Abstract
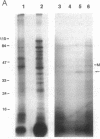
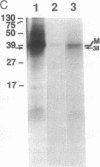
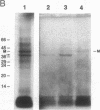
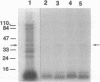
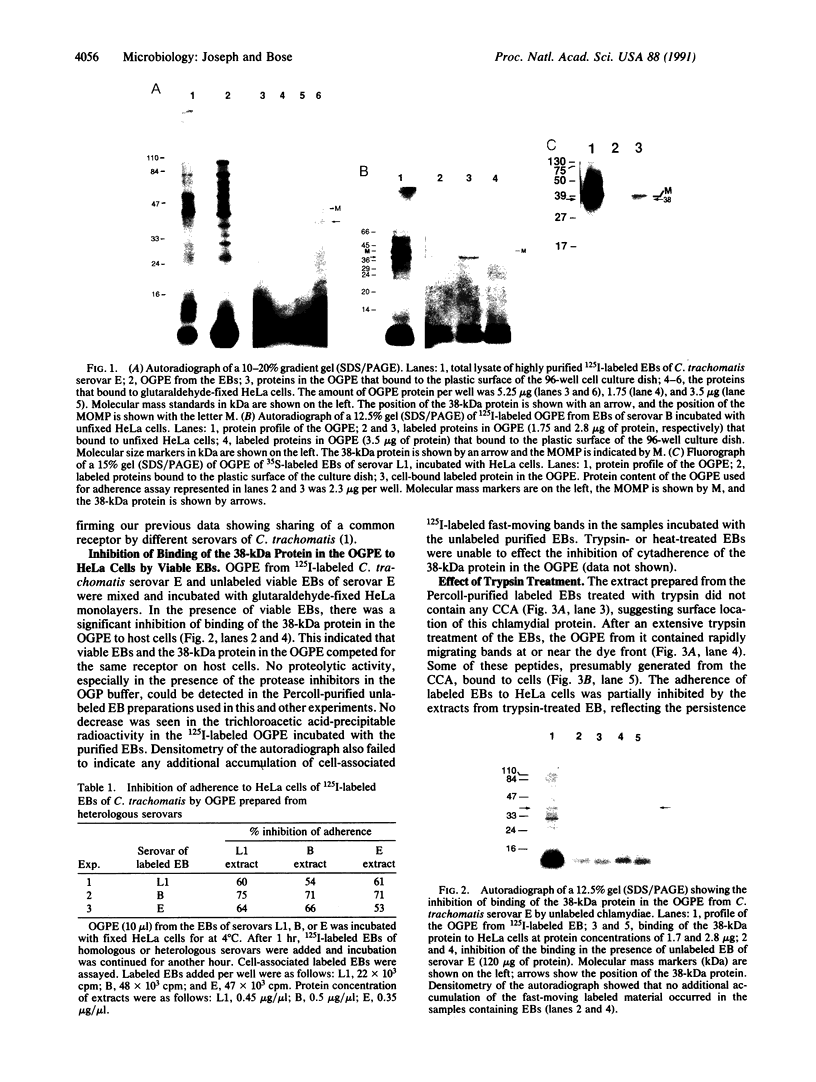
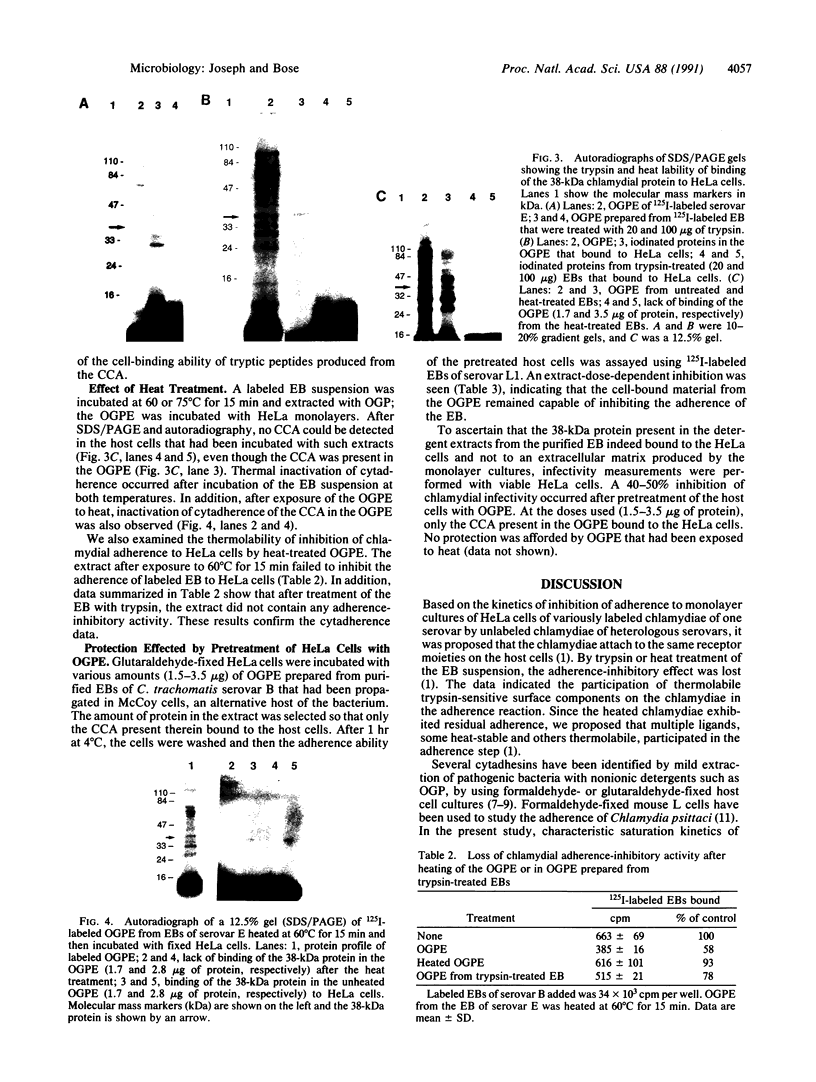
From highly purified elementary bodies (EBs) of Chlamydia trachomatis, we have identified a protein of 38 kDa that selectively binds to monolayer cultures of HeLa cells. This protein, which we have named the chlamydial cytadhesin (CCA), is present on the surface of the EBs of three C. trachomatis serovars (B, E, and L1) that were examined. Localization of the CCA at the surface was confirmed by its ability to be labeled when viable EBs were iodinated and by its absence in preparations from trypsin-treated EBs. Viable EBs, but not heated or trypsin-treated EBs, inhibited the binding of the CCA to HeLa cells, indicating competition for a common receptor on the host cell membrane. A dose-dependent inhibition of adherence of radioactive EBs to HeLa cells was effected by extracts containing the CCA. This inhibition occurred even with extracts prepared from the EB of heterologous serovars. However, no inhibition could be demonstrated with extracts prepared from heat-treated EBs. Heat treatment of the extract resulted in the loss of ability of the CCA to bind to the host cells. HeLa cells preincubated with CCA-containing chlamydial extract showed reduced ability to bind labeled EBs and to develop cytoplasmic inclusions after infection. This protective activity was lost after exposure of the extract to heat. These findings indicate that the CCA is a thermolabile surface-exposed chlamydial adhesin; it may be useful in the development of vaccines for diseases caused by the pathogenic bacterium.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alderete J. F., Garza G. E. Identification and properties of Trichomonas vaginalis proteins involved in cytadherence. Infect Immun. 1988 Jan;56(1):28–33. doi: 10.1128/iai.56.1.28-33.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baseman J. B., Hayes E. C. Molecular characterization of receptor binding proteins and immunogens of virulent Treponema pallidum. J Exp Med. 1980 Mar 1;151(3):573–586. doi: 10.1084/jem.151.3.