Abstract
The gut microbiota is associated with metabolic diseases including obesity, insulin resistance and non-alcoholic fatty liver disease (NAFLD), as demonstrated by correlative studies and by transplant of microbiota from obese humans and mice into germ-free mice. Modification of the microbiota by treatment of high-fat diet (HFD)-fed mice with tempol or antibiotics resulted in decreased adverse metabolic phenotypes. This was due to lower levels of the genera Lactobacillus and decreased bile salt hydrolase (BSH) activity. The decreased BSH resulted in increased levels of tauro-β-muricholic acid (T-β-MCA), a substrate of BSH and a potent farnesoid X receptor (FXR) antagonist. Mice lacking expression of FXR in the intestine were resistant to HFD-induced obesity, insulin resistance and NAFLD thus confirming that intestinal FXR is involved in the potentiation of metabolic disease. A potent intestinal FXR antagonist glycine-β-muricholic acid (Gly-MCA) that is resistant to BSH, was developed that when administered to HFD-treated mice, mimics the effect of the altered microbiota on HFD-induced metabolic disease. Gly-MCA had similar effects on genetically obese leptin-deficient mice. The decreased in adverse metabolic phenotype by tempol, antibiotics and Gly-MCA was due to decreased serum ceramides. Mice lacking FXR in intestine also have lower serum ceramides, are metabolic fit and resistant to HFD-induced metabolic disease, and this is reversed by injection of C16:0 ceramide. In mouse ileum, due to the presence of endogenous FXR agonists produced in the liver, FXR target genes involved in ceramide synthesis are activated and when Gly-MCA is administered, they are repressed, which likely accounts for the decrease in serum ceramides. These studies reveal that ceramides produced in the ileum under control of FXR, influence metabolic diseases.
Non-alcoholic fatty liver disease
The accumulation of ectopic triglycerides in the liver, in the absence of other liver disease or chronic alcohol consumption, is termed non-alcoholic fatty liver disease (NAFLD)1, 2. NAFLD affects approximately 19% of the adult population, and is correlated with the worldwide epidemic of metabolic disease that is associated with concurrent obesity and insulin resistance3. Patients with obesity, type 2 diabetes, dyslipidemia, high blood pressure, and NAFLD are characterized as having metabolic syndrome4 and alarmingly this classification equally applies to children as well as adults5. Persistent NAFLD can lead to non-alcoholic steatohepatitis (NASH), in which fibrosis develops on top of steatosis and is characterized histologically by hepatocyte ballooning, degeneration and inflammation. Fibrosis, although not always observed, is caused by chronic inflammation and oxidative stress that occurs in the presence of elevated hepatic lipids. NASH is associated with an additional increased risk of developing cirrhosis and hepatocellular carcinoma6, 7. NAFLD is linked not only to obesity and insulin resistance, but also to heart disease and atherosclerosis, and there is a significant population (mostly Asian) of metabolically normal individuals with NAFLD that suggest a genetic or environmental influence to this disease8. NAFLD is often called the silent disease due to a lack of clear symptoms that makes it especially dangerous because it is not easily diagnosed. Thus, major efforts have been undertaken to determine the cause of NAFLD, to develop new therapeutic interventions, and to establish curative strategies for NAFLD9. Although the molecular mechanisms of NAFLD pathogenesis have been extensively investigated, there are few clues for the identification of novel targets that can be exploited for NAFLD therapy, and thus this remains an area of high priority.
Influence of gut microbiota on metabolic disease and NAFLD
Accumulating evidence suggests that intestinal bacteria contribute to the development of NAFLD10 based on observations in a variety of animal models11 as well as in humans5, 12, 13. Intestinal bacteria also influence obesity and insulin resistance14, 15. Bacteria community analysis, based on 16S rRNA gene sequencing, has revealed stark differences between metabolically-normal individuals and those with NAFLD and NASH. Broad phyla level (noted shifts in Bacteroidetes, Firmicutes, and Proteobacteria) changes have been reported and some have attempted to implicate specific bacterial strains with disease, although these observations remain preliminary16–18. Interestingly, stool bacterial community profiles of children with NAFLD are unique compared with adults with NAFLD, but this inconsistency might be expected given observations from others19. Using longitudinal sequencing of samples collected over time, it is clear that the gut microbiota is dynamic and ever changing throughout childhood, and only stabilizes once adulthood is reached19. These observations suggest that at the gut bacteria level, NAFLD therapies might need to be tailored based on age, and underscores that the gut microbiota contribution in children may be significantly different in adults. However, it should be noted that these studies are far from complete, as the conclusions are typically derived from small sample sizes and have not been evaluated in other cohorts. It is clear that more long term studies representing different ages, sexes, and ethnic groups will be essential to understand the complex etiology of this disease.
Some of the strongest data linking gut bacteria with NAFLD comes from studies examining the impact of antibiotic treatment on NAFLD pathogenesis and progression. Interestingly, remarkably different outcomes were reported depending on the timing, duration, and dose of antibiotic used either to promote or treat NAFLD. For example, a recent report suggested that chronic exposure to penicillin G (6.8 mg/L in the drinking water) in combination with a high-fat diet (HFD, 45% kcal from fat) resulted in not only metabolic disturbances but also gut dysbiosis that was suggested to be a strong promoter of NAFLD20. This and other studies underscore that early and sustained perturbation of the gut microbiota is strongly associated with metabolic diseases including obesity and NAFLD21, 22. However, other reports suggest that germ free mice are fully resistant to the development of NAFLD (as well as obesity and other related metabolic disorders)23, and that high dose cocktails of antibiotics can reverse NAFLD24. Moreover, antibiotics like rifaximin with poor bioavailability and hence likely to only impact the gut microbiota, were shown to be efficacious against NAFLD in humans25. Antibiotic treatment may even help to prevent the transition towards hepatocellular carcinoma26. While data from low and high dose antibiotic use appear to be contradicting, differences in dose, exposure window, and choice of antibiotic likely direct metabolic processes in the host and gut microbiota either towards health or disease.
The gut microbiota has been directly implicated in the development of obesity and NAFLD in mice and in humans27–29. Notably, in transplant studies, when lean germ-free mice are treated with the cecal microbiota from obese mice, there was an increase in hepatic triglyceride accumulation30. When bacteria from lean humans are transferred to germ-free mice, the mice became resistant to obesity, whereas mice receiving bacteria from obese humans were prone to obesity31. Although it was not directly measured, this correlation may also have extended to insulin resistance and NAFLD that are directly associated with obesity. Indeed, another study revealed that mice receiving bacteria transplanted from their obese counterparts developed hepatic macrovesicular steatosis and showed increased expression of the genes involved in lipid synthesis11.
Correlative studies of gut bacteria transplantation have shown a direct relationship between various genera of bacteria and metabolic disease that may yield hints to mechanism11. For example, obese humans show an enrichment of the energy-harvesting phylum Firmicutes, which have an improved energy yield from intestinal contents (e.g., short chain fatty acids including acetate, butyrate, and propionate) that are thought to accelerate obesity and NAFLD, mostly likely through de novo lipogenesis pathways14. In a study of NAFLD patients, there was an overrepresentation of Lactobacillus spp. and members of the phylum Firmicutes, thus establishing a potential role for gut microbiota in human NAFLD32. While there is an association between populations of gut microbiota and even metabolism in NAFLD, it remains uncertain whether the altered bacteria (as compared to lean individuals) cause obesity or are due to obesity33, 34.
Overall, the gut microbiota was demonstrated to significantly influence, and itself can be shaped by, metabolic diseases such as obesity and type 2 diabetes10, 35. Adding to the complexity in understanding their contribution to NAFLD and other metabolic disease, intestinal bacteria populations vary considerably, depending on factors such as host genetics and health, the environment, and diet36. These variations contribute to an individuals metabolic phenotype or metabotype37–40. Gut bacteria can be directly involved in the metabolism of dietary compounds such as lipids, polysaccharides and xenobiotics, and can influence the uptake of nutrients, which may contribute to host metabolism and metabolic disease. Notably, gut bacteria metabolize bile acids that are produced in the liver, and can metabolize many drugs40, 41. Bile acid metabolites may in part mediate the influence of the microbiota on metabolic disease and may also mediate changes in the composition of the gut bacteria42, 43.
Regulation of bile acid homeostasis
Bile acids are produced in the liver by the oxidation of cholesterol through a series of reactions carried out by cytochromes P450s (CYP), with cholesterol 7α-hydroxylase (CYP7A1) generally considered the rate-limiting enzyme that initiates bile acid synthesis44, 45. After modification by oxidation, most bile acids undergo conjugation with the amino acids taurine and glycine before being transported to the intestine through the canaliculi that merge and form the bile duct46. Taurine conjugation predominantly occurs in mice, while glycine conjugation occurs in hamsters and humans; rats carry out both taurine and glycine conjugation47. Conjugated and unconjugated bile acids are transported out of the liver and form micelles in the small intestine that capture dietary lipids and fat-soluble vitamins from the diet, to facilitate their movement down the gastrointestinal (GI) tract, and promote their metabolism by intestinal lipases and uptake by the epithelial cells prior to transport to the bloodstream. Most bile acids leave the liver in the conjugated form, and in the lower small intestine, they are deconjugated and further metabolized by gut bacteria. Subsequently, approximately 95% of the bile acids are reabsorbed and transported back to the liver, thus completing the process of enterohepatic circulation46, 48, 49.
