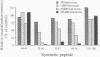Abstract
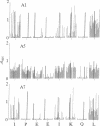
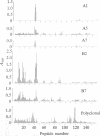
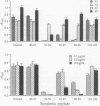
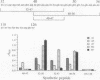
The location of biologically relevant epitopes on recombinant human beta interferon in which Ser-17 replaces Cys-17 (rh[Ser17]IFN-beta) was evaluated by testing the immunoreactivity of antibodies against 159 sequential, overlapping octamer peptides. Three monoclonal antibodies (mAbs) that neutralize rh[Ser17]IFN-beta biologic activity, designated A1, A5, and A7, bound to peptides spanning only residues 39-48, whereas nonneutralizing mAb bound less specifically at multiple sites near the amino terminus. The immunoreactivity of peptides spanning residues 40-47 that contained a series of single amino acid substitutions suggested that residues 41-43 (Pro-Glu-Glu) and 46 (Gln) are important for the binding of neutralizing mAbs. The reactivity of mAbs to larger synthetic peptides containing rh[Ser17]IFN-beta sequences from residue 32 through residue 56 was evaluated. All mAbs except A7 reacted with synthetic peptides representing rh[Ser17]IFN-beta residues 32-47, 40-56, and 32-56, but only mAbs A1 and A5 bound to the core peptide composed of residues 40-47. Peptide 32-56 effectively blocked the binding of mAbs A1 and A5 to rh[Ser17]IFN-beta and markedly inhibited their neutralizing activity. Biologic activity of the peptides was undetectable. Rabbit antisera raised against peptides 32-47 and 40-56 recognized rh[Ser17]IFN-beta but did not neutralize its antiviral activity. Thus, structure-function analysis by peptide mapping has permitted the identification of a linear epitope recognized by neutralizing antibody on a biologically active cytokine. We conclude that the region spanning residues 32-56 is of major importance in the expression of the biologic activity of human IFN-beta.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackerman S. K., Zur Nedden D., Heintzelman M., Hunkapiller M., Zoon K. Biologic activity in a fragment of recombinant human interferon alpha. Proc Natl Acad Sci U S A. 1984 Feb;81(4):1045–1047. doi: 10.1073/pnas.81.4.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amit A. G., Mariuzza R. A., Phillips S. E., Poljak R. J. Three-dimensional structure of an antigen-antibody complex at 2.8 A resolution. Science. 1986 Aug 15;233(4765):747–753. doi: 10.1126/science.2426778. [DOI] [PubMed] [Google Scholar]
- Antoni G., Presentini R., Perin F., Tagliabue A., Ghiara P., Censini S., Volpini G., Villa L., Boraschi D. A short synthetic peptide fragment of human interleukin 1 with immunostimulatory but not inflammatory activity. J Immunol. 1986 Nov 15;137(10):3201–3204. [PubMed] [Google Scholar]
- Arnheiter H., Thomas R. M., Leist T., Fountoulakis M., Gutte B. Physicochemical and antigenic properties of synthetic fragments of human leukocyte interferon. Nature. 1981 Nov 19;294(5838):278–280. doi: 10.1038/294278a0. [DOI] [PubMed] [Google Scholar]
- Barasoain I., Portolés A., Aramburu J. F., Rojo J. M. Antibodies against a peptide representative of a conserved region of human IFN-alpha. Differential effects on the antiviral and antiproliferative effects of IFN. J Immunol. 1989 Jul 15;143(2):507–512. [PubMed] [Google Scholar]
- Benjamin D. C., Berzofsky J. A., East I. J., Gurd F. R., Hannum C., Leach S. J., Margoliash E., Michael J. G., Miller A., Prager E. M. The antigenic structure of proteins: a reappraisal. Annu Rev Immunol. 1984;2:67–101. doi: 10.1146/annurev.iy.02.040184.000435. [DOI] [PubMed] [Google Scholar]
- Berzofsky J. A. Intrinsic and extrinsic factors in protein antigenic structure. Science. 1985 Sep 6;229(4717):932–940. doi: 10.1126/science.2410982. [DOI] [PubMed] [Google Scholar]
- Camble R., Petter N. N., Trueman P., Newton C. R., Carr F. J., Hockney R. C., Moore V. E., Greene A. R., Holland D., Edge M. D. Functionally important conserved amino-acids in interferon-alpha 2 identified with analogues produced from synthetic genes. Biochem Biophys Res Commun. 1986 Feb 13;134(3):1404–1411. doi: 10.1016/0006-291x(86)90405-5. [DOI] [PubMed] [Google Scholar]
