Abstract
Background & Aims
Aspirin use reduces colorectal cancer risk. Aspirin, a nonsteroidal anti-inflammatory drug, inhibits PTGS2 (cyclooxygenase-2); PTGS2 promotes inflammation and suppresses T cell-mediated adaptive immunity. We investigated whether the inverse association of aspirin use with colorectal carcinoma risk was stronger for tumors with lower degrees of lymphocytic infiltrates than for tumors with higher degrees of lymphocytic infiltrates.
Methods
We collected aspirin use data biennially from participants in the Nurses’ Health Study and Health Professionals Follow-up Study. Participants were asked whether they took aspirin in most weeks, the number of tablets taken per week, and years of aspirin use. We collected available tumor specimens (n=1458) from pathology laboratories in the US. A pathologist confirmed the diagnosis of colorectal adenocarcinoma (excluding anal squamous cell carcinoma), and evaluated histopathology features, including patterns and degrees of lymphocytic infiltrates within and around tumor areas. Person-years of follow-up were accrued from the date of return of questionnaires until dates of colorectal cancer diagnosis, death, or the end of follow-up (June 2010). Duplication-method Cox proportional hazards regression was used to assess the association of aspirin with incidence of colorectal carcinoma subgroups according to the degree of tumor-infiltrating lymphocytes (TILs), intratumoral periglandular reaction, peritumoral reaction, or Crohn’s-like reaction.
Results
We documented 1458 rectal and colon cancers. The inverse association between regular aspirin use and colorectal cancer risk significantly differed by concentrations of TILs (Pheterogeneity =0.007). Compared with nonregular use, regular aspirin use was associated with a lower risk of tumors that had low levels of TILs (relative risk, 0.72; 95% CI, 0.63–0.81); strength of the association depended on aspirin dose and duration (both Ptrend <0.001). In contrast, aspirin use was not associated with a risk of tumors having intermediate or high levels TILs. This differential association was consistent regardless of status of tumor microsatellite instability, mutations in BRAF, or expression of PTGS2. Regular aspirin use was associated with a lower risk of tumors that contained low levels of CD3+ T cells, CD8+ T cells, or CD45RO (PTPRC)+ T cells (measured by immunohistochemistry and computer-assisted image analysis).
Conclusions
Based on data from the prospective cohort studies, regular use of aspirin is associated with a lower risk of colorectal carcinomas with low concentrations of TILs. These findings indicate that the immune response in the tumor microenvironment could be involved in the chemopreventive effects of aspirin.
Keywords: immunoprevention, molecular pathological epidemiology, NSAID, pharmacoepidemiology
Introduction
Colorectal cancer is the second leading cause of cancer death in the United States.1 Evidence from epidemiological studies and clinical trials suggests that aspirin can reduce the risk of colorectal cancer;2, 3 however, the mechanisms remain incompletely understood.4–7 Despite the well-recognized importance of the complex interactions between neoplastic and immune cells in the tumor microenvironment,8–11 whether the anti-tumor effect of aspirin might differ by immune status in the tumor microenvironment has been under-explored.
We have previously shown that the benefit of aspirin might be stronger for colorectal cancers with overexpression of prostaglandin-endoperoxide synthase 2 (PTGS2 or cyclooxygenase-2) compared to colorectal cancers lacking PTGS2 overexpression.12 In other words, aspirin appears to inhibit the development of tumors dependent at least in part on PTGS2 for their growth. Given evidence supporting a role of PTGS2 of tumor cells in suppressing T cell-mediated anti-tumor immunity,13–15 we further postulated that aspirin’s role in enhancing anti-tumor immune responses may also underlie its anti-cancer benefit. Thus, we would expect that the inverse association between aspirin use and colorectal cancer risk might be stronger for tumors that arise due to greater suppression of anti-tumor immunity as reflected by low-level lymphocytic infiltrates compared with tumors with more robust anti-tumor immunity as reflected by high-level lymphocytic infiltrates.
To examine this hypothesis, we took a unique approach of integrating longitudinal data on aspirin use with analyses of immune cells in incident cancer tissue, utilizing the resources of two large prospective cohort studies. We investigated the association of regular aspirin use with the risk of colorectal cancer according to the pattern and intensity of histopathological lymphocytic reactions. As an exploratory analysis, we also examined T cell densities in tumor tissue using cases with available tissue microarray (TMA) and image analysis data. In addition, our existing tumor characteristics data enabled us to control for key tumor tissue biomarkers, including PTGS2 expression, BRAF mutation, and microsatellite instability (MSI) status (the latter of which has been associated with immune response in colorectal cancer16, 17).
Methods
Study population
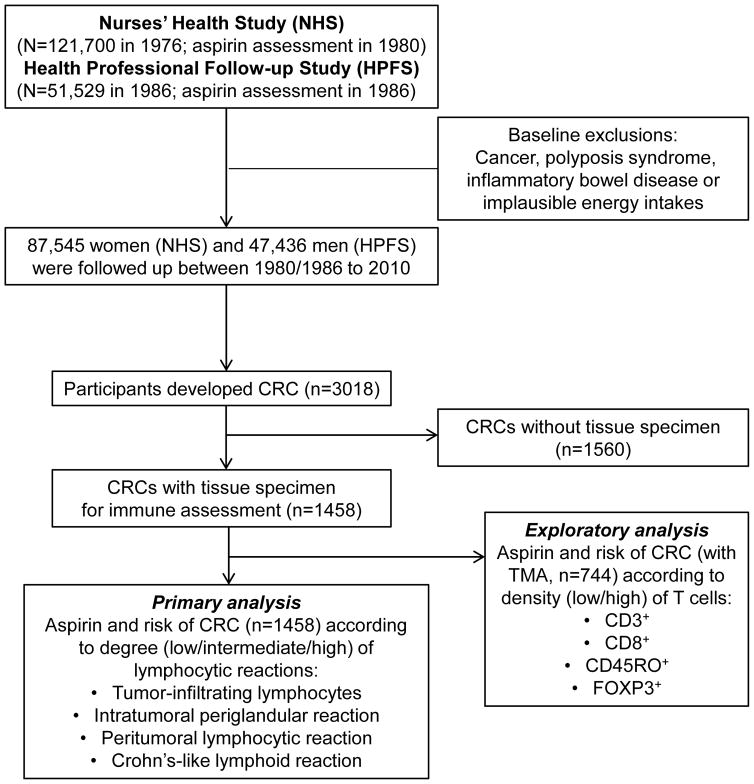
We utilized two ongoing prospective cohort studies; the Nurses’ Health Study (NHS), a cohort study of 121,700 U.S. female nurses aged 30–55 at enrollment in 1976, and the Health Professionals Follow-up Study (HPFS), a cohort study of 51,529 U.S. male health professionals aged 40–75 at enrollment in 1986 (Figure 1). Participants have been mailed questionnaires at enrolment, and every 2 years thereafter, to collect data on demographics, lifestyle factors, medical history, and disease outcomes, and every 4 years to update dietary intake. The follow-up rates in both cohorts have been greater than 90%. The institutional review boards of the Harvard T.H. Chan School of Public Health and Partners Healthcare approved the study protocol.
Figure 1.
Flow diagram of the study population.
Assessment of aspirin use
A detailed description of the collection of information on aspirin use has been published previously.12 Briefly, in the NHS, aspirin use was first assessed in 1980 and every 2 years thereafter, except in 1986. NHS participants were asked whether they took aspirin in most weeks, the number of tablets taken per week, and years of aspirin usage. We updated the information on the number of aspirin tablets taken per week (in categories) every 2 years. Consistent with our prior analyses,12, 18 regular aspirin users were defined as women who reported consumption of 2 or more aspirin tablets per week and nonregular users as women who used fewer than 2 tablets per week, or no aspirin. In the HPFS, in 1986 and every 2 years thereafter, participants were asked whether they used aspirin 2 or more times per week. Beginning in 1994, the mean number of tablets taken per week was assessed.
For both cohorts, participants were specifically asked about standard-dose (325 mg) aspirin tablets. Beginning in 1994, to reflect secular trends in aspirin use, participants were also asked to convert intake of 4 low-dose (81 mg) aspirin (baby aspirin) tablets to 1 standard aspirin tablet in their responses. Since 2000, we asked about low-dose aspirin use separately in both cohorts. The major reasons for aspirin use were arthritis and other musculoskeletal pain, headache, and cardiovascular disease prevention. In addition, we also collected updated information on regular use (2 or more times per week) of other non-steroidal anti-inflammatory drugs (NSAIDs, including Motrin, Advil, Nuprin, Indocin, Dolobid, Aleve, Naprosyn, Anaprox, Relafen, and Ketoprofen).
