Abstract
The pseudotemperate phage PMB12 was isolated from soil on the basis of its ability to enhance the rate of sporulation of Bacillus subtilis 168. PMB12 was subsequently shown to convert the sporulation defect in two genetically distinct classes of sporulation mutants. One class includes those rifampin-resistant mutants that are also spore-negative (mutated at the rif locus). The other class includes a strain carrying the sporulation mutation spoCM-1. The spoCM-1 mutation is linked to cysA15 by PBS1 transduction but is distinct from the rif locus. Several other sporulation mutants were not converted by PMB12. PMB12 is related to phage PBS1. However, PBS1 did not convert the above sporulation mutants. The replication of PBS2, a clear-plaquing derivative of PBS1, is rifampin insensitive, apparently due to a phage-induced rifampin-insensitive RNA polymerase. PMB12 replication is also rifampin insensitive.
Full text
PDF

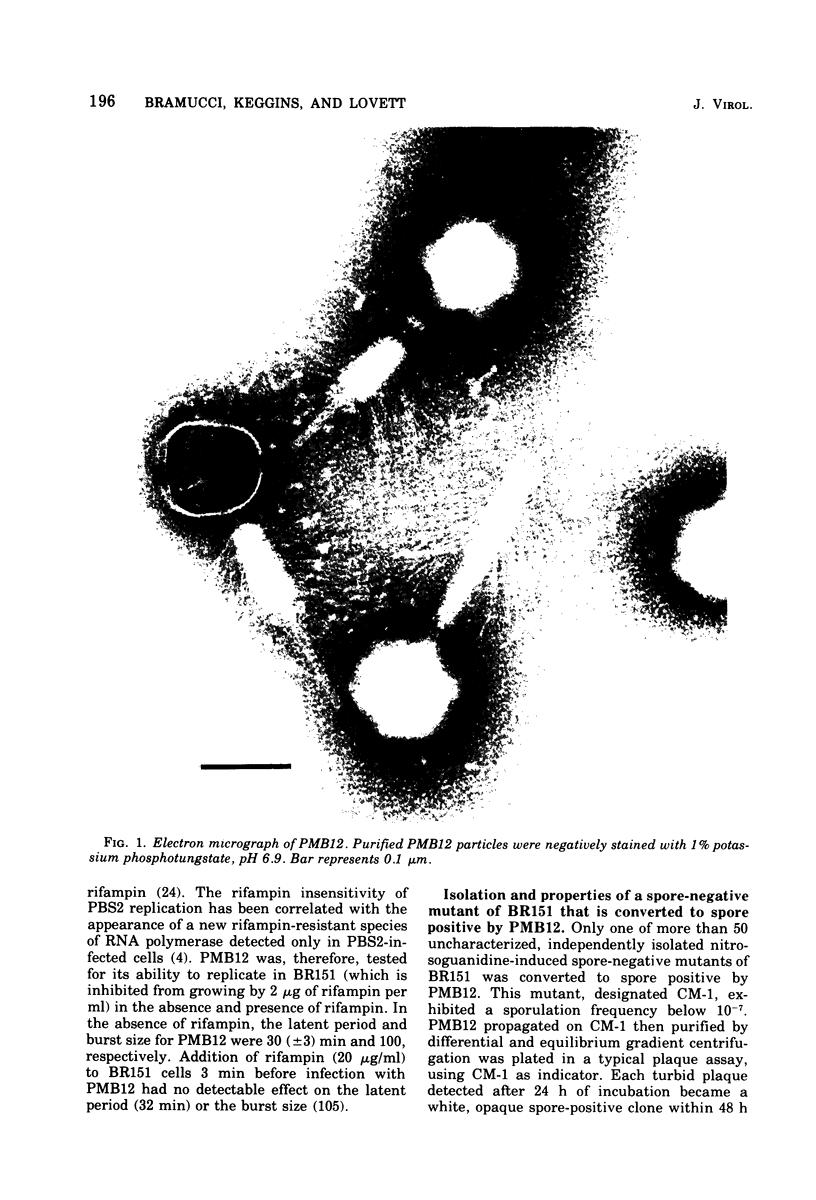




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bouché J. P., Zechel K., Kornberg A. dnaG gene product, a rifampicin-resistant RNA polymerase, initiates the conversion of a single-stranded coliphage DNA to its duplex replicative form. J Biol Chem. 1975 Aug 10;250(15):5995–6001. [PubMed] [Google Scholar]
- Bramucci M. G., Keggins K. M., Lovett P. S. Bacteriophage conversion of spore-negative mutants to spore-positive in Bacillus pumilus. J Virol. 1977 Apr;22(1):194–202. doi: 10.1128/jvi.22.1.194-202.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlin M., McGrath J., Waskell L. New RNA polymerase from Escherichia coli infected with bacteriophage T7. Nature. 1970 Oct 17;228(5268):227–231. doi: 10.1038/228227a0. [DOI] [PubMed] [Google Scholar]
- Clark S., Losick R., Pero J. New RNA polymerase from Bacillus subtilis infected with phage PBS2. Nature. 1974 Nov 1;252(5478):21–24. doi: 10.1038/252021a0. [DOI] [PubMed] [Google Scholar]
- DiCioccio R. A., Strauss N. Patterns of transcription in Bacillus subtilis during sporulation. J Mol Biol. 1973 Jun 25;77(2):325–336. doi: 10.1016/0022-2836(73)90338-0. [DOI] [PubMed] [Google Scholar]
- Doi R. H., Brown L. R., Rodgers G., Hsu Y. Bacillus subtilis mutant altered in spore morphology and in RNA polymerase activity. Proc Natl Acad Sci U S A. 1970 Jun;66(2):404–410. doi: 10.1073/pnas.66.2.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiserling F. A. The structure of Bacillus subtilis bacteriophage PBS 1. J Ultrastruct Res. 1967 Feb;17(3):342–347. doi: 10.1016/s0022-5320(67)80053-4. [DOI] [PubMed] [Google Scholar]