- Carter W. A., Swartz H., Gillespie D. H. Independent evolution of antiviral and growth-modulating activities of interferon. J Biol Response Mod. 1985 Oct;4(5):447–459. [PubMed] [Google Scholar]
- Chow T. P., DeGrado W. F., Knight E., Jr Antibodies to synthetic peptides of human interferon-beta. Use in biosynthetic studies. J Biol Chem. 1984 Oct 10;259(19):12220–12225. [PubMed] [Google Scholar]
- Colman P. M., Laver W. G., Varghese J. N., Baker A. T., Tulloch P. A., Air G. M., Webster R. G. Three-dimensional structure of a complex of antibody with influenza virus neuraminidase. 1987 Mar 26-Apr 1Nature. 326(6111):358–363. doi: 10.1038/326358a0. [DOI] [PubMed] [Google Scholar]
- Geysen H. M., Meloen R. H., Barteling S. J. Use of peptide synthesis to probe viral antigens for epitopes to a resolution of a single amino acid. Proc Natl Acad Sci U S A. 1984 Jul;81(13):3998–4002. doi: 10.1073/pnas.81.13.3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geysen H. M., Rodda S. J., Mason T. J., Tribbick G., Schoofs P. G. Strategies for epitope analysis using peptide synthesis. J Immunol Methods. 1987 Sep 24;102(2):259–274. doi: 10.1016/0022-1759(87)90085-8. [DOI] [PubMed] [Google Scholar]
- Goudsmit J., Boucher C. A., Meloen R. H., Epstein L. G., Smit L., van der Hoek L., Bakker M. Human antibody response to a strain-specific HIV-1 gp120 epitope associated with cell fusion inhibition. AIDS. 1988 Jun;2(3):157–164. [PubMed] [Google Scholar]
- Hoeprich P. D., Jr, Langton B. C., Zhang J. W., Tam J. P. Identification of immunodominant regions of transforming growth factor alpha. Implications of structure and function. J Biol Chem. 1989 Nov 15;264(32):19086–19091. [PubMed] [Google Scholar]
- Kuo L. M., Robb R. J. Structure-function relationships for the IL 2-receptor system. I. Localization of a receptor binding site on IL 2. J Immunol. 1986 Sep 1;137(5):1538–1543. [PubMed] [Google Scholar]
- Kusters J. G., Jager E. J., Lenstra J. A., Koch G., Posthumus W. P., Meloen R. H., van der Zeijst B. A. Analysis of an immunodominant region of infectious bronchitis virus. J Immunol. 1989 Oct 15;143(8):2692–2698. [PubMed] [Google Scholar]
- Lando G., Reichlin M. Antigenic structure of sperm whale myoglobin. II. Characterization of antibodies preferentially reactive with peptides arising in response to immunization with the native protein. J Immunol. 1982 Jul;129(1):212–216. [PubMed] [Google Scholar]
- Leist T., Thomas R. M. Synthesis and physicochemical characterization of major fragments of human leucocyte interferon alpha 1. Biochemistry. 1984 Jun 5;23(12):2541–2547. doi: 10.1021/bi00307a001. [DOI] [PubMed] [Google Scholar]
- Lew A. M., Langford C. J., Anders R. F., Kemp D. J., Saul A., Fardoulys C., Geysen M., Sheppard M. A protective monoclonal antibody recognizes a linear epitope in the precursor to the major merozoite antigens of Plasmodium chabaudi adami. Proc Natl Acad Sci U S A. 1989 May;86(10):3768–3772. doi: 10.1073/pnas.86.10.3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lydon N. B., Favre C., Bove S., Neyret O., Benureau S., Levine A. M., Seelig G. F., Nagabhushan T. L., Trotta P. P. Immunochemical mapping of alpha-2 interferon. Biochemistry. 1985 Jul 16;24(15):4131–4141. doi: 10.1021/bi00336a048. [DOI] [PubMed] [Google Scholar]
- Massone A., Baldari C., Censini S., Bartalini M., Nucci D., Boraschi D., Telford J. L. Mapping of biologically relevant sites on human IL-1 beta using monoclonal antibodies. J Immunol. 1988 Jun 1;140(11):3812–3816. [PubMed] [Google Scholar]
- Meloen R. H., Barteling S. J. Epitope mapping of the outer structural protein VP1 of three different serotypes of foot-and-mouth disease virus. Virology. 1986 Feb;149(1):55–63. doi: 10.1016/0042-6822(86)90086-3. [DOI] [PubMed] [Google Scholar]
- Meloen R. H., Puyk W. C., Meijer D. J., Lankhof H., Posthumus W. P., Schaaper W. M. Antigenicity and immunogenicity of synthetic peptides of foot-and-mouth disease virus. J Gen Virol. 1987 Feb;68(Pt 2):305–314. doi: 10.1099/0022-1317-68-2-305. [DOI] [PubMed] [Google Scholar]
- Redlich P. N., Grossberg S. E. Analysis of antigenic domains on natural and recombinant human IFN-beta by the inhibition of biologic activities with monoclonal antibodies. J Immunol. 1989 Sep 15;143(6):1887–1893. [PubMed] [Google Scholar]