Ascertainment of colorectal cancer cases
We requested written permission to acquire medical records and pathology reports from participants who reported colorectal cancer on biennial questionnaires. We identified unreported lethal colorectal cancer cases through the National Death Index and next-of-kin. For all deaths attributable to colorectal cancer, we requested permission from next-of-kin to review medical records. A study physician, blinded to exposure information, reviewed records to extract information on anatomical location and stage. Cases related to inflammatory bowel diseases and those related to polyposis syndromes were excluded from the current analyses.
Tumor immunity and molecular analyses
We collected available tumor specimens (n=1458) from pathology laboratories across the U.S. (Figure 1). In each case, a study pathologist (S.O.) confirmed the diagnosis of colorectal carcinoma (excluding anal squamous cell carcinoma), and evaluated histopathological features, including patterns and degrees of lymphocytic infiltrates within and around tumor areas. Cases with preoperative treatment were excluded. There were no substantial differences in demographic or clinical features between cases with (n=1458) and without (n=1560) histopathologic immunity data (Supplemental Table 1). The four components of lymphocytic reaction, including tumor-infiltrating lymphocytes (TILs), intratumoral periglandular reaction, peritumoral lymphocytic reaction, and Crohn’s-like lymphoid reaction were recorded as previously described.17 Each component was evaluated as low, intermediate, or high, and an agreement study between independent reviews of more than 400 cases by two pathologists (S.O. and J. Glickman) showed a good concordance.17 We constructed tissue microarrays among a subset of cases (n=744), and performed immunohistochemistry for CD3+ cells, CD8+ cells, CD45RO+ (one of PTPRC protein isoforms) cells, and FOXP3+ cells (Figure 1). We performed image analysis using automated scanning microscope and Ariol image analysis system (Genetix, San Jose, California, USA), to calculate the average density (cells/mm2) of each T-cell subset in tumor tissue, as previously described.19 We dichotomized cases according to the median cutpoint for each marker. We have also analyzed microsatellite instability (MSI), BRAF mutation and PTGS2 expression status, as previously described.12
Statistical analysis
At baseline, we excluded participants who had cancer, polyposis syndrome, or inflammatory bowel disease, or reported implausible energy intakes (<600 or >3500 kcal/d for women, and <800 or >4200 kcal/d for men). Person-years of follow-up were accrued from the date of return of the 1980 questionnaire in the NHS and that of the 1986 questionnaire in the HPFS until the date of either colorectal cancer diagnosis, death, or the end of follow-up (June 2010 for the NHS and January 2010 for the HPFS), whichever came first. We examined the association between regular aspirin use and risk of colorectal cancer cases with histopathologic immunity data (n=1458; 863 cases from nonregular users vs 595 cases from regular users) using Cox proportional hazards regression model that censored cases without immunity data (n=1560; 968 cases from nonregular users vs 592 cases from regular users) at their time of diagnosis. Duplication-method Cox proportional cause-specific hazards regression for competing risks data was used to assess the association of aspirin with tumor subgroups according to the degree (low, intermediate, or high) of each lymphocytic reaction pattern (TILs, intratumoral periglandular reaction, peritumoral reaction, or Crohn’s-like reaction). When examining the association specific to one tumor subgroup, other subgroups were treated as competing events, and tumors of unknown subgroup (i.e., tumors without immunity data) were censored. Hazard ratios as estimates for age-adjusted and multivariable-adjusted relative risks (RRs) with 95% confidence intervals (CIs) were computed. Our primary hypothesis test was heterogeneity test on a difference in the RR for one subgroup (with low reaction), the RR for another subgroup (with intermediate reaction) and the RR for the third subgroup (with high reaction) as an ordinal statistical trend.20 Specifically, we assessed whether the magnitude of the subgroup-specific associations had an increasing or decreasing ordinal trend according to levels of lymphocytic reaction, with the statistical significance of this trend test (one degree of freedom) presented as “Pheterogeneity”. All other assessments were secondary analyses. To account for multiple hypothesis testing for the four lymphocytic reaction components, we used Bonferroni correction to adjust the statistical significance level to α = 0.012 (≈ 0.05/4). All analyses were performed using SAS V.9.3 (SAS Institute Inc, Cary, North Carolina, USA). All statistical tests were two-sided.
The Cox models were also conditioned on age in months, calendar year of the questionnaire cycle (and sex/cohort in the combined cohort analysis). Departures from the proportional hazards assumption were tested by likelihood ratio tests comparing models with and without the interaction terms of age or follow-up cycle by aspirin exposures and no significant violation of the proportionality assumption was found (P>0.05 for all tests). We used time-varying aspirin exposure and covariates (when applicable) such that each individual participant contributed person-time according to the aspirin and covariate data they provided on each biennial questionnaire. We adjusted for the following covariates in the multivariable models: family history of colorectal cancer (yes/no), history of diabetes (yes/no), body mass index (quartile), history of colonoscopy/sigmoidoscopy (yes/no; ever had a colonoscopy/sigmoidoscopy before study baseline and updated every 2 years during follow-up.), smoking in pack-years (never, 0.1–4.9, 5–19.9, 20–39.9, ≥40), physical activity (quartile), alcohol intake (0, 0.1–4.9, 5–14.9, 15–29.9, ≥30 g/d), current multivitamin use (yes/no), regular use of other NSAIDs (yes/no), total energy intake (quartile), folate (quartile), calcium (quartile), red and processed meat intake (quartile), and Alternate Healthy Eating Index-2010 without alcohol (quartile). For women, we additionally adjusted for menopausal status/menopausal hormone therapy (MHT) (premenopausal, postmenopausal and never use of MHT, postmenopausal and past use of MHT, postmenopausal and current use of MHT). To capture potential confounding by diet, we adjusted for Alternate Healthy Eating Index (AHEI)-2010,21 which features higher consumption of vegetables (excluding potatoes), whole fruit, whole grains, nuts and legumes, long chain omega-3 fatty acids, polyunsaturated fatty acids; and a lower consumption of sugar-sweetened beverages, red/processed meat, sodium, trans fat, and moderate alcohol consumption. Adherence to the AHEI-2010 has been associated with reduced risk of cardiovascular disease, diabetes and cancer in our cohorts.22 Because alcohol was included as a separate term in our model, we used a modified AHEI-2010 without alcohol consumption.
We further examined the associations of dose (tablets/week) and duration (years) of aspirin use with risk of colorectal cancer according to levels of TILs. Tests for linear trend were performed using the median of each category of aspirin dose or duration as a continuous variable. Histopathological lymphoid reactions including TILs have been associated with MSI-high colorectal cancers,16, 17 and we have previously shown that the inverse association between regular aspirin use and colorectal cancer risk differed by BRAF mutation status,18 and PTGS2 expression level.12 Thus, we conducted secondary analyses to examine the association between regular aspirin use and colorectal cancer risk according to the levels (low vs. intermediate/high) of TILs stratified by MSI, BRAF or PTGS2 status. We also examined the association between aspirin use and risk of colorectal cancer according to levels of TILs and stage (I/II vs III/IV). As an exploratory analysis, we examined the association between regular aspirin use, levels of TILs, and colorectal cancer-specific mortality (up to January 2012). We also examined the association of regular use of any NSAIDs including aspirin with risk of colorectal cancer according to components of lymphocytic reaction.
In a subset of cases (n=744) with tissue microarray data, we examined whether the association between regular aspirin use and colorectal cancer might differ by densities of CD3+, CD8+, CD45RO+ or FOXP3+ cells.
Results
During 30 years of follow-up with 3,397,324 person-years, we documented 1,458 colorectal cancers with available tissue for characterization of patterns and degrees of lymphocytic infiltrates in tumor tissue. Participants reporting regular aspirin use were more likely to have a history of diabetes, regularly use other non-steroidal anti-inflammatory drugs (NSAIDs) or multivitamins, and consume alcohol (Table 1). Men who used aspirin regularly were also more likely to have a lower gastrointestinal endoscopy. Postmenopausal women who used aspirin regularly were more likely to use menopausal hormone therapy. Consistent with our prior analyses over earlier follow-up,12 regular aspirin use was associated with a significantly lower risk of colorectal cancer compared to nonregular use (RR 0.78; 95% CI 0.70–0.87), with similar associations in women and men (Table 2).
Table 1.