Bile acid synthesis and enterohepatic circulation are under tight control by receptors that modulate the expression of genes encoding enzymes and proteins involved in bile acid synthesis and transport48–50. The major regulator of bile acid homeostasis is the farnesoid X receptor (FXR, NR1H4), a ligand-activated member of the nuclear receptor superfamily51, 52. FXR can be bound by a number of endogenous bile acids, including chenodeoxycholic acid (CDCA), taurocholic acid (TCA), deoxycholic acid (DCA), lithocholic acid (LCA), cholic acid (CA) and muricholic acid at various affinities53, 54, with the potency of FXR activation in reporter gene assays estimated at CDCA>LCA=DCA53. Conjugated bile acids such as glycochenodeoxycholic acid (GCDCA), taurocholic acid (TCA), and taurochenodeoxycholic acid (TCDCA) also weakly activate FXR55. FXR is expressed in the liver and intestine where it regulates the expression of genes involved in the synthesis and transport of bile acids, and thus is a major modulator of bile acid homeostasis and enterohepatic circulation48, 49. In the presence of increased intracellular bile acids, hepatic FXR is activated, resulting in the suppression of bile acid synthesis, enhanced bile acid bile transport to the small intestine, and reduced bile acid uptake from the blood. FXR-mediated gene suppression occurs through the induction of small heterodimeric protein (SHP, NR0B2) that interferes with the positive regulation of gene expression by other nuclear receptors including liver receptor homolog-1 (LRH, NR5A2) and liver X receptor (LXR, NR1H3), both of which control the expression of genes involved in bile acid synthesis, notably CYP7A1, and transport56. Interesting, LXR controls the mouse and rat genes encoding CYP7A1, but does not control the corresponding human CYP7A1 gene57. Hepatocyte nuclear factor 4α (HNF4α, NR2A21) is also a key regulator of CYP7A1, bile acid conjugation enzymes and several transporters58. In the intestine, activation of FXR by increased levels of bile acids in the enterocyte, results in enhanced transport of bile acids to the blood and uptake by the liver, and decreased bile acid synthesis in the liver through induction of fibroblast growth factor (FGF)19 (FGF15 in mice) expression in the enterocyte, which upon transport to the liver and activation of the FGFR4/β-Klotho receptor complex on the hepatocyte plasma membrane, also suppresses the expression of CYP7A1 as recently reviewed59. Hepatic FXR inhibits expression of sodium ion/bile acid cotransporter (NTCP) that transports bile acid into the liver and activates expression of the bile salt export protein (BSEP, ABCb11) that transports bile acids across the canalicular membrane into the bile duct and gall bladder60. Ileal FXR controls bile acid uptake from the small intestine to the portal blood through induction of ASBT (SLC10A2) that transports bile acids into the epithelial cells, bile acid binding protein (I-BABP) that transports in the bile acids across the cell, and OSTα/β (SLC51A/B) that transports bile acids into the portal blood61. FGF15/19 also promotes the emptying of the gall bladder through binding and activating the FGFR2/β-Klotho receptor complex59. Thus, in summary, under conditions of increased bile acid levels in the intestine, FXR is activated in the epithelial cells of the ileum and stimulates the transport of bile acids into the portal blood for delivery back to the liver. Elevated hepatic bile acids activate FXR to increase the export of bile acids into the intestine, decrease uptake of bile acids from the blood and decreased bile acid synthesis. Intestinal FXR activation further increases FXR15/19 to decrease bile acid synthesis in the liver by suppression of CYP7A1 expression. Through mechanisms that are still not well understood, FXR also regulates lipid and glucose levels in the liver and serum62, 63, and influences cardiovascular disease64.
A G protein-coupled receptor, Takeda G-protein coupled receptor-5 (TGR5, GPBAR1) also responds to bile acid ligands produced in the liver50, 65. In contrast, to the expression of FXR which is highly expressed the liver and ileum, and in specific kidney cells, TGR5 is expressed in many tissues including liver, gallbladder, ileum, and skeletal muscle, and other sites that when activated, increases cyclic AMP and associated downstream effects, depending on the cell type65. TGR5 is activated by bile acids including LCA, DCA, CDCA, and CA, some of which also activate FXR. In the intestinal L cells, TGR5 stimulates the release of glucagon-like peptide-1 (GLP-1) that controls glucose levels, and peptide tyrosine tyrosine (PYY) that regulates food intake66. In other tissues, TGR5 has diverse effects that are usually related to metabolism. TGR5 also influences bile acid homeostasis, although the mechanisms are not fully elucidated67. While there is no evidence that the gut microbiota or bacterial-derived metabolites alter TGR5 signaling, this possibility has not yet been thoroughly explored. However, bile acid receptors FXR and TGR5 serve as host conduits by which gut microbiota influence metabolic disease.
FXR mediates the influence of gut microbiota on NAFLD development
Although several mechanisms were proposed to explain how the gut microbiota influence NAFLD development29, there have been a number of hypothesis for the biochemical link and the signal transduction pathway(s) that are responsible for the effects of gut bacteria on hepatic steatosis, including increased intestinal permeability, bacterial endotoxins, and activation of receptors as part of innate immunity68. Indeed, gut bacteria can influence the inflammatory status of the intestine, which is thought to contribute to inflammatory bowel diseases69, but may also influence metabolic disease. Recent evidence for a mechanistic link between intestinal bacteria and metabolic disease in mice, evolved from early studies showing that tempol, a low molecular weight, stable free radical nitroxide, protects cells from oxidative stress and reduces weight gain in mice70. This study established quite definitely, that at least in mice, microbiota, bile acids and intestinal nuclear receptor signaling influences metabolic disease. Tempol-treated mice also have less spontaneous liver cancer and lymphomas, and this is accompanied by increased longevity70, 71. Only in the past few years has the mechanism this remarkable effect of tempol been elucidated. Mice that were given tempol showed an elevated expression of uncoupling protein 2 (UCP2), which is highly expressed in skeletal muscle and correlated with decreased mitochondrial-generated oxidative stress. However, the mechanism by which tempol decreased obesity was not clear until metabolomics studies revealed the presence of elevated levels of molecules known to be associated with increased fatty acid metabolism, and more surprisingly of compounds that reflect metabolism by the gut microbiota72. Mice treated with tempol had lower levels of pantothenic acid and isobutyryl-carnitine in their urine, thus indicating increased fatty acid oxidation73, 74. Pantothenate is the precursor for the synthesis of coenzyme A (CoA), which is required for fatty acid β-oxidation (Figure 1), whereas isobutyryl-L-carnitine is an intermediate in fatty acid β-oxidation. This could partially explain the anti-obesity effects of tempol. In addition, elevated levels of 2,8-dihydroxyquinoline and its glucuronide conjugate were found in the urine of mice that were treated with tempol74. These compounds were also increased in the urine of mice that were treated with the peroxisome proliferator-activated receptor α (PPARα, NR1C1) agonist Wy-14,643, thus indicating that tempol might be a PPARα activator75. PPARα activates genes that are involved in mitochondrial and peroxisomal fatty acid β-oxidation76. However, mice treated with tempol showed no increase in PPARα signaling and there were no tempol metabolites identified that are PPARα activators74.
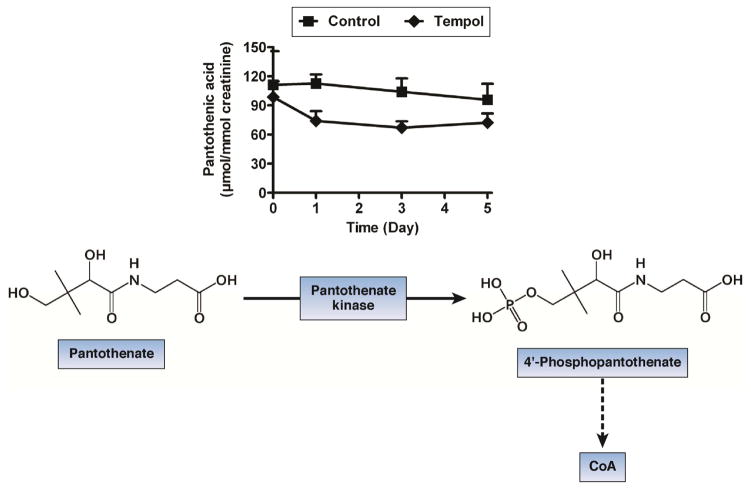
Figure 1.
Levels of pantothenic acid in the urine of mice that were treated with tempol. Pantohthenic acid is converted to 4′phosphopantothenate, which is a precursor for synthesis of coenzyme A (CoA) and is required for the β-oxidation of fatty acids in mitochondria.
2,8-Dihydroxyquinoline (DHQ) and its glucuronide are derived from bacterial metabolism, and are beacons for changes in the gut microbe population, thus suggesting that tempol alters the composition of the gut bacteria; DHQ is produced by Pseudomonas stutzeri and possibly other gut bacteria 72, 77, 78. Indeed, a 16S rRNA gene-based community analysis revealed that tempol administration markedly shifted the cecal bacterial population from the phylum Firmicutes to Bacteriodetes; this occurred within five days of commencing tempol treatment79. There was a notable and specific decrease in the Lactobacillaceae family within the phylum Firmicutes due, in large part, to the reduced levels Lactobacillus spp. Evidence of the mechanistic link between gut bacteria and the metabolic changes attributed to tempol was found when bile acid metabolites were examined. The tempol-treated mice showed higher levels of tauro-β-muricholic acid (T-β-MCA) than did the vehicle-treated mice. T-β-MCA is an antagonist of FXR as demonstrated by its in vivo administration to sterile antibiotic-treated mice and by in vitro reporter gene assays43, 79, 80. T-β-MCA is produced in the liver when ursodeoxycholic acid (UDCA) is hydroxylated in the mouse or rat liver to β-MCA and then conjugated to taurine (Figure 2). As noted above, other bile acid metabolites including CA, taurocholic acid (TCA), CDCA and taurochenodeoxycholic acid (TCDCA), which are potent and weak FXR agonists, respectively, are also produced and enter the intestine. Therefore, a competition exists between bile acid agonist and antagonist for the activation or suppression of FXR signaling in ileum epithelial cells. The levels of agonist and antagonist could be modulated by gut bacterial metabolism of bile acids. In untreated mice, T-β-MCA is rapidly deconjugated to β-MCA by bile salt hydrolase (BSH), an enzyme expressed in some strains of Lactobacillus, Bacteroides, Clostridium, and Bifidobacterium81; this accounts for the observed increase in T-β-MCA following tempol treatment that decreased these bacteria and BSH activity79. Thus, in the absence of tempol, and with high BSH activity, T-β-MCA is almost quantitatively hydrolyzed to β-MCA. When T-β-MCA is low in the gut, FXR can then be weakly activated by other bile acid metabolite agonists that are produced in the liver or by microbiota metabolism in intestine. Thus, mice treated with tempol showed an attenuation of FXR signaling in the ileum, due to the inhibition of FXR by T-β-MCA, which accumulates in the intestine due to lower BSH activity79, 80. Of interest, FXR inhibition was restricted to the intestine, because no decrease in the expression of FXR target genes was found in the livers of tempol-treated mice24, 79. Therefore, a model was proposed in which tempol decreases Lactobacillus spp. through a mechanism that is still not clear. Lower levels of Lactobacillus spp. results in less BSH in the intestine and increased levels of T-β-MCA, which can inhibit FXR signaling in the ileal epithelial cell, the major site of FXR expression in the gut (Figure 3). This model predicts that intestinal FXR mediates the anti-obesity effects of tempol and antibiotics, which also decrease Lactobacillus spp. and BSH, and that the chronic inhibition of FXR would render mice resistant to HFD-induced obesity. Indeed, mice with an intestinal disruption of FXR, did not gain as much weight as their wild-type counterparts, thus establishing that FXR promotes metabolic diseases under treatment with HFD79, 82. This finding is in agreement with an earlier study reporting that mice showed less weight gain and associated increased insulin sensitivity when the Fxr was disrupted leptin-deficient ob/ob mouse background compared to the ob/ob mouse expressing the wild-type FXR, while ob/ob mice lacking FXR expression in the liver were did not display these favorable metabolic effects83. This indirectly implicates intestinal FXR deficiency in the improved metabolic phenotype of ob/ob mice. Mice lacking expression of FXR in the intestine also have decreased insulin resistance and lower fatty livers than their wild-type counterparts79, 82. A recent study found that ob/ob mice had altered gut microbiota compositions, less taurine-conjugated bile acids, increased intestinal FXR expression levels, and elevated FXR signaling compared to their lean ob/+ littermates84, in agreement with the studies in tempol-treated and FXR intestine-deficient mice79.
