Abstract
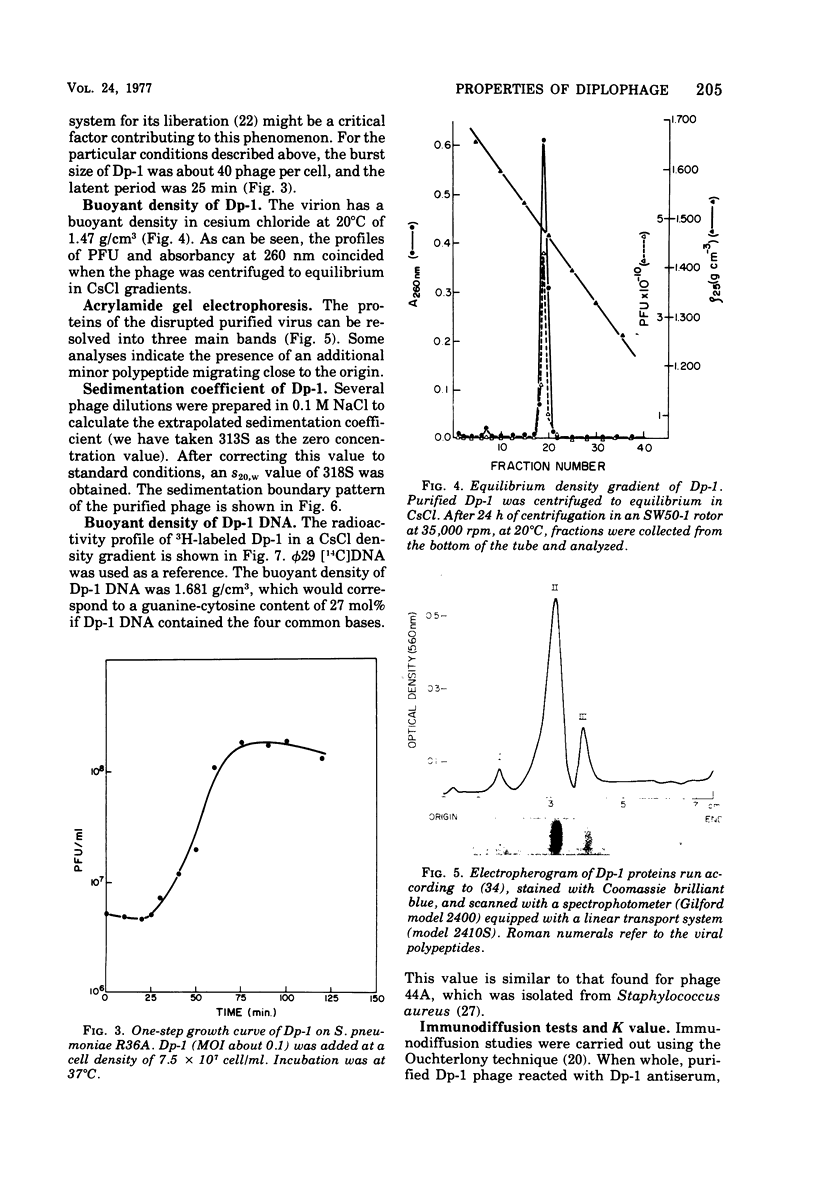
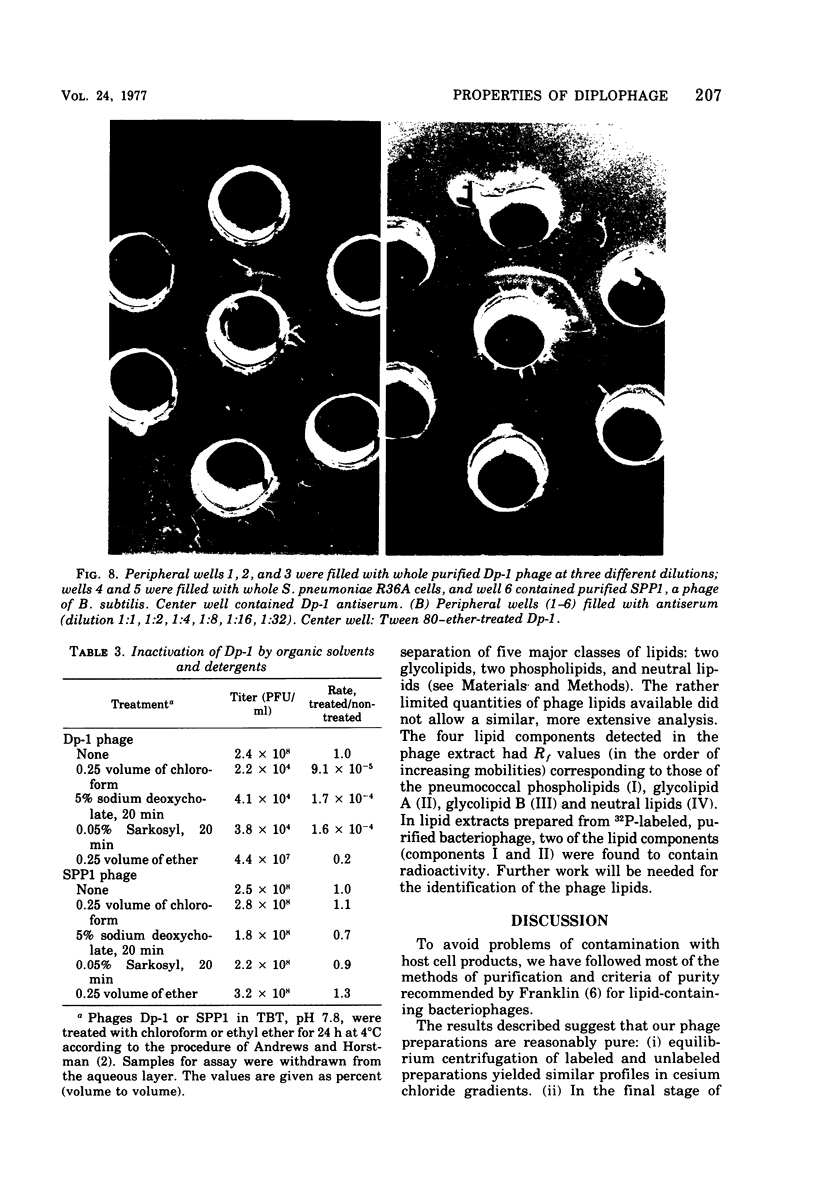
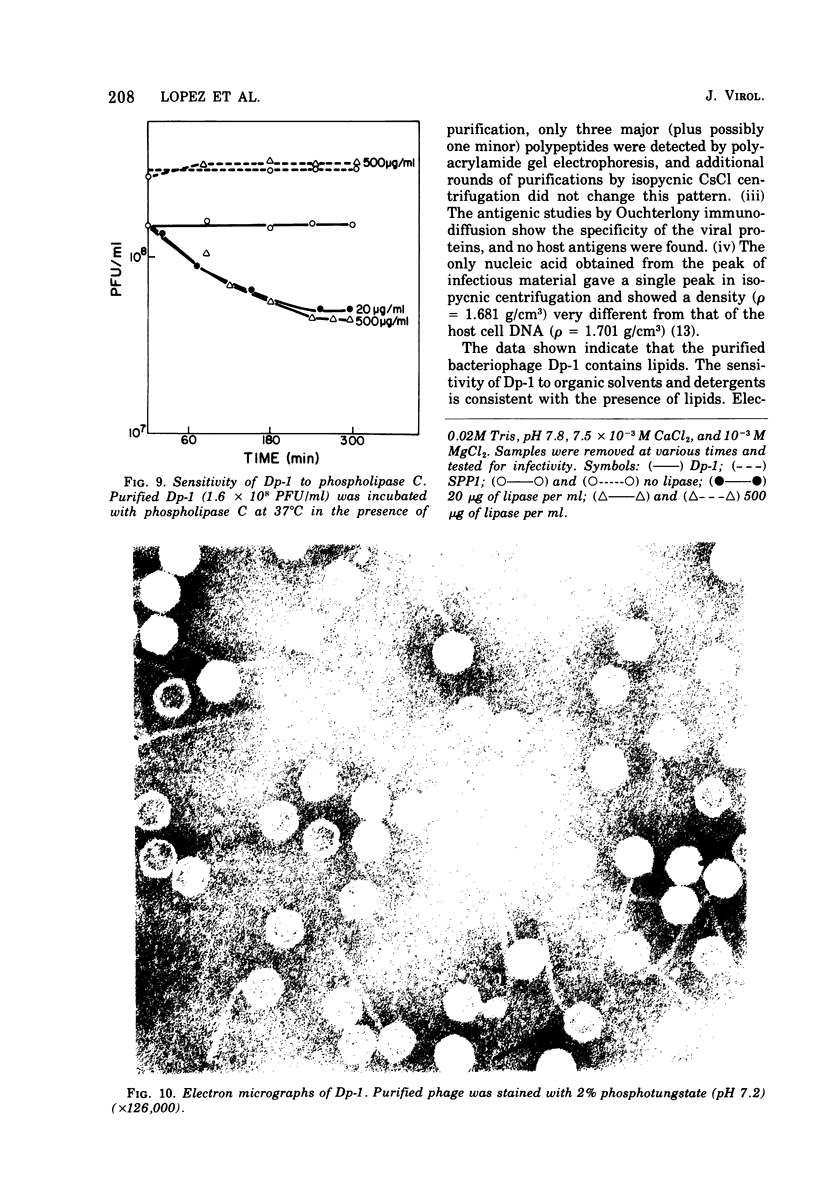
We describe the purification and properties of Dp-1, a bacteriophage isolated from Diplococcus pneumoniae. The phage was sensitive to the organic solvents deoxycholate and Sarkosyl, and its infectivity was reduced by treatment with phospholipase C. Electron microscopy indicated the presence of a double-layered coat around the phage particles. Purified phage preparations contained lipid amounting to about 8.5% of the dry weight of the phage, and thin-layer chromatography resolved the lipids into four components. The phage had a buoyant density in CsCl of 1.47 g/cm3, and a sedimentation constant in 0.1 M NaCl of 313S. Analysis in acrylamide gel electrophoresis indicated the presence of three major proteins. Dp-1 DNA shows a density of 1.681 g/cm3. Neutralizing antisera against the phage have a low potency (K less than 120/min).