- Redlich P. N., Grossberg S. E. Immunochemical characterization of antigenic domains on human interferon-beta: spatially distinct epitopes are associated with both antiviral and antiproliferative activities. Eur J Immunol. 1990 Sep;20(9):1933–1939. doi: 10.1002/eji.1830200910. [DOI] [PubMed] [Google Scholar]
- Ruzicka F. J., Jach M. E., Borden E. C. Binding of recombinant-produced interferon beta ser to human lymphoblastoid cells. Evidence for two binding domains. J Biol Chem. 1987 Nov 25;262(33):16142–16149. [PubMed] [Google Scholar]
- Shafferman A., Velan B., Cohen S., Leitner M., Grosfeld H. Specific residues within an amino-terminal domain of 35 residues of interferon alpha are responsible for recognition of the human interferon alpha cell receptor and for triggering biological effects. J Biol Chem. 1987 May 5;262(13):6227–6237. [PubMed] [Google Scholar]
- Shepard H. M., Leung D., Stebbing N., Goeddel D. V. A single amino acid change in IFN-beta1 abolishes its antiviral activity. Nature. 1981 Dec 10;294(5841):563–565. doi: 10.1038/294563a0. [DOI] [PubMed] [Google Scholar]
- Shi P. T., Riehm J. P., Todd P. E., Leach S. J. The antigenicity of myoglobin-related peptides synthesised on polyacrylamide and polystyrene resin supports. Mol Immunol. 1984 Jun;21(6):489–496. doi: 10.1016/0161-5890(84)90064-6. [DOI] [PubMed] [Google Scholar]
- Socher S. H., Riemen M. W., Martinez D., Friedman A., Tai J., Quintero J. C., Garsky V., Oliff A. Antibodies against amino acids 1-15 of tumor necrosis factor block its binding to cell-surface receptor. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8829–8833. doi: 10.1073/pnas.84.24.8829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart A. G., Adair J. R., Catlin G., Hynes C., Hall J., Davies J., Dawson K., Porter A. G. Chemical mutagenesis of human interferon-beta: construction, expression in E. coli, and biological activity of sodium bisulfite-induced mutations. DNA. 1987 Apr;6(2):119–128. doi: 10.1089/dna.1987.6.119. [DOI] [PubMed] [Google Scholar]
- Streuli M., Hall A., Boll W., Stewart W. E., 2nd, Nagata S., Weissmann C. Target cell specificity of two species of human interferon-alpha produced in Escherichia coli and of hybrid molecules derived from them. Proc Natl Acad Sci U S A. 1981 May;78(5):2848–2852. doi: 10.1073/pnas.78.5.2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor-Papadimitriou J., Shearer M., Griffin D. Epitopes of human interferon-alpha defined by the reaction of monoclonal antibodies with alpha interferons and interferon analogues. J Immunol. 1987 Nov 15;139(10):3375–3381. [PubMed] [Google Scholar]
- Tymms M. J., McInnes B., Waine G. J., Cheetham B. F., Linnane A. W. Functional significance of amino acid residues within conserved hydrophilic regions in human interferons-alpha. Antiviral Res. 1989 Aug;12(1):37–47. doi: 10.1016/0166-3542(89)90066-1. [DOI] [PubMed] [Google Scholar]
- Van der Zee R., Van Eden W., Meloen R. H., Noordzij A., Van Embden J. D. Efficient mapping and characterization of a T cell epitope by the simultaneous synthesis of multiple peptides. Eur J Immunol. 1989 Jan;19(1):43–47. doi: 10.1002/eji.1830190108. [DOI] [PubMed] [Google Scholar]
- Velan B., Cohen S., Grosfeld H., Leitner M., Shafferman A. Bovine interferon alpha genes. Structure and expression. J Biol Chem. 1985 May 10;260(9):5498–5504. [PubMed] [Google Scholar]
- Ziltener H. J., Clark-Lewis I., Fazekas de St Groth B., Orban P. C., Hood L. E., Kent S. B., Schrader J. W. Monoclonal antipeptide antibodies recognize IL-3 and neutralize its bioactivity in vivo. J Immunol. 1988 Feb 15;140(4):1182–1187. [PubMed] [Google Scholar]
- Zoon K., Zur Nedden D., Arnheiter H. Specific binding of human alpha interferon to a high affinity cell surface binding site on bovine kidney cells. J Biol Chem. 1982 May 10;257(9):4695–4697. [PubMed] [Google Scholar]
- de Vos A. M., Tong L., Milburn M. V., Matias P. M., Jancarik J., Noguchi S., Nishimura S., Miura K., Ohtsuka E., Kim S. H. Three-dimensional structure of an oncogene protein: catalytic domain of human c-H-ras p21. Science. 1988 Feb 19;239(4842):888–893. doi: 10.1126/science.2448879. [DOI] [PubMed] [Google Scholar]