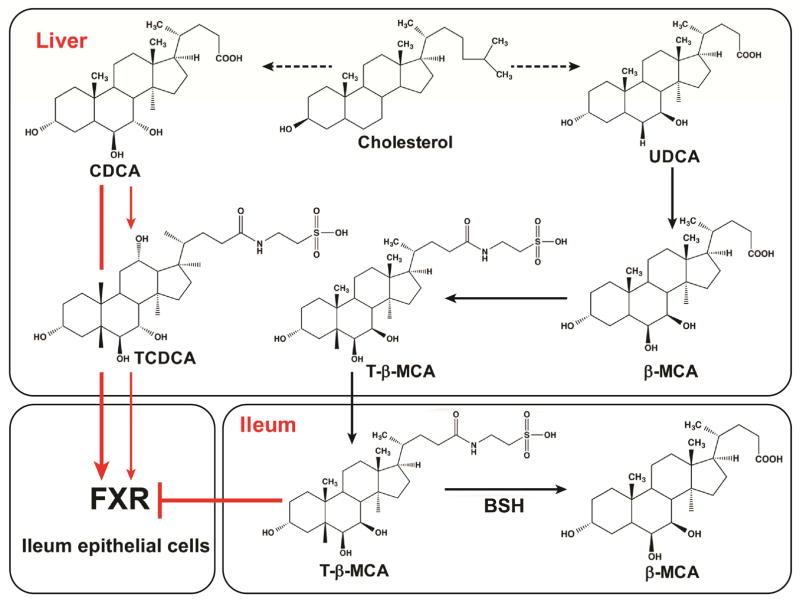
Figure 2.
Bile acids are synthesized in the liver and intestine to produced FXR agonist and antagonist. In the liver CDCA is synthesized and then conjugated with taurine to produce TCDCA, or hydroxylated to produce α-MCA which can also be conjugated with taurine to produce T-α-MCA (not shown). UDCA, an isomer of CDCA, is made and hydroxylated to produce β-MCA that is then conjugated with taurine to yield T-β-MCA. TCDCA and T-β-MCA and some ot their free unconjugated forms are exported to the intestine. In the intestine T-β-MCA, an FXR antagonist, and can by deconjugated to taurine and β-MCA by bacterial BSH. CDCA resulting from TCDCA deconjugation, is a potent FXR agonist. The concentrations of CDCA, T-β-MCA and other bile acids in the lower small intestine, likely determine the extent of FXR signaling in ileal epithelial cells.
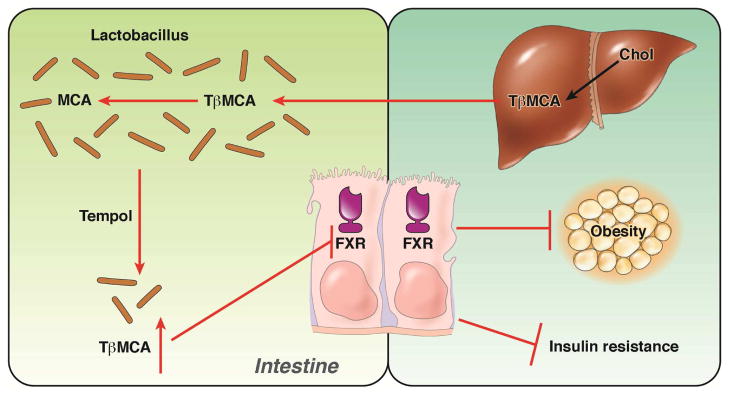
Figure 3.
The role of gut bacterial and intestinal FXR in tempol-induced weight loss. Cholesterol is converted to T-β-MCA that is secreted into to intestine. In the lower small intestine of mice it is hydrolyzed to β-MCA and taurine by BSH. When mice are administered tempol, the levels of Lactobacillus and BSH activity are decreased resulting in increased T-β-MCA that inhibits FXR in the ilium epithelial cells. This results in lower obesity and insulin resistance.
Further evidence on the role of gut bacteria in metabolic disease was obtained by treating mice with antibiotics and showing that the bile acid pool was modified coincident with decreased intestinal bacteria, and that FXR signaling was markedly suppressed. Treatment with the potent combination of bacitracin, neomycin, and streptomycin resulted in marked changes in the intestinal bacterial population, including decreased Lactobacillus spp., and this was correlated with lower levels of the FXR inhibitor T-β-MCA and decreased FXR signaling in the intestine24. Similar results were obtained when using ampicillin, another broad-spectrum antibiotic. A link between antibiotics, conjugated bile acids, and FXR signaling was indicated by earlier studies showing that decreased TCA deconjugation to cholic acid was correlated with decreased FXR signaling as revealed by monitoring expression of the FXR target genes Fgf15 and Shp in the ileum85, 86. In these studies, the lower FXR signaling was likely due, in large part, to the increased levels of T-β-MCA in the intestine as found with tempol79. While these earlier studies with ampicillin, did not address the effects of antibiotics, bile acids, and FXR signaling on metabolic diseases, more recent work revealed that antibiotic-treated mice on a HFD were metabolically fit as indicated by reduced weight, increased insulin sensitivity and decreased hepatic steatosis compared with controls that were not treated with antibiotics24. These studies suggest that there is an endogenous pathway that controls metabolic fitness involving hepatic synthesis of bile acids, gut bacteria and intestinal FXR. The diet, notably a high fat diet, also contributes to this pathway. While gut bacteria can metabolize bile acids and contribute to the balance of FXR bile acid activators and inhibitors, gut bacteria can also produce FXR ligands that are not derived from bile acids. A recent study found that extracts from select gut bacteria have FXR agonist activity that when administered to HFD-fed mice produced some improvements in metabolic parameters, although the metabolites activated (not inhibited) intestinal and hepatic FXR 87. While a challenging proposition, this work suggests that gut bacteria administered as probiotics could improve metabolic fitness.
The inhibition of intestinal FXR signaling by an endogenous bile acid T-β-MCA results in decreased HFD-induced obesity, insulin resistance and NAFLD, which suggests that FXR could be a potential drug target. However, T-β-MCA is not a viable candidate drug because it is rapidly hydrolyzed by BSH in the intestine of mice that are on a HFD or mice that are genetically obese as these mice have abundant BSH-expressing bacteria. Humans also have bacterial-derived BSH activity88, which indicates that T-β-MCA would also be unstable in the human intestine. For a compound to be a potential drug candidate, a derivative of T-β-MCA that is resistant to BSH but retains FXR antagonism activity would need to be produced. In silico modeling of the T-β-MCA binding to FXR revealed that a closely related derivative, glycine-β-muricholic acid (Gly-MCA), could potentially bind to the FXR active site, similar to T-β-MCA82. Reporter gene assays show that Gly-MCA is a more potent inhibitor of FXR than T-β-MCA. However, unlike T-β-MCA, Gly-MCA is resistant to BSH and is thus stable in the intestine. Indeed, in mice that were orally administered Gly-MCA, which has no significant oral bioavailability, Gly-MCA was found un-metabolized in the ileum and feces with no accumulation in the liver82. The latter finding is of notable importance since inhibition of hepatic FXR could result in hepatic steatosis and toxicity, as seen in the whole body knockout mice89, and in mice with liver-specific disruption of FXR (unpublished results). Indeed, orally administered Gly-MCA does not inhibit hepatic FXR signaling as revealed by the lack of decreased expression of the FXR target gene Shp82. When administered to HFD-fed mice at a dose of 10 mg kg−1, Gly-MCA inhibited FXR signaling in the ileum and decreased obesity, insulin resistance and fatty liver82, similar to the effects observed in both tempol- and antibiotic-treated mice24, 79. Gly-MCA also decreased these metabolic parameters both in mice that were made obese with HFD feeding, and in genetically-obese mice, thus indicating that this pathway could be a novel mechanism to treat metabolic disease82.
Role of intestinal FXR in the regulation ceramides
Ceramides at high levels can cause adverse effects in many tissues. In humans, there is an inverse correlation between metabolic disease and serum ceramides90, 91. In rodent models, ceramide accumulation is correlated with toxicity that leads to metabolic diseases, in particular the promotion of insulin resistance92. Inhibitors of ceramide synthesis protected rats from diabetes, leading to the concept of toxic lipids93, 94. Increased serum and tissue ceramides also contribute to obesity and NAFLD, as was demonstrated with studies on transgenic models. Mice that were heterozygous for dihydroceramide desaturase 1 showed lower levels of ceramides, and enhanced insulin sensitivity, and were refractory to dexamethasone-induced insulin resistance95. Mice that lacked the expression of ceramide synthase S6 and had reduced C16:0 ceramides were protected from HFD-induced obesity and glucose intolerance96. In contrast, transgenic mice over-expressing N-acylsphingosine amidohydrolase, a potent ceramidase, in liver or adipose tissue and were fed a HFD, had lower levels of serum ceramides, improved insulin sensitivity and decreased fatty livers97. Thus, ceramide levels have been correlated with metabolic disease in humans, and high serum and tissue ceramide levels directly cause metabolic diseases in mouse models.