Age-standardized characteristics according to person-years of regular aspirin use
| Characteristic | NHS | HPFS | Combined | |||
|---|---|---|---|---|---|---|
|
| ||||||
| Nonregular users | Regular users | Nonregular users | Regular users | Nonregular users | Regular users | |
| Age, y* | 59.1 (51.3–66.7) | 61.3 (53.3–69.2) | 61.3 (53.2–69.6) | 65.4 (58.3–73.1) | 59.6 (51.8–67.4) | 62.8 (54.8–70.6) |
| Family history of cancer, % | 13 | 13 | 12 | 12 | 13 | 13 |
| History of diabetes, % | 6.2 | 8.1 | 6.3 | 8.2 | 6.3 | 8.1 |
| BMI, kg/m2 | 24.0 (21.9–27.1) | 24.6 (22.2–28.0) | 25.2 (23.5–27.3) | 25.5 (23.8–27.6) | 24.4 (22.3–27.2) | 25.0 (22.7–27.8) |
| Postmenopause, % | 76 | 78 | - | - | 77 | 77 |
| Menopausal hormone therapy, % | 26 | 31 | - | - | 26 | 31 |
| History of colonoscopy/sigmoidoscopy, % | 36 | 38 | 47 | 57 | 39 | 44 |
| Current use of multivitamin, % | 48 | 55 | 40 | 54 | 46 | 54 |
| Regular use of NSAIDs, % | 25 | 34 | 13 | 17 | 20 | 27 |
| Physical activity, MET-hrs/wk | 11.9 (5.4–22.1) | 11.3 (5.1–21.3) | 21.2 (9.4–40.3) | 23.2 (11.3–41.7) | 14.3 (6.3–27.6) | 14.9 (6.6–28.8) |
| Pack-year among ever smokers | 18 (7–35) | 20 (7–37) | 20 (10–35) | 20 (10–35) | 19 (8–35) | 20 (8–36) |
| Total calorie, kcal/d | 1625 (1349–1936) | 1664 (1386–1977) | 1890 (1557–2303) | 1924 (1586–2323) | 1690 (1395–2036) | 1736 (1436–2086) |
| Alcohol intake, g/d | 1.9 (0.2–7.6) | 2.2 (0.3–8.1) | 5.4 (0.9–14.3) | 7.0 (1.5–16.3) | 2.5 (0.3–9.6) | 3.3 (0.5–11.2) |
| Red and processed meat, servings/wk | 6.0 (4.0–8.4) | 6.2 (4.3–8.6) | 5.7 (3.2–8.8) | 5.5 (3.2–8.5) | 5.9 (3.9–8.5) | 6.0 (4.0–8.6) |
| Calcium, mg/d | 856 (648–1112) | 888 (672–1147) | 830 (656–1.93) | 863 (691–1115) | 850 (651–1109) | 878 (678–1136) |
| Folate, μg/d/ | 366 (263–520) | 393 (277–549) | 446 (334–645) | 510 (370–701) | 389 (282–550) | 427 (302–596) |
| Alternate Healthy Eating Index (AHEI) 2010† | 46.0 (39.6–52.9) | 45.4 (39.0–52.0) | 47.5 (40.6–54.8) | 48.1(41.4–55.0) | 46.5 (39.9–53.4) | 46.2 (39.7–53.0) |
All values other than age have been directly standardized to age distribution (in 5-year age group) of all the participants. Median (25th–75th percentile) was presented for continuous variables.
Without alcohol intake.
Table 2.
Regular aspirin use and risk of colorectal cancer overall and by components of lymphocytic reaction
| NHS | HPFS | Combined | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Nonregular users | Regular users | Nonregular users | Regular users | Nonregular users | Regular users | Pheterogenity† | |
| Total colorectal cancer | |||||||
| Person-years | 1455499 | 966281 | 519815 | 455729 | 1975314 | 1422010 | |
| Cases, No. | 526 | 304 | 337 | 291 | 863 | 595 | |
| Age-adjusted RR (95% CI) | 1 (reference) | 0.75 (0.65–0.87) | 1 (reference) | 0.82 (0.70–0.97) | 1 (reference) | 0.78 (0.70–0.87) | |
| Multivariable RR (95% CI)* | 1 (reference) | 0.75 (0.65–0.87) | 1 (reference) | 0.83 (0.71–0.98) | 1 (reference) | 0.78 (0.70–0.87) | |
|
| |||||||
| Tumor-infiltrating lymphocytes (TILs) | |||||||
| Low | |||||||
| Cases, No. | 387 | 204 | 278 | 218 | 665 | 422 | |
| Age-adjusted RR (95% CI) | 1 (reference) | 0.69 (0.58–0.82) | 1 (reference) | 0.75 (0.62–0.89) | 1 (reference) | 0.72 (0.63–0.81) | 0.007 |
| Multivariable RR (95% CI)* | 1 (reference) | 0.69 (0.58–0.82) | 1 (reference) | 0.75 (0.63–0.91) | 1 (reference) | 0.72 (0.63–0.81) | 0.007 |
| Intermediate | |||||||
| Cases, No. | 83 | 59 | 39 | 40 | 122 | 99 | |
| Age-adjusted RR (95% CI) | 1 (reference) | 0.91 (0.65–1.27) | 1 (reference) | 1.01 (0.64–1.59) | 1 (reference) | 0.94 (0.72–1.23) | |
| Multivariable RR (95% CI)* | 1 (reference) | 0.91 (0.65–1.27) | 1 (reference) | 1.02 (0.65–1.61) | 1 (reference) | 0.95 (0.72–1.24) | |
| High | |||||||
| Cases, No. | 55 | 40 | 19 | 32 | 74 | 72 | |
| Age-adjusted RR (95% CI) | 1 (reference) | 0.90 (0.60–1.35) | 1 (reference) | 1.56 (0.88–2.78) | 1 (reference) | 1.08 (0.78–1.51) | |
| Multivariable RR (95% CI)* | 1 (reference) | 0.91 (0.60–1.37) | 1 (reference) | 1.57 (0.88–2.79) | 1 (reference) | 1.09 (0.78–1.51) | |
|
| |||||||
| Intratumoral periglandular reaction | |||||||
| Low | |||||||
| Cases, No. | 68 | 45 | 41 | 37 | 109 | 82 | |
| Age-adjusted RR (95% CI) | 1 (reference) | 0.85 (0.58–1.24) | 1 (reference) | 0.71 (0.46–1.12) | 1 (reference) | 0.79 (0.59–1.05) | 0.37 |
| Multivariable RR (95% CI)* | 1 (reference) | 0.84 (0.57–1.23) | 1 (reference) | 0.72 (0.46–1.13) | 1 (reference) | 0.78 (0.59–1.05) | 0.36 |
| Intermediate | |||||||
| Cases, No. | 385 | 217 | 266 | 209 | 651 | 426 | |
| Age-adjusted RR (95% CI) | 1 (reference) | 0.74 (0.63–0.88) | 1 (reference) | 0.78 (0.65–0.94) | 1 (reference) | 0.76 (0.67–0.86) | |
| Multivariable RR (95% CI)* | 1 (reference) | 0.74 (0.62–0.87) | 1 (reference) | 0.79 (0.66–0.96) | 1 (reference) | 0.76 (0.67–0.86) | |
| High | |||||||
| Cases, No. | 69 | 42 | 30 | 45 | 99 | 87 | |
| Age-adjusted RR (95% CI) | 1 (reference) | 0.76 (0.52–1.12) | 1 (reference) | 1.33 (0.83–2.12) | 1 (reference) | 0.95 (0.71–1.28) | |
| Multivariable RR (95% CI)* | 1 (reference) | 0.77 (0.52–1.13) | 1 (reference) | 1.31 (0.82–2.10) | 1 (reference) | 0.95 (0.71–1.27) | |
|
| |||||||
| Peritumoral lymphocytic reaction | |||||||
| Low | |||||||
| Cases, No. | 72 | 43 | 44 | 46 | 116 | 89 | |
| Age-adjusted RR (95% CI) | 1 (reference) | 0.76 (0.52–1.11) | 1 (reference) | 0.84 (0.55–1.28) | 1 (reference) | 0.80 (0.60–1.05) | 0.15 |
| Multivariable RR (95% CI)* | 1 (reference) | 0.76 (0.52–1.11) | 1 (reference) | 0.84 (0.55–1.28) | 1 (reference) | 0.79 (0.60–1.05) | 0.15 |
| Intermediate | |||||||
| Cases, No. | 370 | 206 | 256 | 180 | 626 | 386 | |
| Age-adjusted RR (95% CI) | 1 (reference) | 0.73 (0.62–0.87) | 1 (reference) | 0.71 (0.59–0.87) | 1 (reference) | 0.73 (0.64–0.83) | |
| Multivariable RR (95% CI)* | 1 (reference) | 0.73 (0.61–0.87) | 1 (reference) | 0.73 (0.60–0.88) | 1 (reference) | 0.73 (0.64–0.83) | |
| High | |||||||
| Cases, No. | 78 | 55 | 36 | 62 | 114 | 117 | |
| Age-adjusted RR (95% CI) | 1 (reference) | 0.87 (0.62–1.23) | 1 (reference) | 1.44 (0.95–2.19) | 1 (reference) | 1.07 (0.83–1.40) | |
| Multivariable RR (95% CI)* | 1 (reference) | 0.88 (0.62–1.24) | 1 (reference) | 1.43 (0.94–2.17) | 1 (reference) | 1.07 (0.82–1.39) | |
|
| |||||||
| Crohn’s-like lymphoid reaction | |||||||
| Low | |||||||
| Cases, No. | 339 | 186 | 203 | 171 | 542 | 357 | |
| Age-adjusted RR (95% CI) | 1 (reference) | 0.71 (0.60–0.85) | 1 (reference) | 0.78 (0.63–0.96) | 1 (reference) | 0.74 (0.65–0.85) | 0.36 |
| Multivariable RR (95% CI)* | 1 (reference) | 0.71 (0.59–0.85) | 1 (reference) | 0.78 (0.63–0.97) | 1 (reference) | 0.74 (0.64–0.85) | 0.42 |
| Intermediate | |||||||
| Cases, No. | 72 | 39 | 49 | 45 | 121 | 84 | |
| Age-adjusted RR (95% CI) | 1 (reference) | 0.69 (0.46–1.02) | 1 (reference) | 0.97 (0.64–1.47) | 1 (reference) | 0.81 (0.61–1.07) | |
| Multivariable RR (95% CI)* | 1 (reference) | 0.68 (0.46–1.01) | 1 (reference) | 0.95 (0.62–1.43) | 1 (reference) | 0.80 (0.60–1.06) | |
| High | |||||||
| Cases, No. | 32 | 21 | 15 | 18 | 47 | 39 | |
| Age-adjusted RR (95% CI) | 1 (reference) | 0.80 (0.46–1.39) | 1 (reference) | 1.07 (0.53–2.14) | 1 (reference) | 0.89 (0.58–1.37) | |
| Multivariable RR (95% CI)* | 1 (reference) | 0.80 (0.46–1.39) | 1 (reference) | 1.02 (0.51–2.07) | 1 (reference) | 0.87 (0.57–1.34) | |
Abbreviations: CI, confidence interval; HPFS, Health Professionals Follow-up Study; NHS, Nurses’ Health Study; NSAIDs, non-steroidal anti-inflammatory drugs; RR, relative risk.