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose S. K., Paul R. G. Purification of Chlamydia trachomatis lymphogranuloma venereum elementary bodies and their interaction with HeLa cells. J Gen Microbiol. 1982 Jun;128(6):1371–1379. doi: 10.1099/00221287-128-6-1371. [DOI] [PubMed] [Google Scholar]
- Hackstadt T. Identification and properties of chlamydial polypeptides that bind eucaryotic cell surface components. J Bacteriol. 1986 Jan;165(1):13–20. doi: 10.1128/jb.165.1.13-20.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch T. P., Vance D. W., Jr, Al-Hossainy E. Attachment of Chlamydia psittaci to formaldehyde-fixed and unfixed L cells. J Gen Microbiol. 1981 Aug;125(2):273–283. doi: 10.1099/00221287-125-2-273. [DOI] [PubMed] [Google Scholar]
- Krause D. C., Baseman J. B. Mycoplasma pneumoniae proteins that selectively bind to host cells. Infect Immun. 1982 Jul;37(1):382–386. doi: 10.1128/iai.37.1.382-386.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Su H., Watkins N. G., Zhang Y. X., Caldwell H. D. Chlamydia trachomatis-host cell interactions: role of the chlamydial major outer membrane protein as an adhesin. Infect Immun. 1990 Apr;58(4):1017–1025. doi: 10.1128/iai.58.4.1017-1025.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuszynski G. P., Knight L., Piperno J. R., Walsh P. N. A rapid method for removal of [125I]iodide following iodination of protein solutions. Anal Biochem. 1980 Jul 15;106(1):118–122. doi: 10.1016/0003-2697(80)90126-8. [DOI] [PubMed] [Google Scholar]
- Vretou E., Goswami P. C., Bose S. K. Adherence of multiple serovars of Chlamydia trachomatis to a common receptor on HeLa and McCoy cells is mediated by thermolabile protein(s). J Gen Microbiol. 1989 Dec;135(12):3229–3237. doi: 10.1099/00221287-135-12-3229. [DOI] [PubMed] [Google Scholar]
- Wenman W. M., Meuser R. U. Chlamydia trachomatis elementary bodies possess proteins which bind to eucaryotic cell membranes. J Bacteriol. 1986 Feb;165(2):602–607. doi: 10.1128/jb.165.2.602-607.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]