virus. XI. Identification and relative molar rates of synthesis of structural and nonstructural herpes virus polypeptides in the infected cell. J Virol. 1973 Dec;12(6):1347–1365. doi: 10.1128/jvi.12.6.1347-1365.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honess R. W., Roizman B. Regulation of herpesvirus macromolecular synthesis. I. Cascade regulation of the synthesis of three groups of viral proteins. J Virol. 1974 Jul;14(1):8–19. doi: 10.1128/jvi.14.1.8-19.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honess R. W., Roizman B. Regulation of herpesvirus macromolecular synthesis: sequential transition of polypeptide synthesis requires functional viral polypeptides. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1276–1280. doi: 10.1073/pnas.72.4.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang E. S., Chen S. T., Pagano J. S. Human cytomegalovirus. I. Purification and characterization of viral DNA. J Virol. 1973 Dec;12(6):1473–1481. doi: 10.1128/jvi.12.6.1473-1481.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang E. S. Human cytomegalovirus. IV. Specific inhibition of virus-induced DNA polymerase activity and viral DNA replication by phosphonoacetic acid. J Virol. 1975 Dec;16(6):1560–1565. doi: 10.1128/jvi.16.6.1560-1565.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeor S. C., Albrecht T. B., Funk F. D., Rapp F. Stimulation of cellular DNA synthesis by human cytomegalovirus. J Virol. 1974 Feb;13(2):353–362. doi: 10.1128/jvi.13.2.353-362.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan A. S. A brief review of the biochemistry of herpesvirus-host cell interaction. Cancer Res. 1973 Jun;33(6):1393–1398. [PubMed] [Google Scholar]
- Kim K. S., Sapienza V. J., Carp R. I., Moon H. M. Analysis of structural polypeptides of purified human cytomegalovirus. J Virol. 1976 Dec;20(3):604–611. doi: 10.1128/jvi.20.3.604-611.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lamb R. A., Mahy B. W., Choppin P. W. The synthesis of sendai virus polypeptides in infected cells. Virology. 1976 Jan;69(1):116–131. doi: 10.1016/0042-6822(76)90199-9. [DOI] [PubMed] [Google Scholar]
- Mao J. C., Robishaw E. E., Overby L. R. Inhibition of DNA polymerase from herpes simplex virus-infected wi-38 cells by phosphonoacetic Acid. J Virol. 1975 May;15(5):1281–1283. doi: 10.1128/jvi.15.5.1281-1283.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NISONOFF A., WISSLER F. C., LIPMAN L. N., WOERNLEY D. L. Separation of univalent fragments from the bivalent rabbit antibody molecule by reduction of disulfide bonds. Arch Biochem Biophys. 1960 Aug;89:230–244. doi: 10.1016/0003-9861(60)90049-7. [DOI] [PubMed] [Google Scholar]
- Overby L. R., Robishaw E. E., Schleicher J. B., Rueter A., Shipkowitz N. L., Mao J. C. Inhibition of herpes simplex virus replication by phosphonoacetic acid. Antimicrob Agents Chemother. 1974 Sep;6(3):360–365. doi: 10.1128/aac.6.3.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell K. L., Courtney R. J. Polypeptide synthesized in herpes simplex virus type 2-infected HEp-2 cells. Virology. 1975 Jul;66(1):217–228. doi: 10.1016/0042-6822(75)90192-0. [DOI] [PubMed] [Google Scholar]
- Rakusanova T., Ben-Porat T., Kaplan A. S. Effect of herpesvirus infection on the synthesis of cell-specific RNA. Virology. 1972 Aug;49(2):537–548. doi: 10.1016/0042-6822(72)90505-3. [DOI] [PubMed] [Google Scholar]
- Spear P. G. Membrane proteins specified by herpes simplex viruses. I. Identification of four glycoprotein precursors and their products in type 1-infected cells. J Virol. 1976 Mar;17(3):991–1008. doi: 10.1128/jvi.17.3.991-1008.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinski M. F., Ginsberg H. S. Hexon peptides of type 2, 3, and 5 adenoviruses and their relationship to hexon structure. J Virol. 1975 Apr;15(4):898–905. doi: 10.1128/jvi.15.4.898-905.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinski M. F. Human cytomegalovirus: glycoproteins associated with virions and dense bodies. J Virol. 1976 Aug;19(2):594–609. doi: 10.1128/jvi.19.2.594-609.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strnad B. C., Aurelian L. Proteins of herpesvirus type 2: I. Virion, nonvirion, and antigenic polypeptides in infected cells. Virology. 1976 Feb;69(2):438–452. doi: 10.1016/0042-6822(76)90475-x. [DOI] [PubMed] [Google Scholar]
- Tanaka S., Furukawa T., Plotkin S. A. Human cytomegalovirus stimulates host cell RNA synthesis. J Virol. 1975 Feb;15(2):297–304. doi: 10.1128/jvi.15.2.297-304.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Wengler G. Protein synthesis in BHK-21 cells infected with semliki forest virus. J Virol. 1975 Jan;17(1):10–19. doi: 10.1128/jvi.17.1.10-19.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wentworth B. B., French L. Plaque assay of cytomegalovirus strains of human origin. Proc Soc Exp Biol Med. 1970 Nov;135(2):253–258. doi: 10.3181/00379727-135-35031. [DOI] [PubMed] [Google Scholar]