Adjusted for family history of colorectal cancer (yes/no), history of diabetes (yes/no), body mass index (quartile), history of colonoscopy/sigmoidoscopy (yes/no), smoking in pack-years (never, 0.1–4.9, 5–19.9, 20–39.9, ≥40), physical activity (quartile), alcohol intake (0, 0.1–4.9, 5–14.9, 15–29.9, ≥30 g/d), current multivitamin use (yes/no), regular use of NSAIDs (yes/no), total energy intake (quartile), folate (quartile), calcium (quartile), red and processed meat intake (quartile), and Alternate Healthy Eating Index-2010 without alcohol (quartile). For women, we additionally adjusted for menopause status/menopausal hormone therapy (MHT) (premenopausal, postmenopausal and never use of MHT, postmenopausal and past use of MHT, postmenopausal and current use of MHT). The Cox models were also conditioned on age in months, calendar year of the questionnaire cycle and sex/cohort (in the combined cohort analysis).
We assessed whether the magnitude of the subtype-specific associations had an increasing or decreasing ordinal trend according to the subtyping marker using a trend test with one degree of freedom, and the statistical significance of this test was presented as Pheterogeneity.
In testing our primary hypothesis, the inverse association of regular aspirin use with risk of colorectal cancer significantly differed by the density of tumor-infiltrating lymphocytes (TILs) after correction for multiple testing (Pheterogeneity=0.007, with adjusted α level of 0.012) (Table 2). Compared with nonregular use, regular aspirin use was associated with lower risk of the tumor subgroup with low-level TILs (RR 0.72; 95% CI 0.63–0.81), but not with risk of tumor subgroups with intermediate-level (RR 0.95; 95% CI 0.72–1.24) or high-level TILs (RR 1.09; 95% CI 0.78–1.51). The differential association was similarly observed in women and men. Although similar differential associations of aspirin use with colorectal cancer risk according to levels of intratumoral periglandular reaction (and peritumoral reaction) were observed, the differences were not statistically significant (Pheterogeneity ≥0.15) (Table 2).
We further explored the heterogeneous association according to the degree of TILs across tablets of aspirin consumed each week and duration of aspirin use. We observed a lower risk of TIL-low colorectal cancer with increasing aspirin dosage per week (Ptrend <0.001). In contrast, aspirin dosage per week was not significantly associated with tumors with intermediate or high-level TILs (Ptrend >0.28) (Table 3). Similarly, the inverse association of aspirin with TIL-low colorectal cancer risk became stronger with longer duration of use (Ptrend <0.001), but duration of aspirin use was not significantly associated with colorectal cancer with intermediate or high-level TILs (Ptrend >0.5) (Table 4).
Table 3.
Dose of regular aspirin use and risk of colorectal cancer overall and by tumor-infiltrating lymphocytes
| Tablets/wk | Ptrend* | Pheterogenity‡ | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| 0 | 0.5–1.5 | 2–5 | ≥6 | |||
| Total colorectal cancer | ||||||
| Person-years | 625399 | 1148842 | 680674 | 599431 | ||
| Cases, No. | 226 | 544 | 293 | 236 | ||
| Age-adjusted RR (95% CI) | 1 (reference) | 1.09 (0.92–1.28) | 0.87 (0.73–1.03) | 0.78 (0.65–0.94) | <0.001 | |
| Multivariable RR (95% CI)† | 1 (reference) | 1.07 (0.91–1.26) | 0.86 (0.72–1.03) | 0.76 (0.63–0.92) | <0.001 | |
|
| ||||||
| Tumor-infiltrating lymphocytes | ||||||
| Low | ||||||
| Cases, No. | 178 | 399 | 215 | 164 | ||
| Age-adjusted RR (95% CI) | 1 (reference) | 1.05 (0.87–1.26) | 0.83 (0.68–1.01) | 0.70 (0.56–0.87) | <0.001 | 0.04 |
| Multivariable RR (95% CI)† | 1 (reference) | 1.03 (0.86–1.25) | 0.82 (0.67–1.01) | 0.69 (0.55–0.85) | <0.001 | 0.04 |
| Intermediate | ||||||
| Cases, No. | 30 | 89 | 47 | 40 | ||
| Age-adjusted RR (95% CI) | 1 (reference) | 1.20 (0.78–1.84) | 0.98 (0.61–1.57) | 0.94 (0.58–1.52) | 0.31 | |
| Multivariable RR (95% CI)† | 1 (reference) | 1.18 (0.77–1.82) | 0.97 (0.61–1.55) | 0.92 (0.57–1.49) | 0.29 | |
| High | ||||||
| Cases, No. | 18 | 56 | 31 | 32 | ||
| Age-adjusted RR (95% CI) | 1 (reference) | 1.30 (0.75–2.25) | 1.05 (0.58–1.89) | 1.26 (0.70–2.26) | 0.83 | |
| Multivariable RR (95% CI)† | 1 (reference) | 1.28 (0.74–2.21) | 1.05 (0.58–1.90) | 1.24 (0.69–2.23) | 0.85 | |
Abbreviations: CI, confidence interval; HPFS, Health Professionals Follow-up Study; NHS, Nurses’ Health Study; RR, relative risk.
Tests for trend were conducted using the median value of each category as a continuous variable.
Adjusted for the same set of covariates as in Table 2.
We assessed whether the magnitude of the subtype-specific associations had an increasing or decreasing ordinal trend according to levels of TILs, using a trend test with one degree of freedom, and the statistical significance of this test was presented as Pheterogeneity.
Table 4.