The mechanism by which the inhibition of intestinal FXR signaling decreased diet- and genetically-induced metabolic disease involves modulation of ceramide synthesis. A signaling pathway linking intestinal FXR with liver and adipose tissue metabolism was uncovered in which FXR controls the expression of genes encoding ceramides synthesis enzymes24, 82. Intestinal FXR was also found to control the levels of serum ceramides, which can adversely affect multiple tissues, such as liver and adipose. Decreased hepatic steatosis in HFD-fed obese mice following antibiotic treatment was correlated with lower ileal and serum ceramides24. The decreased steatosis that was observed with antibiotics was reversed by the injection of C16:0 ceramide. The ileum and serum ceramide levels were also lower in mice that were administered the intestinal FXR antagonist Gly-MCA, and this was also reversed by the injection of C16:0 ceramide82. Treating mice with the potent FXR agonist GW4064 induced RNAs for a number of genes involved in ceramide metabolism in the ileum. Notably, the genes that increase ceramide production in the ileum, including sphingomyelin phosphodiesterase 3 (Smpd3) and serine palmitoyltransferase long chain base subunit 2 (Sptlc2), have upstream FXR binding sites (http://bit.ly/1W9cvJf) and were identified as direct FXR target genes in reporter gene assays82 In the mouse ileum, due to the presence of an endogenous FXR agonist that is produced in the liver, these genes are activated; when Gly-MCA is administered, they are repressed, which likely accounts for the decrease in serum ceramide levels82 (Figure 4).
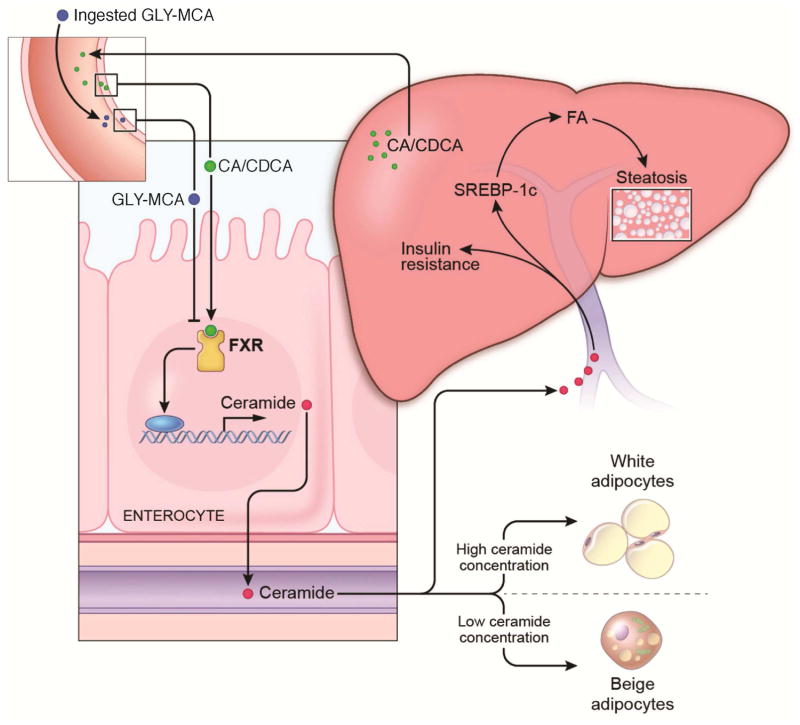
Figure 4.
The role FXR signaling and ceramides in the modulation of metabolic disease. In obese mice, a bile acid agonist produced in the liver constitutively stimulates FXR in the ileum resulting in increased production of ceramides. Ceramides then cause lipid toxicity in the liver resulting in increased ER stress and elevated fatty acid synthesis due to increased SREBP-1 signaling. Ceramides also impair adipose function through increase ER stress, decreasing the ration of beige to white adipocytes. In the presence of the FXR antagonist Gly-MCA, ceramide levels are lowered resulting in decreased hepatic lipid synthesis and steatosis and increased adipose beiging.
The mechanism underlying the adverse impact of ceramides on cells has received some attention. High concentrations of ceramides cause mitochondrial damage98, through producing mitochondrial permeability transition pores that are involved in the induction of apoptosis99, 100. However, it is unclear whether ceramides can enter cells and be incorporated into the mitochondrial membrane or whether de novo ceramide synthesis is required for channel production. Elevated levels of ceramides in pancreatic β-cells cause increased mitochondrial permeability leading to apoptosis and lower insulin production by the pancreas101, 102. Ceramides can also stimulate ER stress that, in turn, can increase apoptosis103, 104. High serum and tissue ceramides also adversely affect the liver, which can lead to NAFLD105. In human NAFLD livers, the expression of genes involved in ceramide metabolism and signaling is increased and the resultant elevated ceramides have been measured in serum from those with NAFLD106; however, it is not clear whether NAFLD is the cause of or the result of increased cellular ceramides107. Because hepatic lipid precursors are elevated in NAFLD, it follows that hepatic ceramide synthesis could increase, which can then cause cell damage. Therefore, this raises the possibility that ceramides produced in extrahepatic tissues can influence liver pathology. The increase in serum ceramides as a result of synthesis in intestinal epithelial cells, that was found after inhibition of FXR signaling, was also correlated with NAFLD24. Treatment of primary hepatocytes with ceramides at the concentration levels found in the serum of obese mice caused an increase in lipid accumulation, and in SREBP1N mRNA and protein, a transcription factor that controls fatty acid synthesis108, and in its target genes, which suggests that the effects of ceramides are direct24. HFD-fed mice showed an increased expression of fatty acid synthesis gene mRNAs and proteins, but the expression of fatty acid β-oxidation was unchanged in the livers of these mice with and without FXR antagonism and altered ceramide levels24, 79. However, the precise mechanistic link between ceramides and SREBP-1c activation and increased signaling is not currently known.
Mice subjected to intestinal FXR antagonism by Gly-MCA also showed increased white adipose beiging that likely mediated weight loss through increased energy expenditure and heat production as revealed by increased VO2 production after Gly-MCA administration82, 109, 110. The increased beiging is due in part to lower the levels of serum ceramides as induction of beiging in cultured white adipocyte cultures was inhibited by treatment with ceramides as revealed by reduction in the activation of UCP1 and other beige-specific genes82. Increased adipose beiging likely accounts for the increased energy expenditure and weight loss that were observed in the obese mice treated with Gly-MCA. Although it was not determined, the decreased hyperglycemia Gly-MCA-treated mice could also be due to the effects of ceramides which, as noted above, impair β-cell function.
Finally, it cannot be ruled out that there may be other mechanisms in addition to the alteration of ceramides that contribute the the favorable metabolic effects resulting from intestinal FXR inhibition. For example inhibition of FXR signaling in enteroendocrine L cells increases glucagon-like peptide-1 (GLP-1) production111. Since increased GLP-1 secreation positively influences glucose metabolism that can affect insulin resistance, fatty liver and obesity, this might contribute in part to the favorable metabolic phenotyped seen with Gly-MCA.
FXR as a drug target
Mice lacking expression of FXR show increased levels of serum and hepatic bile acids, cholesterol, and triglycerides, and a pro-atherogenic serum lipoprotein profile89. This suggest that activation of FXR could ameliorate these symptoms, notably liver cholestasis, because FXR is responsible for regulating bile acid synthesis and transport49. A highly potent FXR agonist, 6α-ethyl-chenodeoxycholic acid (6-ECDCA, obetacholic acid), a derivative of the FXR agonist CDCA112, was recently approved for the treatment of primary biliary cirrhosis (PBC) and is in late clinical trials for NASH113–115. PBC is currently treated with UDCA116. A phase 3 “FLINT” trial has shown an improved NASH score, although some patients developed pruritus, and there was a trend toward increased plasma LDL cholesterol and decreased HDL-cholesterol; the latter result suggests that further studies are warranted to assess the cardiovascular outcomes in patients under long term treatment with obetacholic acid113, 114. Although these studies have indicated that hepatic FXR is a viable therapeutic target for the development of drugs to treat liver diseases in humans, the potential for intestinal FXR as a drug target has not been explored in humans. However, a recent clinical study suggested that UDCA might have direct or indirect (may compete with endogenous agonist FXR) inhibitory activity toward intestinal FXR117, 118. While UDCA binds to FXR, it shows partial agonist or no FXR agonist activity119, 120. It remains to be determined if UDCA is an FXR antagonist.
The question arises whether intestinal FXR signaling is altered in humans with metabolic disease, thus suggesting that modulation of FXR could be a viable therapeutic strategy in humans. A recent study revealed that levels of serum FGF19 were lower in obese humans compared to lean patients121. Others found that FGF19 levels were inversely correlated with NAFLD122. These studies suggest that FXR signaling was suppressed under conditions of obesity and NAFLD which are not in agreement with studies in mice showing that reduced FXR signaling by tempol, antibiotics and Gly-MCA decreased obesity and NAFLD24, 79, 82. The reason for these discrepancies in FGF19 expression in human metabolic disease is not yet known. However, the pattern of expression of FGF19 is different between humans and mice. Notably, in humans FGF19 is expressed in the gallbladder and common bile duct123, 124. Other correlative evidence of altered intestinal FXR signaling was obtained with in ileum biopsies in a population of Chinese patients undergoing colonoscopies showing that the expression of FXR mRNA, and mRNAs from FXR target genes SHP and FGF19 in ileum biopsies were correlated with body mass index82. This study also revealed that obese mice had increased Fxr, Shp and Fgf15 mRNA expression as compared to lean mice. However, the mechanism of improvement in metabolic endpoints in the mouse studies were likely not due to lower FGF15 levels and modestly increased CYP7A1 expression, but were related to decreased levels of serum ceramides as discussed above. A number of studies revealed that ceramides levels are correlated with insulin resistance in humans that were unrelated to obesity92. Others found increased levels of ceramides in muscle tissue of obese125 and diabetic126 patients. More studies are needed to establish whether ceramide levels are associated with obesity and NAFLD in humans.