- Fukuda R., Doi R. H. Two polypeptides associated with the ribonucleic acid polymerase core of Bacillus subtilis during sporulation. J Bacteriol. 1977 Jan;129(1):422–432. doi: 10.1128/jb.129.1.422-432.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harford N., Sueoka N. Chromosomal location of antibiotic resistance markers in Bacillus subtilis. J Mol Biol. 1970 Jul 28;51(2):267–286. doi: 10.1016/0022-2836(70)90142-7. [DOI] [PubMed] [Google Scholar]
- Hemphill H. E., Whiteley H. R. Bacteriophages of Bacillus subtilis. Bacteriol Rev. 1975 Sep;39(3):257–315. doi: 10.1128/br.39.3.257-315.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoch J. A. Genetics of bacterial sporulation. Adv Genet. 1976;18:69–98. doi: 10.1016/s0065-2660(08)60437-x. [DOI] [PubMed] [Google Scholar]
- Hranueli D., Piggot P. J., Mandelstam J. Statistical estimate of the total number of operons specific for Bacillus subtilis sporulation. J Bacteriol. 1974 Sep;119(3):684–690. doi: 10.1128/jb.119.3.684-690.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter B. I., Yamagishi H., Takahashi I. Molecular weight of bacteriophage PBS 1 deoxyribonucleic acid. J Virol. 1967 Aug;1(4):841–842. doi: 10.1128/jvi.1.4.841-842.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JANSSEN F. W., LUND A. J., ANDERSON L. E. Colorimetric assay for dipicolinic acid in bacterial spores. Science. 1958 Jan 3;127(3288):26–27. doi: 10.1126/science.127.3288.26. [DOI] [PubMed] [Google Scholar]
- Joys T. M. Correlation between susceptibility to bacteriophage PBS1 and motility in Bacillus subtilis. J Bacteriol. 1965 Dec;90(6):1575–1577. doi: 10.1128/jb.90.6.1575-1577.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leighton T. J. An RNA polymerase mutation causing temperature-sensitive sporulation in Bacillus subtilis. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1179–1183. doi: 10.1073/pnas.70.4.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linn T., Greenleaf A. L., Losick R. RNA polymerase from sporulating Bacillus subtilis. Purification and properties of a modified form of the enzyme containing two sporulation polypeptides. J Biol Chem. 1975 Dec 25;250(24):9256–9261. [PubMed] [Google Scholar]
- Linn T., Losick R., Sonenshein A. L. Rifampin resistance mutation of Bacillus subtilis altering the electrophoretic mobility of the beta subunit of ribonucleic acid polymerase. J Bacteriol. 1975 Jun;122(3):1387–1390. doi: 10.1128/jb.122.3.1387-1390.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losick R., Sonenshein A. L. Change in the template specificity of RNA polymerase during sporulation of Bacillus subtilis. Nature. 1969 Oct 4;224(5214):35–37. doi: 10.1038/224035a0. [DOI] [PubMed] [Google Scholar]