Duration of regular aspirin use and risk of colorectal cancer overall and by tumor-infiltrating lymphocytes
| Years of Regular Aspirin Use | Ptrend* | Pheterogenity‡ | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| 0 | 1–5 | 6–10 | 11–15 | ≥16 | |||
| Total colorectal cancer | |||||||
| Person-years | 1246298 | 628463 | 585563 | 278593 | 644154 | ||
| Cases, No. | 486 | 296 | 306 | 128 | 236 | ||
| Age-adjusted RR (95% CI) | 1 (reference) | 1.00 (0.87–1.16) | 0.92 (0.79–1.06) | 0.74 (0.61–0.91) | 0.74 (0.63–0.87) | <0.001 | |
| Multivariable RR (95% CI)† | 1 (reference) | 1.01 (0.88–1.18) | 0.93 (0.80–1.08) | 0.75 (0.61–0.92) | 0.74 (0.62–0.87) | <0.001 | |
|
| |||||||
| Tumor-infiltrating lymphocytes | |||||||
| Low | |||||||
| Cases, No. | 385 | 223 | 233 | 80 | 164 | ||
| Age-adjusted RR (95% CI) | 1 (reference) | 0.95 (0.81–1.13) | 0.88 (0.75–1.04) | 0.60 (0.47–0.77) | 0.68 (0.56–0.82) | <0.001 | 0.03 |
| Multivariable RR (95% CI)† | 1 (reference) | 0.96 (0.81–1.14) | 0.90 (0.76–1.06) | 0.61 (0.47–0.78) | 0.68 (0.56–0.82) | <0.001 | 0.04 |
| Intermediate | |||||||
| Cases, No. | 60 | 44 | 43 | 30 | 44 | ||
| Age-adjusted RR (95% CI) | 1 (reference) | 1.20 (0.81–1.78) | 1.04 (0.70–1.56) | 1.32 (0.84–2.08) | 0.90 (0.60–1.35) | 0.52 | |
| Multivariable RR (95% CI)† | 1 (reference) | 1.22 (0.82–1.82) | 1.06 (0.71–1.58) | 1.34 (0.85–2.10) | 0.90 (0.60–1.36) | 0.52 | |
| High | |||||||
| Cases, No. | 41 | 29 | 30 | 18 | 28 | ||
| Age-adjusted RR (95% CI) | 1 (reference) | 1.18 (0.73–1.90) | 1.09 (0.67–1.76) | 1.11 (0.63–1.97) | 1.02 (0.62–1.69) | 0.96 | |
| Multivariable RR (95% CI)† | 1 (reference) | 1.18 (0.73–1.92) | 1.10 (0.68–1.78) | 1.13 (0.64–2.00) | 1.01 (0.61–1.68) | 0.95 | |
Abbreviations: CI, confidence interval; HPFS, Health Professionals Follow-up Study; NHS, Nurses’ Health Study; RR, relative risk.
Tests for trend were conducted using the median value of each category as a continuous variable.
Adjusted for the same set of covariates as in Table 2.
We assessed whether the magnitude of the subtype-specific associations had an increasing or decreasing ordinal trend according to levels of TILs, using a trend test with one degree of freedom, and the statistical significance of this test was presented as Pheterogeneity.
The differential association between regular aspirin use and risk of colorectal cancer according to levels of TILs appeared to be consistent in strata of tumor MSI status, tumor BRAF mutation status, PTGS2 expression status (Table 5), and stage (I/II vs III/IV) (Supplemental Table 2), although statistical power was limited in these subgroup analyses.
Table 5.
Regular aspirin use and risk of colorectal cancer by microsatellite instability, BRAF mutation, PTGS2 expression and tumor-infiltrating lymphocytes
| Aspirin use | ||||
|---|---|---|---|---|
|
| ||||
| Nonregular users | Regular users | |||
| Microsatellite instability (MSI) | Microsatellite stable (MSS) | Cases, No. | 662 | 448 |
| Multivariable RR (95% CI)* | 1 (reference) | 0.78 (0.69–0.88) | ||
| MSI-high | Cases, No. | 122 | 93 | |
| Multivariable RR (95% CI)* | 1 (reference) | 0.84 (0.64–1.11) | ||
|
| ||||
| MSS | Tumor-infiltrating lymphocytes | |||
| Low | ||||
| Cases, No. | 528 | 345 | ||
| Multivariable RR (95% CI)* | 1 (reference) | 0.75 (0.66–0.87) | ||
| Intermediate/high | ||||
| Cases, No. | 110 | 84 | ||
| Multivariable RR (95% CI)* | 1 (reference) | 0.93 (0.70–1.25) | ||
|
| ||||
| MSI-high | Tumor-infiltrating lymphocytes | |||
| Low | ||||
| Cases, No. | 47 | 17 | ||
| Multivariable RR (95% CI)* | 1 (reference) | 0.41 (0.23–0.71) | ||
| Intermediate/high | ||||
| Cases, No. | 74 | 73 | ||
| Multivariable RR (95% CI)* | 1 (reference) | 1.03 (0.74–1.44) | ||
|
| ||||
| BRAF mutation | Wild-type | Cases, No. | 682 | 455 |
| Multivariable RR (95% CI)* | 1 (reference) | 0.76 (0.68–0.86) | ||
| Mutant | Cases, No. | 112 | 93 | |
| Multivariable RR (95% CI)* | 1 (reference) | 0.94 (0.71–1.24) | ||
|
| ||||
| Wild-type | Tumor-infiltrating lymphocytes | |||
| Low | ||||
| Cases, No. | 519 | 338 | ||
| Multivariable RR (95% CI)* | 1 (reference) | 0.75 (0.65–0.86) | ||
| Intermediate/high | ||||
| Cases, No. | 135 | 98 | ||
| Multivariable RR (95% CI)* | 1 (reference) | 0.87 (0.66–1.13) | ||
|
| ||||
| Mutant | Tumor-infiltrating lymphocytes | |||
| Low | ||||
| Cases, No. | 64 | 30 | ||
| Multivariable RR (95% CI)* | 1 (reference) | 0.54 (0.35–0.84) | ||
| Intermediate/high | ||||
| Cases, No. | 48 | 58 | ||
| Multivariable RR (95% CI)* | 1 (reference) | 1.27 (0.86–1.88) | ||
|
| ||||
| PTGS2 expression | Negative | Cases, No. | 267 | 213 |
| Multivariable RR (95% CI)* | 1 (reference) | 0.90 (0.75–1.08) | ||
| Positive | Cases, No. | 488 | 292 | |
| Multivariable RR (95% CI)* | 1 (reference) | 0.70 (0.61–0.82) | ||
| Negative | Tumor-infiltrating lymphocytes | |||
| Low | ||||
| Cases, No. | 187 | 133 | ||
| Multivariable RR (95% CI)* | 1 (reference) | 0.79 (0.63–0.99) | ||
| Intermediate/high | ||||
| Cases, No. | 71 | 67 | ||
| Multivariable RR (95% CI)* | 1 (reference) | 1.07 (0.76–1.50) | ||
| Positive | Tumor-infiltrating lymphocytes | |||
| Low | ||||
| Cases, No. | 378 | 203 | ||
| Multivariable RR (95% CI)* | 1 (reference) | 0.64 (0.53–0.76) | ||
| Intermediate/high | ||||
| Cases, No. | 89 | 76 | ||
| Multivariable RR (95% CI)* | 1 (reference) | 1.01 (0.74–1.37) | ||
Abbreviations: CI, confidence interval; HPFS, Health Professionals Follow-up Study; NHS, Nurses’ Health Study; RR, relative risk.
Adjusted for the same set of covariates as in Table 2.
We assessed whether the magnitude of the subtype-specific associations had an increasing or decreasing ordinal trend according to levels of TILs, using a trend test with one degree of freedom, and the statistical significance of this test was presented as Pheterogeneity.
Regular aspirin use was not differentially associated with colorectal cancer-specific mortality according to the degree of TILs (low vs intermediate/high) (Pinteraction=0.17) (Supplemental Table 3). Nonetheless, it is difficult to determine lack of statistical interaction due to limited statistical power. Additional studies are warranted to examine the interactive effects of aspirin and TILs that may modify clinical outcome of colorectal cancer patients.
Statistical power was limited in our cohorts to examine the association between non-aspirin NSAIDs and colorectal cancer according to tumor immunity status. We thus analyzed the association between any NSAIDs including aspirin and risk of colorectal cancer according to components of lymphocytic reaction (Supplemental Table 4). The findings were generally consistent with the findings in our primary analysis of regular aspirin use as an exposure variable.
In a subset of cases with tissue microarray data, inverse associations of regular aspirin use with cancer risk were observed for tumors with low densities of CD3+ (RR 0.73; 95% CI 0.58–0.91), CD8+ (RR 0.73; 95% CI 0.58–0.91) and CD45RO+ cells (RR 0.74; 95% CI 0.60–0.92), but not for tumors with high densities of CD3+, CD8+ or CD45RO+ cells (Table 6). The association of aspirin with colorectal cancer risk appeared to be similar according to tumor FOXP3+ cell density.
Table 6.