Activation of intestinal FXR improves metabolic disorders in HFD-fed mice
Contrary to the results of the inhibition of ileal FXR through bacterial-associated T-β-MCA and exogenous Gly-MCA, others reported that activation of intestinal FXR improves metabolic disorders in mice127. Oral gavage of the intestine-restricted compound fexeramine, an FXR agonist128, resulted in activation of FXR in the ileum, as revealed by induction of the target genes Shp and Fgf15. This was accompanied by less weight gain, lower insulin resistance, and decreased hepatic steatosis after feeding mice a HFD. Similar to the results obtained with Gly-MCA82, fexeramine-treated mice showed increased beiging of adipose tissue, as noted by histology and the elevated expression of the brown adipose marker UCP1. However, data were presented demonstrating that the metabolic effects of fexeramine were mediated in part by the TGR5. Notably, when fed a HFD, mice lacking Tgr5 gained as much weight as their wild-type counterparts and did not show improved insulin sensitivity127. Unlike fexaramine, Gly-MCA had no effects on intestinal TGR5-cAMP-GLP1 signaling in vivo, and the brown and beige fat TGR5-cAMP-DIO2 pathway. Luciferase reporter gene assays further demonstrated that Gly-MCA does not activate TGR5 signaling. Thus, the metabolic improvements after Gly-MCA administration were largely due to inhibition of the intestinal FXR-ceramide axis. This study also found, that among the bile acids produced in these mice, lithocholic acid was elevated and it is important to note that at high concentrations, LCA can cause hepatic toxicity and cancer129, 130. It is also noteworthy that doses of 100 mg kg−1 were required for the positive metabolic effects of fexeramine, a dose that is ten times higher than Gly-MCA24. Thus, development of an intestine-restricted FXR agonist with a higher affinity for FXR is warranted.
Predicted safety of modulating FXR
Chronic activation of FXR in transgenic constitutively-activated FXR-expressing mice triggered spontaneous liver toxicity, growth delay, and even partial neonatal death, which suggested that chronic FXR-activating agents treatment may lead to unwanted side effects131. In contrast, mice lacking expression of FXR have increased spontaneous liver cancer. Both male and female Fxr-null mice had a high incidence of liver tumors including hepatocellular adenoma, carcinoma and hepatocholangiocellular carcinoma at 12-months of age132, 133. These findings suggest that the global inhibition of FXR signaling could result in liver cancer. While the mechanism of carcinogenesis in FXR-deficient mice is not known, it could be the result of increased inflammation and cell proliferation in the toxic environment of the cholestatic liver. In agreement with these mouse studies, the expression of FXR in human hepatocellular carcinomas was markedly reduced compared to tumor-adjacent normal liver tissue, and the extent of expression was inversely related with large tumor size, and poor differentiation134. In human colon cancer cells, forced expression of FXR reduced cell proliferation in vitro and in vivo in xenotransplantation studies. Another study found that genetically (Apcmin mice)- and chemically-induced colon cancers were observed in Fxr-null mice and suggested that lack of FXR also promotes cell proliferation, inflammation, and tumorigenesis in the intestine, further suggesting that a functioning FXR may protect against intestinal carcinogenesis135. However, it is not clear from this study whether the intestinal cancer is due to FXR deficiency in liver or intestinal tissues. Others found that the re-expression of FXR in the intestine of Fxr-null mice inhibited spontaneous liver cancer and the authors propose that this was due to increased FGF15 production and the lowering of hepatic bile acids through suppression of CYP7A1136. This study suggests that inhibition of intestinal FXR could increase colon cancer. However, it is not clear what the physiological or pharmaceutical significance of this would be because the model that was used also lacked hepatic FXR and the intestinal FXR was likely highly expressed throughout the intestine, because the villin promoter was controlling the FXR transgene. FXR expression is highest in the lower ileum. In the case of inhibition of intestinal FXR by altering the gut microbiota, or by treatment with Gly-MCA, no significant changes were found in the bile acid pool and composition in the liver or intestine, other than altering the levels of conjugated bile acid metabolites as a result of BSH inhibition24, 79, 82. Furthermore, Gly-MCA does not result in complete repression of FXR signaling. To determine the potential for increased liver and colon cancer in mice lacking intestinal FXR expression, long-term studies in intestine-specific Fxr-null mice and mice that are chronically treated with an intestine FXR antagonist are needed.
There also should be some concern about the long term effects of inhibiting ileal FXR on bile acid homeostasis. Does inhibition of intestinal FXR block bile acid enterohepatic circulation? The intestinal FXR knockout mice have no adverse phenotypes and do not have a marked alteration in the quantity and distribution of bile acids with no increase in toxic bile acids such as LCA24, 79, 82. This is like due to the fact that these mice have constitutive expression of the ileal epithelium bile acid transporters ASBT and OSTα/β, and the I-BABP; inhibition FXR does not significantly reduce the expression of these transporters so that bile acid flow from the intestine to the portal blood remains intact.
Conclusions, caviats and future prospects
Based on studies in mice where the gut microbiota can be manipulated leading to altered intestinal bile acids and FXR signaling, it may not be possible to stably alter human the human gut flora population by a probiotic approach. The used of diet to manipulate the gut microbiota populations would also be difficult due to compliance issues. Manipulating the gut microbiota population by chronic treatment of humans with antibiotics could also be unsafe, possibly resulting in drug-resistance and the proliferation of pathogens. A recent clinical trial using vacomycin treatment altered the gut microbiota, short chain fatty acid levels and bile acid concentrations, but did not reveal any improvement in metabolism that would be expected to lead to less metabolic disease, but the study was for only seven days137. More importantly, human and mouse bile acids markedly differ thus bringing into question the ability to extrapolate results in mice to humans. Finally, it should also be noted that mice make MCA and taurine conjugates, while humans produce CA and glycine conjugates. A more direct inhibition of FXR by use of stable synthetic derivatives such as Gly-MCA and it analogs would be preferable.
Targeting a receptor in the intestine for the treatment of metabolic disease avoids systemic exposure to the resultant compound thus making it a promising strategy. However, it remains to be determined whether activation127 or inhibition82 of FXR will be viable approaches for therapy. It also questionable as to whether the effects of altering FXR signaling on beige adipose as found in the mouse models will translate to humans as there is no evidence that beiging can be altered by drugs, although there is some evidence for cold induced increased brown adipose in humans110. However, the influence of FXR in the ileum on NAFLD and type 2 diabetes may more likely be viable disease targets in humans.
Figure 5.
Potential mechanism by which ceramides increase NAFLD. Ceramides enter the cell and incorporate into the ER and mitochondrial membranes causing increased ER stress and mitochondrial permeability, respectively. Mitochondrial damage can give rise to increased reactive oxygen species and cause apoptosis. Through a mechanism that is still unclear, ceramides can activate SREBP-1c leading to increased fatty acid synthesis and NAFLD.
Acknowledgments
Grant support: This work was supported by the Intramural Research Program, Center for Cancer Research, National Cancer Institute, National Institutes of Health to FJG, the National Institutes of Environmental Health Sciences (ES022186) to ADP, and by the National Natural Science Foundation of China [81403007 and 81470554] to CJ.
Abbreviations
- BSH
bile salt hydrolase
- ER
endoplasmic reticulum
- CDCA
chenodeoxycholic acid
- CA
cholic acid
- FXR
farnesoid X receptor
- FGF
fibroblast growth factor
- Gly-MCA
glycine-β-muricholic acid
- GCDCA
glycochenodeoxycholic acid
- HFD
high-fat diet
- NAFLD
non-alcoholic fatty liver disease
- T-β-MCA
tauro-β-muricholic acid
- TCDCA
taurochenodeoxycholic acid
Footnotes
Disclosures: The authors declare no competing financial interest or conflict of interest.