- Lovett P. S., Duvall E. J., Keggins K. M. Bacillus pumilus plasmid pPL10: properties and insertion into Bacillus subtilis 168 by transformation. J Bacteriol. 1976 Aug;127(2):817–828. doi: 10.1128/jb.127.2.817-828.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovett P. S., Young F. E. Genetic analysis in Bacillus pumilus by PBSI-mediated transduction. J Bacteriol. 1970 Feb;101(2):603–608. doi: 10.1128/jb.101.2.603-608.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piggot P. J., Coote J. G. Genetic aspects of bacterial endospore formation. Bacteriol Rev. 1976 Dec;40(4):908–962. doi: 10.1128/br.40.4.908-962.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price A. R., Hitzeman R., Frato J., Lombardi K. Rifampicin-resistant bacteriophage PBS2 infection and RNA polymerase in Bacillus subtilis. Nucleic Acids Res. 1974 Nov;1(11):1497–1502. doi: 10.1093/nar/1.11.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHILDKRAUT C. L., MARMUR J., DOTY P. Determination of the base composition of deoxyribonucleic acid from its buoyant density in CsCl. J Mol Biol. 1962 Jun;4:430–443. doi: 10.1016/s0022-2836(62)80100-4. [DOI] [PubMed] [Google Scholar]
- STUDIER F. W. SEDIMENTATION STUDIES OF THE SIZE AND SHAPE OF DNA. J Mol Biol. 1965 Feb;11:373–390. doi: 10.1016/s0022-2836(65)80064-x. [DOI] [PubMed] [Google Scholar]
- Sonenshein A. L., Cami B., Brevet J., Cote R. Isolation and characterization of rifampin-resistant and streptolydigin-resistant mutants of Bacillus subtilis with altered sporulation properties. J Bacteriol. 1974 Oct;120(1):253–265. doi: 10.1128/jb.120.1.253-265.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonenshein A. L., Losick R. RNA polymerase mutants blocked in sporulation. Nature. 1970 Aug 29;227(5261):906–909. doi: 10.1038/227906a0. [DOI] [PubMed] [Google Scholar]
- Sumida-Yasumoto C., Doi R. H. Bacillus subtilis ribonucleic acid polymerase mutants conditionally temperature sensitive at various stages of sporulation. J Bacteriol. 1977 Jan;129(1):433–444. doi: 10.1128/jb.129.1.433-444.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumida-Yasumoto C., Doi R. H. Transcription from the complementary deoxyribonucleic acid strands of Bacillus subtilis during various stages of sporulation. J Bacteriol. 1974 Feb;117(2):775–782. doi: 10.1128/jb.117.2.775-782.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipper D. J., Johnson C. W., Ginther C. L., Leighton T., Wittmann H. G. Erythromycin resistant mutations in Bacillus subtilis cause temperature sensitive sporulation. Mol Gen Genet. 1977 Jan 18;150(2):147–159. doi: 10.1007/BF00695395. [DOI] [PubMed] [Google Scholar]
- Yamagishi H. Single strand interruptions in PBS 1 bacteriophage DNA molecule. J Mol Biol. 1968 Aug 14;35(3):623–633. doi: 10.1016/s0022-2836(68)80018-x. [DOI] [PubMed] [Google Scholar]