Regular aspirin use and risk of colorectal cancer by tumor-infiltrating T-cell subset density*
| NHS | HPFS | Combined | Pheterogenity‡ | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Nonregular users | Regular users | Nonregular users | Regular users | Nonregular users | Regular users | ||
| Total colorectal cancer | |||||||
| Person-years | 1455729 | 966385 | 519658 | 455572 | 1975387 | 1421957 | |
| Cases, No. | 285 | 183 | 154 | 122 | 439 | 305 | |
| Age-adjusted RR (95% CI) | 1 (reference) | 0.86 (0.71–1.03) | 1 (reference) | 0.83 (0.65–1.06) | 1 (reference) | 0.85 (0.73–0.98) | |
| Multivariable RR (95% CI)† | 1 (reference) | 0.85 (0.70–1.02) | 1 (reference) | 0.85 (0.67–1.09) | 1 (reference) | 0.85 (0.73–0.98) | |
|
| |||||||
| CD3+ cells | |||||||
| Low | |||||||
| Age-adjusted RR (95% CI) | 1 (reference) | 0.80 (0.61–1.05) | 1 (reference) | 0.63 (0.43–0.91) | 1 (reference) | 0.73 (0.59–0.91) | 0.04 |
| Multivariable RR (95% CI)† | 1 (reference) | 0.79 (0.60–1.04) | 1 (reference) | 0.65 (0.44–0.94) | 1 (reference) | 0.73 (0.58–0.91) | 0.03 |
| High | |||||||
| Age-adjusted RR (95% CI) | 1 (reference) | 0.95 (0.72–1.24) | 1 (reference) | 1.12 (0.80–1.57) | 1 (reference) | 1.01 (0.82–1.25) | |
| Multivariable RR (95% CI)† | 1 (reference) | 0.94 (0.72–1.24) | 1 (reference) | 1.14 (0.81–1.60) | 1 (reference) | 1.01 (0.82–1.25) | |
|
| |||||||
| CD8+ cells | |||||||
| Low | |||||||
| Age-adjusted RR (95% CI) | 1 (reference) | 0.71 (0.55–0.93) | 1 (reference) | 0.76 (0.51–1.12) | 1 (reference) | 0.73 (0.58–0.91) | 0.04 |
| Multivariable RR (95% CI)† | 1 (reference) | 0.70 (0.53–0.92) | 1 (reference) | 0.78 (0.53–1.16) | 1 (reference) | 0.73 (0.58–0.91) | 0.04 |
| High | |||||||
| Age-adjusted RR (95% CI) | 1 (reference) | 1.06 (0.81–1.41) | 1 (reference) | 0.92 (0.65–1.28) | 1 (reference) | 1.00 (0.81–1.24) | |
| Multivariable RR (95% CI)† | 1 (reference) | 1.06 (0.80–1.40) | 1 (reference) | 0.95 (0.68–1.33) | 1 (reference) | 1.00 (0.81–1.24) | |
|
| |||||||
| CD45RO+ cells | |||||||
| Low | |||||||
| Age-adjusted RR (95% CI) | 1 (reference) | 0.72 (0.54–0.97) | 1 (reference) | 0.75 (0.54–1.04) | 1 (reference) | 0.74 (0.59–0.92) | 0.05 |
| Multivariable RR (95% CI)† | 1 (reference) | 0.72 (0.53–0.97) | 1 (reference) | 0.78 (0.56–1.08) | 1 (reference) | 0.74 (0.60–0.92) | 0.06 |
| High | |||||||
| Age-adjusted RR (95% CI) | 1 (reference) | 0.98 (0.76–1.26) | 1 (reference) | 1.03 (0.70–1.51) | 1 (reference) | 0.99 (0.80–1.23) | |
| Multivariable RR (95% CI)† | 1 (reference) | 0.97 (0.75–1.25) | 1 (reference) | 1.06 (0.72–1.56) | 1 (reference) | 0.99 (0.80–1.22) | |
|
| |||||||
| FOXP3+ | |||||||
| Low | |||||||
| Age-adjusted RR (95% CI) | 1 (reference) | 0.82 (0.62–1.08) | 1 (reference) | 0.87 (0.61–1.23) | 1 (reference) | 0.84 (0.67–1.04) | 0.89 |
| Multivariable RR (95% CI)† | 1 (reference) | 0.80 (0.60–1.07) | 1 (reference) | 0.91 (0.64-1.29) | 1 (reference) | 0.84 (0.67–1.04) | 0.86 |
| High | |||||||
| Age-adjusted RR (95% CI) | 1 (reference) | 0.86 (0.66–1.12) | 1 (reference) | 0.75 (0.51–1.10) | 1 (reference) | 0.82 (0.66–1.02) | |
| Multivariable RR (95% CI)† | 1 (reference) | 0.83 (0.64–1.09) | 1 (reference) | 0.76 (0.52–1.12) | 1 (reference) | 0.81 (0.65–1.02) | |
Abbreviations: CI, confidence interval; HPFS, Health Professionals Follow-up Study; NHS, Nurses’ Health Study; RR, relative risk.
Cut-off for low and high tumor-infiltrating T cell subset density (cells/mm2): 244.97 for CD3+ cells, 236.65 for CD8+ cells, 376.97 for CD45RO+ cells and 26.36 for FOXP3+ cells.
Adjusted for the same set of covariates as in Table 2.
We assessed whether the magnitude of the subtype-specific associations had an increasing or decreasing ordinal trend according to density of T cells, using a trend test with one degree of freedom, and the statistical significance of this test was presented as Pheterogeneity.
Discussion
In two large prospective cohort studies, we observed an inverse association between regular aspirin use and risk of colorectal cancers with low-level tumor-infiltrating lymphocytes (TILs) but not with risk of colorectal cancers with high-level TILs. The apparent benefit of aspirin use for tumors with low-level TILs increased with dose and duration of aspirin use. Our findings provide the first line of population-based evidence for the role of host immunity in mediating the effect of aspirin in colorectal cancer chemoprevention. Aspirin, through either prostaglandin-dependent or independent pathways, may enhance anti-tumor immunity, thereby exerting a stronger effect on tumors that more strongly depend on suppression of tumor immune response for their growth. Overall, these results improve our understanding of the mechanisms through which aspirin may exert its antineoplastic effects and also provide broad support for the potential of exploiting immune mechanisms for disease prevention (i.e., immunoprevention).23, 24 Nonetheless, further functional studies to more fully elucidate the immune mechanisms of anti-tumor effect of aspirin are warranted.
The observed differential association of aspirin and colorectal cancer according to tumor immunity status is biologically plausible. Evidence suggests that aspirin may exert multiple effects on different components of innate and adaptive immunity through modulation of immune and inflammatory cytokines.23, 25–29 For example, aspirin may induce tolerogenic activity in dendritic cells and inhibit their subsequent immunostimulatory function.30 In addition, aspirin can induce apoptosis in neutrophils and monocytes,31 and trigger a lipoxin-driven immune counter-regulation.32 For T lymphocytes, aspirin can disrupt the integrin- and SELL (selectin L)-mediated binding of T cells to the endothelium,33, 34 directly suppress T cell activation or proliferation, and/or inhibit cytokine production related to the T cell-mediated adaptive immune response.25 Our data support the possibility that aspirin may cooperate with the host immune system, in particular, lymphocytes, to interrupt the development or growth of colorectal neoplasia.
Integrated analysis of tumor characteristics is increasingly important in cancer research.35–37 Tumor MSI status should be analyzed in the current study of aspirin use and risk of colorectal cancer according to lymphocytic infiltrates, since MSI-high tumor cells have many frameshift mutations in coding sequences throughout the genome, resulting in abundant neoantigens that elicit intense and more diverse immune responses.16, 38–41 Recently, some MSI-high colorectal cancers have been shown to respond to immunotherapy blocking the PDCD1 (programmed cell death-1, PD-1) immune checkpoint, supporting the importance of the interplay between MSI-high tumor cells and immune cells.42 However, MSI status is not the sole determinant of tumoral immune response, as the levels of tumor-infiltrating T cells overlap considerably between MSI-high and MSS colorectal cancers.17, 19, 43, 44 In the current study, we found that the differential association between aspirin and cancer risk according to levels of TILs appeared to be largely independent of MSI status, further supporting a distinct role of host immunity in mediating the association between aspirin and colorectal cancer.