Writing Assistance: N/A
Author contributions: F.J.G., C.J., and A.D.P. wrote the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55:2005–23. doi: 10.1002/hep.25762. [DOI] [PubMed] [Google Scholar]
- 2.Sattar N, Forrest E, Preiss D. Non-alcoholic fatty liver disease. BMJ. 2014;349:g4596. doi: 10.1136/bmj.g4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Younossi ZM, Stepanova M, Negro F, et al. Nonalcoholic fatty liver disease in lean individuals in the United States. Medicine (Baltimore) 2012;91:319–27. doi: 10.1097/MD.0b013e3182779d49. [DOI] [PubMed] [Google Scholar]
- 4.Grundy SM. Metabolic syndrome update. Trends Cardiovasc Med. 2015 doi: 10.1016/j.tcm.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 5.Michail S, Lin M, Frey MR, et al. Altered gut microbial energy and metabolism in children with non-alcoholic fatty liver disease. FEMS Microbiol Ecol. 2015;91:1–9. doi: 10.1093/femsec/fiu002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baffy G, Brunt EM, Caldwell SH. Hepatocellular carcinoma in non-alcoholic fatty liver disease: an emerging menace. J Hepatol. 2012;56:1384–91. doi: 10.1016/j.jhep.2011.10.027. [DOI] [PubMed] [Google Scholar]
- 7.Dyson J, Day C. Treatment of non-alcoholic fatty liver disease. Dig Dis. 2014;32:597–604. doi: 10.1159/000360511. [DOI] [PubMed] [Google Scholar]
- 8.Loomba R, Sanyal AJ. The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol. 2013;10:686–90. doi: 10.1038/nrgastro.2013.171. [DOI] [PubMed] [Google Scholar]
- 9.Bhatia LS, Curzen NP, Calder PC, et al. Non-alcoholic fatty liver disease: a new and important cardiovascular risk factor? Eur Heart J. 2012;33:1190–200. doi: 10.1093/eurheartj/ehr453. [DOI] [PubMed] [Google Scholar]
- 10.Aqel B, DiBaise JK. Role of the gut microbiome in nonalcoholic fatty liver disease. Nutr Clin Pract. 2015;30:780–6. doi: 10.1177/0884533615605811. [DOI] [PubMed] [Google Scholar]
- 11.Le Roy T, Llopis M, Lepage P, et al. Intestinal microbiota determines development of non-alcoholic fatty liver disease in mice. Gut. 2013;62:1787–94. doi: 10.1136/gutjnl-2012-303816. [DOI] [PubMed] [Google Scholar]
- 12.Del Chierico F, Nobili V, Vernocchi P, et al. Gut microbiota profiling of pediatric NAFLD and obese patients unveiled by an integrated meta-omics based approach. Hepatology. 2016 doi: 10.1002/hep.28572. [DOI] [PubMed] [Google Scholar]
- 13.Boursier J, Mueller O, Barret M, et al. The severity of nonalcoholic fatty liver disease is associated with gut dysbiosis and shift in the metabolic function of the gut microbiota. Hepatology. 2016;63:764–75. doi: 10.1002/hep.28356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Turnbaugh PJ, Ley RE, Mahowald MA, et al. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–31. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 15.Arora T, Backhed F. The gut microbiota and metabolic disease: current understanding and future perspectives. J Intern Med. 2016 doi: 10.1111/joim.12508. [DOI] [PubMed] [Google Scholar]
- 16.Ng M, Fleming T, Robinson M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:766–81. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Minicis S, Rychlicki C, Agostinelli L, et al. Dysbiosis contributes to fibrogenesis in the course of chronic liver injury in mice. Hepatology. 2014;59:1738–49. doi: 10.1002/hep.26695. [DOI] [PubMed] [Google Scholar]
- 18.Elshaghabee FM, Bockelmann W, Meske D, et al. Ethanol Production by Selected Intestinal Microorganisms and Lactic Acid Bacteria Growing under Different Nutritional Conditions. Front Microbiol. 2016;7:47. doi: 10.3389/fmicb.2016.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yatsunenko T, Rey FE, Manary MJ, et al. Human gut microbiome viewed across age and geography. Nature. 2012;486:222–7. doi: 10.1038/nature11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mahana D, Trent CM, Kurtz ZD, et al. Antibiotic perturbation of the murine gut microbiome enhances the adiposity, insulin resistance, and liver disease associated with high-fat diet. Genome Med. 2016;8:48. doi: 10.1186/s13073-016-0297-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nobel YR, Cox LM, Kirigin FF, et al. Metabolic and metagenomic outcomes from early-life pulsed antibiotic treatment. Nat Commun. 2015;6:7486. doi: 10.1038/ncomms8486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cox LM, Yamanishi S, Sohn J, et al. Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell. 2014;158:705–21. doi: 10.1016/j.cell.2014.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suarez-Zamorano N, Fabbiano S, Chevalier C, et al. Microbiota depletion promotes browning of white adipose tissue and reduces obesity. Nat Med. 2015;21:1497–501. doi: 10.1038/nm.3994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang C, Xie C, Li F, et al. Intestinal farnesoid X receptor signaling promotes nonalcoholic fatty liver disease. J Clin Invest. 2015;125:386–402. doi: 10.1172/JCI76738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gangarapu V, Ince AT, Baysal B, et al. Efficacy of rifaximin on circulating endotoxins and cytokines in patients with nonalcoholic fatty liver disease. Eur J Gastroenterol Hepatol. 2015;27:840–5. doi: 10.1097/MEG.0000000000000348. [DOI] [PubMed] [Google Scholar]
- 26.Yoshimoto S, Loo TM, Atarashi K, et al. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature. 2013;499:97–101. doi: 10.1038/nature12347. [DOI] [PubMed] [Google Scholar]
- 27.Park JS, Seo JH, Youn HS. Gut microbiota and clinical disease: obesity and nonalcoholic Fatty liver disease. Pediatr Gastroenterol Hepatol Nutr. 2013;16:22–7. doi: 10.5223/pghn.2013.16.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nguyen TL, Vieira-Silva S, Liston A, et al. How informative is the mouse for human gut microbiota research? Dis Model Mech. 2015;8:1–16. doi: 10.1242/dmm.017400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quigley EM, Monsour HP. The gut microbiota and nonalcoholic fatty liver disease. Semin Liver Dis. 2015;35:262–9. doi: 10.1055/s-0035-1562946. [DOI] [PubMed] [Google Scholar]
- 30.Bäckhed F, Ding H, Wang T, et al. The gut microbiota as an environmental factor that regulates fat storage. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:15718–15723. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ridaura VK, Faith JJ, Rey FE, et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science. 2013;341:1241214. doi: 10.1126/science.1241214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raman M, Ahmed I, Gillevet PM, et al. Fecal microbiome and volatile organic compound metabolome in obese humans with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2013;11:868–75. e1–3. doi: 10.1016/j.cgh.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 33.Dumas ME, Barton RH, Toye A, et al. Metabolic profiling reveals a contribution of gut microbiota to fatty liver phenotype in insulin-resistant mice. Proc Natl Acad Sci U S A. 2006;103:12511–6. doi: 10.1073/pnas.0601056103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spencer MD, Hamp TJ, Reid RW, et al. Association between composition of the human gastrointestinal microbiome and development of fatty liver with choline deficiency. Gastroenterology. 2011;140:976–986. doi: 10.1053/j.gastro.2010.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barlow GM, Yu A, Mathur R. Role of the gut microbiome in obesity and dabetes mellitus. Nutr Clin Pract. 2015;30:787–97. doi: 10.1177/0884533615609896. [DOI] [PubMed] [Google Scholar]
- 36.Sonnenburg JL, Backhed F. Diet-microbiota interactions as moderators of human metabolism. Nature. 2016;535:56–64. doi: 10.1038/nature18846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Claus SP, Tsang TM, Wang Y, et al. Systemic multicompartmental effects of the gut microbiome on mouse metabolic phenotypes. Mol Syst Biol. 2008;4:219. doi: 10.1038/msb.2008.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Waldram A, Holmes E, Wang Y, et al. Top-down systems biology modeling of host metabotype-microbiome associations in obese rodents. J Proteome Res. 2009;8:2361–75. doi: 10.1021/pr8009885. [DOI] [PubMed] [Google Scholar]
- 39.Lozupone CA, Stombaugh JI, Gordon JI, et al. Diversity, stability and resilience of the human gut microbiota. Nature. 2012;489:220–30. doi: 10.1038/nature11550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Patterson AD, Turnbaugh PJ. Microbial determinants of biochemical individuality and their impact on toxicology and pharmacology. Cell Metab. 2014;20:761–8. doi: 10.1016/j.cmet.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fang ZZ, Zhang D, Cao YF, et al. Irinotecan (CPT-11)-induced elevation of bile acids potentiates suppression of IL-10 expression. Toxicol Appl Pharmacol. 2015;291:21–27. doi: 10.1016/j.taap.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wahlstrom A, Sayin SI, Marschall HU, et al. Intestinal Crosstalk between Bile Acids and Microbiota and Its Impact on Host Metabolism. Cell Metab. 2016;24:41–50. doi: 10.1016/j.cmet.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 43.Parseus A, Sommer N, Sommer F, et al. Microbiota-induced obesity requires farnesoid X receptor. Gut. 2016 doi: 10.1136/gutjnl-2015-310283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Russell DW. The enzymes, regulation, and genetics of bile acid synthesis. Annu Rev Biochem. 2003;72:137–74. doi: 10.1146/annurev.biochem.72.121801.161712. [DOI] [PubMed] [Google Scholar]
- 45.Chiang JY. Bile acids: regulation of synthesis. J Lipid Res. 2009;50:1955–66. doi: 10.1194/jlr.R900010-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hofmann AF. The enterohepatic circulation of bile acids in mammals: form and functions. Front Biosci (Landmark Ed) 2009;14:2584–98. doi: 10.2741/3399. [DOI] [PubMed] [Google Scholar]
- 47.Sakakura H, Kimura N, Takeda H, et al. Simultaneous determination of bile acids in rat liver tissue by high-performance liquid chromatography. J Chromatogr B Biomed Sci Appl. 1998;718:33–40. doi: 10.1016/s0378-4347(98)00342-9. [DOI] [PubMed] [Google Scholar]
- 48.Gonzalez FJ. Nuclear receptor control of enterohepatic circulation. Compr Physiol. 2012;2:2811–28. doi: 10.1002/cphy.c120007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Matsubara T, Li F, Gonzalez FJ. FXR signaling in the enterohepatic system. Mol Cell Endocrinol. 2013;368:17–29. doi: 10.1016/j.mce.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stanimirov B, Stankov K, Mikov M. Bile acid signaling through farnesoid X and TGR5 receptors in hepatobiliary and intestinal diseases. Hepatobiliary Pancreat Dis Int. 2015;14:18–33. doi: 10.1016/s1499-3872(14)60307-6. [DOI] [PubMed] [Google Scholar]
- 51.Forman BM, Goode E, Chen J, et al. Identification of a nuclear receptor that is activated by farnesol metabolites. Cell. 1995;81:687–93. doi: 10.1016/0092-8674(95)90530-8. [DOI] [PubMed] [Google Scholar]
- 52.Seol W, Choi HS, Moore DD. Isolation of proteins that interact specifically with the retinoid X receptor: two novel orphan receptors. Mol Endocrinol. 1995;9:72–85. doi: 10.1210/mend.9.1.7760852. [DOI] [PubMed] [Google Scholar]
- 53.Makishima M, Okamoto AY, Repa JJ, et al. Identification of a nuclear receptor for bile acids. Science. 1999;284:1362–5. doi: 10.1126/science.284.5418.1362. [DOI] [PubMed] [Google Scholar]
- 54.Parks DJ, Blanchard SG, Bledsoe RK, et al. Bile acids: natural ligands for an orphan nuclear receptor. Science. 1999;284:1365–8. doi: 10.1126/science.284.5418.1365. [DOI] [PubMed] [Google Scholar]
- 55.Vaquero J, Monte MJ, Dominguez M, et al. Differential activation of the human farnesoid X receptor depends on the pattern of expressed isoforms and the bile acid pool composition. Biochem Pharmacol. 2013;86:926–39. doi: 10.1016/j.bcp.2013.07.022. [DOI] [PubMed] [Google Scholar]
- 56.Zhang Y, Hagedorn CH, Wang L. Role of nuclear receptor SHP in metabolism and cancer. Biochim Biophys Acta. 2011;1812:893–908. doi: 10.1016/j.bbadis.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen JY, Levy-Wilson B, Goodart S, et al. Mice expressing the human CYP7A1 gene in the mouse CYP7A1 knock-out background lack induction of CYP7A1 expression by cholesterol feeding and have increased hypercholesterolemia when fed a high fat diet. J Biol Chem. 2002;277:42588–95. doi: 10.1074/jbc.M205117200. [DOI] [PubMed] [Google Scholar]
- 58.Chiang JY. Hepatocyte nuclear factor 4alpha regulation of bile acid and drug metabolism. Expert Opin Drug Metab Toxicol. 2009;5:137–47. doi: 10.1517/17425250802707342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kliewer SA, Mangelsdorf DJ. Bile acids as hormones: The FXR-FGF15/19 pathway. Dig Dis. 2015;33:327–31. doi: 10.1159/000371670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kullak-Ublick GA, Stieger B, Meier PJ. Enterohepatic bile salt transporters in normal physiology and liver disease. Gastroenterology. 2004;126:322–42. doi: 10.1053/j.gastro.2003.06.005. [DOI] [PubMed] [Google Scholar]
- 61.Dawson PA. Role of the intestinal bile acid transporters in bile acid and drug disposition. Handb Exp Pharmacol. 2011:169–203. doi: 10.1007/978-3-642-14541-4_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cipriani S, Mencarelli A, Palladino G, et al. FXR activation reverses insulin resistance and lipid abnormalities and protects against liver steatosis in Zucker (fa/fa) obese rats. J Lipid Res. 2010;51:771–84. doi: 10.1194/jlr.M001602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Porez G, Prawitt J, Gross B, et al. Bile acid receptors as targets for the treatment of dyslipidemia and cardiovascular disease. J Lipid Res. 2012;53:1723–37. doi: 10.1194/jlr.R024794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mencarelli A, Fiorucci S. FXR an emerging therapeutic target for the treatment of atherosclerosis. J Cell Mol Med. 2010;14:79–92. doi: 10.1111/j.1582-4934.2009.00997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stepanov V, Stankov K, Mikov M. The bile acid membrane receptor TGR5: a novel pharmacological target in metabolic, inflammatory and neoplastic disorders. J Recept Signal Transduct Res. 2013;33:213–23. doi: 10.3109/10799893.2013.802805. [DOI] [PubMed] [Google Scholar]
- 66.Duan H, Ning M, Zou Q, et al. Discovery of Intestinal Targeted TGR5 Agonists for the Treatment of Type 2 Diabetes. J Med Chem. 2015;58:3315–28. doi: 10.1021/jm500829b. [DOI] [PubMed] [Google Scholar]
- 67.Donepudi AC, Boehme S, Li F, et al. G-protein-coupled bile acid receptor plays a key role in bile acid metabolism and fasting-induced hepatic steatosis in mice. Hepatology. 2016 doi: 10.1002/hep.28707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Henao-Mejia J, Elinav E, Jin C, et al. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature. 2012;482:179–85. doi: 10.1038/nature10809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hansen JJ. Immune responses to intestinal microbes in inflammatory bowel diseases. Curr Allergy Asthma Rep. 2015;15:61. doi: 10.1007/s11882-015-0562-9. [DOI] [PubMed] [Google Scholar]
- 70.Mitchell JB, Xavier S, DeLuca AM, et al. A low molecular weight antioxidant decreases weight and lowers tumor incidence. Free Radic Biol Med. 2003;34:93–102. doi: 10.1016/s0891-5849(02)01193-0. [DOI] [PubMed] [Google Scholar]
- 71.Erker L, Schubert R, Yakushiji H, et al. Cancer chemoprevention by the antioxidant tempol acts partially via the p53 tumor suppressor. Hum Mol Genet. 2005;14:1699–708. doi: 10.1093/hmg/ddi181. [DOI] [PubMed] [Google Scholar]
- 72.Wikoff WR, Anfora AT, Liu J, et al. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc Natl Acad Sci U S A. 2009;106:3698–703. doi: 10.1073/pnas.0812874106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Patterson AD, Slanar O, Krausz KW, et al. Human urinary metabolomic profile of PPARalpha induced fatty acid beta-oxidation. J Proteome Res. 2009;8:4293–300. doi: 10.1021/pr9004103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li F, Pang X, Krausz KW, et al. Stable isotope- and mass spectrometry-based metabolomics as tools in drug metabolism: A study expanding tempol pharmacology. J Proteome Res. 2013 doi: 10.1021/pr301023x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhen Y, Krausz KW, Chen C, et al. Metabolomic and genetic analysis of biomarkers for peroxisome proliferator-activated receptor alpha expression and activation. Mol Endocrinol. 2007;21:2136–51. doi: 10.1210/me.2007-0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kersten S. Integrated physiology and systems biology of PPARalpha. Mol Metab. 2014;3:354–71. doi: 10.1016/j.molmet.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shukla OP. Microbial transformation of quinoline by a Pseudomonas sp. Appl Environ Microbiol. 1986;51:1332–42. doi: 10.1128/aem.51.6.1332-1342.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shukla OP. Microbiological degradation of quinoline by Pseudomonas stutzeri: the coumarin pathway of quinoline catabolism. Microbios. 1989;59:47–63. [PubMed] [Google Scholar]
- 79.Li F, Jiang C, Krausz KW, et al. Microbiome remodelling leads to inhibition of intestinal farnesoid X receptor signalling and decreased obesity. Nat Commun. 2013;4:2384. doi: 10.1038/ncomms3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sayin SI, Wahlstrom A, Felin J, et al. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab. 2013;17:225–235. doi: 10.1016/j.cmet.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 81.Tanaka H, Doesburg K, Iwasaki T, et al. Screening of lactic acid bacteria for bile salt hydrolase activity. J Dairy Sci. 1999;82:2530–5. doi: 10.3168/jds.S0022-0302(99)75506-2. [DOI] [PubMed] [Google Scholar]
- 82.Jiang C, Xie C, Lv Y, et al. Intestine-selective farnesoid X receptor inhibition improves obesity-related metabolic dysfunction. Nat Commun. 2015;6:10166. doi: 10.1038/ncomms10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Prawitt J, Abdelkarim M, Stroeve JH, et al. Farnesoid X receptor deficiency improves glucose homeostasis in mouse models of obesity. Diabetes. 2011;60:1861–71. doi: 10.2337/db11-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Park MY, Kim SJ, Ko EK, et al. Gut microbiota-associated bile acid deconjugation accelerates hepatic steatosis in ob/ob mice. J Appl Microbiol. 2016 doi: 10.1111/jam.13158. [DOI] [PubMed] [Google Scholar]
- 85.Miyata M, Takamatsu Y, Kuribayashi H, et al. Administration of ampicillin elevates hepatic primary bile acid synthesis through suppression of ileal fibroblast growth factor 15 expression. J Pharmacol Exp Ther. 2009;331:1079–85. doi: 10.1124/jpet.109.160093. [DOI] [PubMed] [Google Scholar]
- 86.Kuribayashi H, Miyata M, Yamakawa H, et al. Enterobacteria-mediated deconjugation of taurocholic acid enhances ileal farnesoid X receptor signaling. Eur J Pharmacol. 2012;697:132–8. doi: 10.1016/j.ejphar.2012.09.048. [DOI] [PubMed] [Google Scholar]
- 87.Zhang X, Osaka T, Tsuneda S. Bacterial metabolites directly modulate farnesoid X receptor activity. Nutr Metab (Lond) 2015;12:48. doi: 10.1186/s12986-015-0045-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ridlon JM, Kang DJ, Hylemon PB. Bile salt biotransformations by human intestinal bacteria. J Lipid Res. 2006;47:241–59. doi: 10.1194/jlr.R500013-JLR200. [DOI] [PubMed] [Google Scholar]
- 89.Sinal CJ, Tohkin M, Miyata M, et al. Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell. 2000;102:731–744. doi: 10.1016/s0092-8674(00)00062-3. [DOI] [PubMed] [Google Scholar]
- 90.Kremer GJ, Atzpodien W, Schnellbacher E. Plasma glycosphingolipids in diabetics and normals. Klin Wochenschr. 1975;53:637–8. doi: 10.1007/BF01469685. [DOI] [PubMed] [Google Scholar]
- 91.Promrat K, Longato L, Wands JR, et al. Weight loss amelioration of non-alcoholic steatohepatitis linked to shifts in hepatic ceramide expression and serum ceramide levels. Hepatol Res. 2011;41:754–62. doi: 10.1111/j.1872-034X.2011.00815.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chaurasia B, Summers SA. Ceramides - lipotoxic inducers of metabolic disorders. Trends Endocrinol Metab. 2015;26:538–50. doi: 10.1016/j.tem.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 93.Unger RH. Lipotoxic diseases. Annu Rev Med. 2002;53:319–36. doi: 10.1146/annurev.med.53.082901.104057. [DOI] [PubMed] [Google Scholar]
- 94.Ahammed SK, Chowdhury A. Insulin resistance and “lipotoxic liver diseases”. Trop Gastroenterol. 2013;34:1–4. doi: 10.7869/tg.2012.82. [DOI] [PubMed] [Google Scholar]