Cancer immunity status reflects molecular interactions between tumor and immune cells, occurring in the tumor microenvironment.45, 46 Compared to the other components of lymphocytic reaction, lymphocytes in the TIL component are present close to surfaces of tumor cells and hence in more direct contact with the tumor cells containing somatic mutations. The degree of TIL, especially tumor-specific cytotoxic T cells, has been associated with a good prognosis in colorectal cancer.47–50 As immunotherapy has emerged as an attractive strategy in the treatment of cancer, integrated analyses of tumor molecular characteristics, host factors (including dietary, lifestyle, and environmental exposures), and immune cells in the tumor microenvironment are increasingly important.8 Our data strengthen the causal link between aspirin and the prevention of colorectal neoplasia by identifying a subgroup of colorectal cancer that may be sensitive to aspirin chemoprevention, and enhance our understanding of the mechanisms through which aspirin may exert its antineoplastic effects.
The strengths of our study include prospective and updated assessments of aspirin use during up to 30 years of follow-up. In addition, we collected detailed information on potential confounders and had high follow-up rates. Importantly, cancer immunity status, which has rarely been examined in epidemiological studies, provides important information on interactions between tumor and host immune cells, which cannot be obtained from peripheral blood biomarkers.51 In addition, our integrative molecular pathological epidemiology approach enabled us to attribute the risk reduction to the tumor subgroup, refine effect estimates for the tumor subgroup, and provide evidence in support of causality.
Our study has limitations. Firstly, the study was observational and subject to the influence of confounding. However, adjustment for a wide range of risk factors for colorectal cancer had minimal impact on our results. Secondly, because the majority of participants were non-Hispanic health professionals, generalizability to other ethnic or socioeconomic groups remains to be assessed. In addition, we were not able to retrieve tissue specimens from all incident cancers; however, the characteristics of those participants from whom we could collect tissue data were largely similar to those from whom we could not. Finally, replication of our findings is needed and studies that examine macrophages, myeloid-derived suppressor cells, NK cells, Th2 cells, and other types of immune cells in tumor tissue may provide additional insights to the potential role of host immunity in mediating the chemopreventive effect of aspirin.
In conclusion, regular aspirin use, by dose and duration, is associated with a lower risk of colorectal cancer with low-level tumor-infiltrating lymphocytes (TILs), but not with risk of colorectal cancer with more intense patterns of TILs. This differential association appeared to be consistent across strata of tumor MSI, BRAF mutation or PTGS2 expression status. Our findings highlight the potential importance of host immunity in mediating the activity of aspirin in colorectal cancer chemoprevention.
Supplementary Material
Acknowledgments
Grant support: This work was supported by U.S. National Institutes of Health (NIH) grants [P01 CA87969 to M.J. Stampfer; UM1 CA186107 to M.J. Stampfer; P01 CA55075 to W.C. Willett; UM1 CA167552 to W.C. Willett; P50 CA127003 to C.S.F.; R01 CA137178 to A.T.C.; K24 DK098311 to A.T.C., R01 CA151993 to S.O.; R35 CA197735 to S.O.; K07 CA190673 to R.N., and K07 CA188126 to X.Z.]; Nodal Award (to S.O.) from the Dana-Farber Harvard Cancer Center; and by grants from The Raymond P. Lavietes Foundation (to Y.C.), The Project P Fund, The Friends of the Dana-Farber Cancer Institute, the Bennett Family Fund, and the Entertainment Industry Foundation through National Colorectal Cancer Research Alliance. K.M. is supported by a fellowship grant from Uehara Memorial Foundation and a grant from Program for Advancing Strategic International Networks to Accelerate the Circulation of Talented Researchers from Japan Society for the Promotion of Science. The content is solely the responsibility of the authors and does not necessarily represent the official views of NIH. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
We would like to thank the participants and staff of the Nurses’ Health Study and the Health Professionals Follow-up Study for their valuable contributions as well as the following state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, WY. The authors assume full responsibility for analyses and interpretation of these data.
Abbreviations
- AHEI
Alternate Healthy Eating Index
- CI
confidence interval
- HPFS
Health Professionals Follow-up Study
- MET
metabolic equivalent task
- MSI
microsatellite instability
- MSS
microsatellite stable
- NHS
Nurses’ Health Study
- NSAIDs
non-steroidal anti-inflammatory drugs
- RR
relative risk
- TIL
tumor infiltrating lymphocytes
- TMA
tissue microarray
Footnotes
Role of sponsor: The study sponsors have no role in the study design, collection, analysis, and interpretation of data.
Use of standardized official symbols: We use HUGO (Human Genome Organisation)-approved official symbols for genes and gene products, including BRAF, CD3, CD8, FOXP3, PDCD1, PTGS2, PTPRC, and SELL, all of which are described at www.genenames.org. Gene names are italicised, and gene product names are non-italicized.
Disclosures: Andrew T. Chan previously served as a consultant for Bayer Healthcare, Pozen Inc, and Pfizer Inc. This study was not funded by Bayer Healthcare, Pozen Inc, or Pfizer Inc. No other conflict of interest exists. The other authors declare that they have no conflicts of interest.
Author Contributions: Drs Cao and Ogino had full access to all of the data in the study, and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Y.C., E.L.G., C.S.F., A.T.C., S.O.
Acquisition of data: Y.C., R.N., Z.R.Q., M.S., E.L.G., C.S.F., A.T.C., S.O.
Analysis and interpretation of data: all coauthors
Drafting of the manuscript: Y.C.
Critical revision of the manuscript for important intellectual content: all coauthors
Statistical analysis: Y.C.
Obtained funding: Y.C., R.N., X.Z., C.S.F., A.T.C., S.O.
Administrative, technical, or material support: S.O.
Study supervision: A.T.C., S.O.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
Author names in bold designate shared co-first authorship.
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Cuzick J, Thorat MA, Bosetti C, et al. Estimates of benefits and harms of prophylactic use of aspirin in the general population. Ann Oncol. 2015;26:47–57. doi: 10.1093/annonc/mdu225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cao Y, Nishihara R, Wu K, et al. Population-wide Impact of Long-term Use of Aspirin and the Risk for Cancer. JAMA Oncol. 2016 doi: 10.1001/jamaoncol.2015.6396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thun MJ, Jacobs EJ, Patrono C. The role of aspirin in cancer prevention. Nat Rev Clin Oncol. 2012;9:259–67. doi: 10.1038/nrclinonc.2011.199. [DOI] [PubMed] [Google Scholar]
- 5.Drew DA, Cao Y, Chan AT. Aspirin and colorectal cancer: the promise of precision chemoprevention. Nat Rev Cancer. 2016;16:173–86. doi: 10.1038/nrc.2016.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Umar A, Steele VE, Menter DG, et al. Mechanisms of nonsteroidal anti-inflammatory drugs in cancer prevention. Semin Oncol. 2016;43:65–77. doi: 10.1053/j.seminoncol.2015.09.010. [DOI] [PubMed] [Google Scholar]
- 7.Xia D, Wang D, Kim SH, et al. Prostaglandin E2 promotes intestinal tumor growth via DNA methylation. Nat Med. 2012;18:224–6. doi: 10.1038/nm.2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galon J, Mlecnik B, Bindea G, et al. Towards the introduction of the Immunoscore in the classification of malignant tumors. J Pathol. 2014;232:199–209. doi: 10.1002/path.4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Palmqvist R, Wikberg ML, Ling A, et al. The association of immune cell infiltration and prognosis in colorectal cancer. Curr Colorectal Cancer Rep. 2013;9:372–379. [Google Scholar]
- 10.Joyce JA, Fearon DT. T cell exclusion, immune privilege, and the tumor microenvironment. Science. 2015;348:74–80. doi: 10.1126/science.aaa6204. [DOI] [PubMed] [Google Scholar]
- 11.Fridman WH, Pages F, Sautes-Fridman C, et al. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. 2012;12:298–306. doi: 10.1038/nrc3245. [DOI] [PubMed] [Google Scholar]
- 12.Chan AT, Ogino S, Fuchs CS. Aspirin and the risk of colorectal cancer in relation to the expression of COX-2. New England Journal of Medicine. 2007;356:2131–2142. doi: 10.1056/NEJMoa067208. [DOI] [PubMed] [Google Scholar]
- 13.Gobel C, Breitenbuecher F, Kalkavan H, et al. Functional expression cloning identifies COX-2 as a suppressor of antigen-specific cancer immunity. Cell Death Dis. 2014;5:e1568. doi: 10.1038/cddis.2014.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burkholder B, Huang RY, Burgess R, et al. Tumor-induced perturbations of cytokines and immune cell networks. Biochim Biophys Acta. 2014;1845:182–201. doi: 10.1016/j.bbcan.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 15.Zelenay S, van der Veen AG, Bottcher JP, et al. Cyclooxygenase-Dependent Tumor Growth through Evasion of Immunity. Cell. 2015;162:1257–1270. doi: 10.1016/j.cell.2015.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rooney MS, Shukla SA, Wu CJ, et al. Molecular and genetic properties of tumors associated with local immune cytolytic activity. Cell. 2015;160:48–61. doi: 10.1016/j.cell.2014.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ogino S, Nosho K, Irahara N, et al. Lymphocytic reaction to colorectal cancer is associated with longer survival, independent of lymph node count, microsatellite instability, and CpG island methylator phenotype. Clinical Cancer Research. 2009;15:6412–6420. doi: 10.1158/1078-0432.CCR-09-1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nishihara R, Lochhead P, Kuchiba A, Jung S, et al. Aspirin Use and Risk of Colorectal Cancer According to BRAF Mutation Status. Jama-Journal of the American Medical Association. 2013;309:2563–2571. doi: 10.1001/jama.2013.6599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nosho K, Baba Y, Tanaka N, et al. Tumour-infiltrating T-cell subsets, molecular changes in colorectal cancer, and prognosis: cohort study and literature review. J Pathol. 2010;222:350–66. doi: 10.1002/path.2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang M, Spiegelman D, Kuchiba A, et al. Statistical methods for studying disease subtype heterogeneity. Stat Med. 2016;35:782–800. doi: 10.1002/sim.6793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCullough ML, Feskanich D, Stampfer MJ, et al. Diet quality and major chronic disease risk in men and women: moving toward improved dietary guidance. American Journal of Clinical Nutrition. 2002;76:1261–1271. doi: 10.1093/ajcn/76.6.1261. [DOI] [PubMed] [Google Scholar]
- 22.Chiuve SE, Fung TT, Rimm EB, et al. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr. 2012;142:1009–18. doi: 10.3945/jn.111.157222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marzbani E, Inatsuka C, Lu HL, et al. The Invisible Arm of Immunity in Common Cancer Chemoprevention Agents. Cancer Prevention Research. 2013;6:764–773. doi: 10.1158/1940-6207.CAPR-13-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kensler TW, Spira A, Garber JE, et al. Transforming Cancer Prevention through Precision Medicine and Immune-oncology. Cancer Prev Res (Phila) 2016;9:2–10. doi: 10.1158/1940-6207.CAPR-15-0406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hussain M, Javeed A, Ashraf M, et al. Aspirin and immune system. International Immunopharmacology. 2012;12:10–20. doi: 10.1016/j.intimp.2011.11.021. [DOI] [PubMed] [Google Scholar]
- 26.Chia WK, Ali R, Toh HC. Aspirin as adjuvant therapy for colorectal cancer-reinterpreting paradigms. Nature Reviews Clinical Oncology. 2012;9:561–570. doi: 10.1038/nrclinonc.2012.137. [DOI] [PubMed] [Google Scholar]
- 27.Tougeron D, Sha D, Manthravadi S, et al. Aspirin and colorectal cancer: back to the future. Clinical Cancer Research. 2014;20:1087–94. doi: 10.1158/1078-0432.CCR-13-2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li PW, Wu H, Zhang HH, et al. Aspirin use after diagnosis but not prediagnosis improves established colorectal cancer survival: a meta-analysis. Gut. 2015;64:1419–1425. doi: 10.1136/gutjnl-2014-308260. [DOI] [PubMed] [Google Scholar]
- 29.Roy HK, Turzhitsky V, Wali R, et al. Spectral biomarkers for chemoprevention of colonic neoplasia: a placebo-controlled double-blinded trial with aspirin. Gut. 2015 doi: 10.1136/gutjnl-2015-309996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matasic R, Dietz AB, Vuk-Pavlovic S. Cyclooxygenase-independent inhibition of dendritic cell maturation by aspirin. Immunology. 2000;101:53–60. doi: 10.1046/j.1365-2567.2000.00065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Negrotto S, Malaver E, Alvarez ME, et al. Aspirin and salicylate suppress polymorphonuclear apoptosis delay mediated by proinflammatory stimuli. Journal of Pharmacology and Experimental Therapeutics. 2006;319:972–979. doi: 10.1124/jpet.106.109389. [DOI] [PubMed] [Google Scholar]
- 32.El Kebir D, Jozsef L, Khreiss T, et al. Aspirin-triggered lipoxins override the apoptosis-delaying action of serum amyloid A in human neutrophils: a novel mechanism for resolution of inflammation. J Immunol. 2007;179:616–22. doi: 10.4049/jimmunol.179.1.616. [DOI] [PubMed] [Google Scholar]
- 33.Gerli R, Gresele P, Bistoni O, et al. Salicylates inhibit T cell adhesion on endothelium under nonstatic conditions: Induction of L-selectin shedding by a tyrosine kinase-dependent mechanism. Journal of Immunology. 2001;166:832–840. doi: 10.4049/jimmunol.166.2.832. [DOI] [PubMed] [Google Scholar]
- 34.Gerli R, Paolucci C, Gresele P, et al. Salicylates inhibit adhesion and transmigration of T lymphocytes by preventing integrin activation induced by contact with endothelial cells. Blood. 1998;92:2389–98. [PubMed] [Google Scholar]
- 35.Okugawa Y, Grady WM, Goel A. Epigenetic Alterations in Colorectal Cancer: Emerging Biomarkers. Gastroenterology. 2015;149:1204. doi: 10.1053/j.gastro.2015.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Colussi D, Brandi G, Bazzoli F, et al. Molecular Pathways Involved in Colorectal Cancer: Implications for Disease Behavior and Prevention. International Journal of Molecular Sciences. 2013;14:16365–16385. doi: 10.3390/ijms140816365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carethers JM, Jung BH. Genetics and Genetic Biomarkers in Sporadic Colorectal Cancer. Gastroenterology. 2015;149:1177. doi: 10.1053/j.gastro.2015.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saeterdal I, Bjorheim J, Lislerud K, et al. Frameshift-mutation-derived peptides as tumor-specific antigens in inherited and spontaneous colorectal cancer. Proc Natl Acad Sci U S A. 2001;98:13255–13260. doi: 10.1073/pnas.231326898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schwitalle Y, Kloor M, Eiermann S, et al. Immune response against frameshift-induced neopeptides in HNPCC patients and healthy HNPCC mutation carriers. Gastroenterology. 2008;134:988–997. doi: 10.1053/j.gastro.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 40.Brown SD, Warren RL, Gibb EA, et al. Neo-antigens predicted by tumor genome meta-analysis correlate with increased patient survival. Genome Res. 2014;24:743–50. doi: 10.1101/gr.165985.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boissiere-Michot F, Lazennec G, Frugier H, et al. Characterization of an adaptive immune response in microsatellite-instable colorectal cancer. Oncoimmunology. 2014;3:e29256. doi: 10.4161/onci.29256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Le DT, Uram JN, Wang H, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med. 2015;372:2509–20. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dahlin AM, Henriksson ML, Van Guelpen B, et al. Colorectal cancer prognosis depends on T-cell infiltration and molecular characteristics of the tumor. Mod Pathol. 2011;24:671–682. doi: 10.1038/modpathol.2010.234. [DOI] [PubMed] [Google Scholar]
- 44.Llosa NJ, Cruise M, Tam A, et al. The vigorous immune microenvironment of microsatellite instable colon cancer is balanced by multiple counter-inhibitory checkpoints. Cancer Discov. 2015;5:43–51. doi: 10.1158/2159-8290.CD-14-0863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ogino S, Galon J, Fuchs CS, et al. Cancer immunology-analysis of host and tumor factors for personalized medicine. Nat Rev Clin Oncol. 2011;8:711–719. doi: 10.1038/nrclinonc.2011.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lal N, Beggs AD, Willcox BE, et al. An immunogenomic stratification of colorectal cancer: Implications for development of targeted immunotherapy. Oncoimmunology. 2015:4. doi: 10.4161/2162402X.2014.976052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Galon J, Costes A, Sanchez-Cabo F, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–4. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 48.Di Caro G, Marchesi F, Laghi L, et al. Immune cells: plastic players along colorectal cancer progression. J Cell Mol Med. 2013;17:1088–95. doi: 10.1111/jcmm.12117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mei Z, Liu Y, Liu C, et al. Tumour-infiltrating inflammation and prognosis in colorectal cancer: systematic review and meta-analysis. Br J Cancer. 2014;110:1595–605. doi: 10.1038/bjc.2014.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reissfelder C, Stamova S, Gossmann C, et al. Tumor-specific cytotoxic T lymphocyte activity determines colorectal cancer patient prognosis. J Clin Invest. 2015;125:739–51. doi: 10.1172/JCI74894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Koshiol J, Lin SW. Can tissue-based immune markers be used for studying the natural history of cancer? Ann Epidemiol. 2012;22:520–30. doi: 10.1016/j.annepidem.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.