- 95.Holland WL, Brozinick JT, Wang LP, et al. Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metab. 2007;5:167–79. doi: 10.1016/j.cmet.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 96.Turpin SM, Nicholls HT, Willmes DM, et al. Obesity-induced CerS6-dependent C16:0 ceramide production promotes weight gain and glucose intolerance. Cell Metab. 2014;20:678–86. doi: 10.1016/j.cmet.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 97.Xia JY, Holland WL, Kusminski CM, et al. Targeted induction of ceramide degradation leads to improved systemic metabolism and reduced hepatic steatosis. Cell Metab. 2015;22:266–78. doi: 10.1016/j.cmet.2015.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kolesnick RN, Kronke M. Regulation of ceramide production and apoptosis. Annu Rev Physiol. 1998;60:643–65. doi: 10.1146/annurev.physiol.60.1.643. [DOI] [PubMed] [Google Scholar]
- 99.Colombini M. Ceramide channels and mitochondrial outer membrane permeability. J Bioenerg Biomembr. 2016 doi: 10.1007/s10863-016-9646-z. [DOI] [PubMed] [Google Scholar]
- 100.Siskind LJ, Kolesnick RN, Colombini M. Ceramide channels increase the permeability of the mitochondrial outer membrane to small proteins. J Biol Chem. 2002;277:26796–803. doi: 10.1074/jbc.M200754200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lang F, Ullrich S, Gulbins E. Ceramide formation as a target in beta-cell survival and function. Expert Opin Ther Targets. 2011;15:1061–71. doi: 10.1517/14728222.2011.588209. [DOI] [PubMed] [Google Scholar]
- 102.Boslem E, Meikle PJ, Biden TJ. Roles of ceramide and sphingolipids in pancreatic beta-cell function and dysfunction. Islets. 2012;4:177–87. doi: 10.4161/isl.20102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Summers SA. Ceramides in insulin resistance and lipotoxicity. Prog Lipid Res. 2006;45:42–72. doi: 10.1016/j.plipres.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 104.Galadari S, Rahman A, Pallichankandy S, et al. Role of ceramide in diabetes mellitus: evidence and mechanisms. Lipids Health Dis. 2013;12:98. doi: 10.1186/1476-511X-12-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Alkhouri N, Dixon LJ, Feldstein AE. Lipotoxicity in nonalcoholic fatty liver disease: not all lipids are created equal. Expert Rev Gastroenterol Hepatol. 2009;3:445–51. doi: 10.1586/egh.09.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gorden DL, Myers DS, Ivanova PT, et al. Biomarkers of NAFLD progression: a lipidomics approach to an epidemic. J Lipid Res. 2015;56:722–36. doi: 10.1194/jlr.P056002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Greco D, Kotronen A, Westerbacka J, et al. Gene expression in human NAFLD. Am J Physiol Gastrointest Liver Physiol. 2008;294:G1281–7. doi: 10.1152/ajpgi.00074.2008. [DOI] [PubMed] [Google Scholar]
- 108.Xu X, So JS, Park JG, et al. Transcriptional control of hepatic lipid metabolism by SREBP and ChREBP. Semin Liver Dis. 2013;33:301–11. doi: 10.1055/s-0033-1358523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kajimura S, Spiegelman BM, Seale P. Brown and beige fat: Physiological roles beyond heat generation. Cell Metab. 2015;22:546–59. doi: 10.1016/j.cmet.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kim SH, Plutzky J. Brown Fat and Browning for the Treatment of Obesity and Related Metabolic Disorders. Diabetes Metab J. 2016;40:12–21. doi: 10.4093/dmj.2016.40.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Trabelsi MS, Daoudi M, Prawitt J, et al. Farnesoid X receptor inhibits glucagon-like peptide-1 production by enteroendocrine L cells. Nat Commun. 2015;6:7629. doi: 10.1038/ncomms8629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pellicciari R, Fiorucci S, Camaioni E, et al. 6alpha-ethyl-chenodeoxycholic acid (6-ECDCA), a potent and selective FXR agonist endowed with anticholestatic activity. J Med Chem. 2002;45:3569–72. doi: 10.1021/jm025529g. [DOI] [PubMed] [Google Scholar]
- 113.Sanyal AJ. Use of farnesoid X receptor agonists to treat nonalcoholic fatty liver disease. Dig Dis. 2015;33:426–32. doi: 10.1159/000371698. [DOI] [PubMed] [Google Scholar]
- 114.Neuschwander-Tetri BA, Loomba R, Sanyal AJ, et al. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet. 2015;385:956–65. doi: 10.1016/S0140-6736(14)61933-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Copple BL, Li T. Pharmacology of bile acid receptors: Evolution of bile acids from simple detergents to complex signaling molecules. Pharmacol Res. 2016;104:9–21. doi: 10.1016/j.phrs.2015.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Silveira MG, Lindor KD. Obeticholic acid and budesonide for the treatment of primary biliary cirrhosis. Expert Opin Pharmacother. 2014;15:365–72. doi: 10.1517/14656566.2014.873404. [DOI] [PubMed] [Google Scholar]
- 117.Mueller M, Thorell A, Claudel T, et al. Ursodeoxycholic acid exerts farnesoid X receptor-antagonistic effects on bile acid and lipid metabolism in morbid obesity. J Hepatol. 2015;62:1398–404. doi: 10.1016/j.jhep.2014.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gonzalez FJ, Jiang C, Bisson WH, et al. Inhibition of farnesoid X receptor signaling shows beneficial effects in human obesity. J Hepatol. 2015;62:1234–6. doi: 10.1016/j.jhep.2015.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sharma R, Prichard D, Majer F, et al. Ursodeoxycholic acid amides as novel glucocorticoid receptor modulators. J Med Chem. 2011;54:122–30. doi: 10.1021/jm100860s. [DOI] [PubMed] [Google Scholar]
- 120.Yu DD, Andrali SS, Li H, et al. Novel FXR (farnesoid X receptor) modulators: Potential therapies for cholesterol gallstone disease. Bioorg Med Chem. 2016 doi: 10.1016/j.bmc.2016.06.039. [DOI] [PubMed] [Google Scholar]
- 121.Gallego-Escuredo JM, Gomez-Ambrosi J, Catalan V, et al. Opposite alterations in FGF21 and FGF19 levels and disturbed expression of the receptor machinery for endocrine FGFs in obese patients. Int J Obes (Lond) 2015;39:121–9. doi: 10.1038/ijo.2014.76. [DOI] [PubMed] [Google Scholar]
- 122.Wojcik M, Janus D, Dolezal-Oltarzewska K, et al. A decrease in fasting FGF19 levels is associated with the development of non-alcoholic fatty liver disease in obese adolescents. J Pediatr Endocrinol Metab. 2012;25:1089–93. doi: 10.1515/jpem-2012-0253. [DOI] [PubMed] [Google Scholar]
- 123.Song KH, Li T, Owsley E, et al. Bile acids activate fibroblast growth factor 19 signaling in human hepatocytes to inhibit cholesterol 7alpha-hydroxylase gene expression. Hepatology. 2009;49:297–305. doi: 10.1002/hep.22627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zweers SJ, Booij KA, Komuta M, et al. The human gallbladder secretes fibroblast growth factor 19 into bile: towards defining the role of fibroblast growth factor 19 in the enterobiliary tract. Hepatology. 2012;55:575–83. doi: 10.1002/hep.24702. [DOI] [PubMed] [Google Scholar]
- 125.Adams JM, 2nd, Pratipanawatr T, Berria R, et al. Ceramide content is increased in skeletal muscle from obese insulin-resistant humans. Diabetes. 2004;53:25–31. doi: 10.2337/diabetes.53.1.25. [DOI] [PubMed] [Google Scholar]
- 126.Straczkowski M, Kowalska I, Baranowski M, et al. Increased skeletal muscle ceramide level in men at risk of developing type 2 diabetes. Diabetologia. 2007;50:2366–73. doi: 10.1007/s00125-007-0781-2. [DOI] [PubMed] [Google Scholar]
- 127.Fang S, Suh JM, Reilly SM, et al. Intestinal FXR agonism promotes adipose tissue browning and reduces obesity and insulin resistance. Nat Med. 2015;21:159–65. doi: 10.1038/nm.3760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Downes M, Verdecia MA, Roecker AJ, et al. A chemical, genetic, and structural analysis of the nuclear bile acid receptor FXR. Mol Cell. 2003;11:1079–92. doi: 10.1016/s1097-2765(03)00104-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hofmann AF. Detoxification of lithocholic acid, a toxic bile acid: relevance to drug hepatotoxicity. Drug Metab Rev. 2004;36:703–22. doi: 10.1081/dmr-200033475. [DOI] [PubMed] [Google Scholar]
- 130.Matsubara T, Tanaka N, Patterson AD, et al. Lithocholic acid disrupts phospholipid and sphingolipid homeostasis leading to cholestasis in mice. Hepatology. 2011;53:1282–93. doi: 10.1002/hep.24193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Cheng Q, Inaba Y, Lu P, et al. Chronic activation of FXR in transgenic mice caused perinatal toxicity and sensitized mice to cholesterol toxicity. Mol Endocrinol. 2015;29:571–582. doi: 10.1210/me.2014-1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kim I, Ahn S-H, Inagaki T, et al. Differential regulation of bile acid homeostasis by the farnesoid X receptor in liver and intestine. Journal of lipid research. 2007;48:2664–2672. doi: 10.1194/jlr.M700330-JLR200. [DOI] [PubMed] [Google Scholar]
- 133.Yang F, Huang X, Yi T, et al. Spontaneous development of liver tumors in the absence of the bile acid receptor farnesoid X receptor. Cancer Res. 2007;67:863–7. doi: 10.1158/0008-5472.CAN-06-1078. [DOI] [PubMed] [Google Scholar]
- 134.Su H, Ma C, Liu J, et al. Downregulation of nuclear receptor FXR is associated with multiple malignant clinicopathological characteristics in human hepatocellular carcinoma. Am J Physiol Gastrointest Liver Physiol. 2012;303:G1245–53. doi: 10.1152/ajpgi.00439.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Maran RR, Thomas A, Roth M, et al. Farnesoid X receptor deficiency in mice leads to increased intestinal epithelial cell proliferation and tumor development. J Pharmacol Exp Ther. 2009;328:469–77. doi: 10.1124/jpet.108.145409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Degirolamo C, Modica S, Vacca M, et al. Prevention of spontaneous hepatocarcinogenesis in farnesoid X receptor-null mice by intestinal-specific farnesoid X receptor reactivation. Hepatology. 2015;61:161–70. doi: 10.1002/hep.27274. [DOI] [PubMed] [Google Scholar]
- 137.Reijnders D, Goossens GH, Hermes GD, et al. Effects of Gut Microbiota Manipulation by Antibiotics on Host Metabolism in Obese Humans: A Randomized Double-Blind Placebo-Controlled Trial. Cell Metab. 2016;24:63–74. doi: 10.1016/j.cmet.2016.06.016. [DOI] [PubMed] [Google Scholar]