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Braunstein S. N., Franklin R. M. Structure and synthesis of a lipid-containing bacteriophage. V. Phospholipids of the host BAL-31 and of the bacteriophage PM2. Virology. 1971 Mar;43(3):685–695. doi: 10.1016/0042-6822(71)90292-3. [DOI] [PubMed] [Google Scholar]
- Espejo R. T., Canelo E. S. Properties of bacteriophage PM2: a lipid-containing bacterial virus. Virology. 1968 Apr;34(4):738–747. doi: 10.1016/0042-6822(68)90094-9. [DOI] [PubMed] [Google Scholar]
- Franklin R. M. Structure and synthesis of bacteriophage PM2, with particular emphasis on the viral lipid bilayer. Curr Top Microbiol Immunol. 1974;(68):107–159. doi: 10.1007/978-3-642-66044-3_5. [DOI] [PubMed] [Google Scholar]
- Goldfine H. Comparative aspects of bacterial lipids. Adv Microb Physiol. 1972;8:1–58. doi: 10.1016/s0065-2911(08)60187-3. [DOI] [PubMed] [Google Scholar]
- Gross-Bellard M., Oudet P., Chambon P. Isolation of high-molecular-weight DNA from mammalian cells. Eur J Biochem. 1973 Jul 2;36(1):32–38. doi: 10.1111/j.1432-1033.1973.tb02881.x. [DOI] [PubMed] [Google Scholar]
- Harrison S. C., Caspar D. L., Camerini-Otero R. D., Franklin R. M. Lipid and protein arrangement in bacteriophage PM2. Nat New Biol. 1971 Feb 17;229(7):197–201. doi: 10.1038/newbio229197a0. [DOI] [PubMed] [Google Scholar]
- Hinnen R., Schäfer R., Franklin R. M. Structure and synthesis of a lipid-containing bacteriophage. Preparation of virus and localization of the structural proteins. Eur J Biochem. 1974 Dec 16;50(1):1–14. doi: 10.1111/j.1432-1033.1974.tb03867.x. [DOI] [PubMed] [Google Scholar]
- Hirokawa H. Transfecting deoxyribonucleic acid of Bacillus bacteriophage phi 29 that is protease sensitive. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1555–1559. doi: 10.1073/pnas.69.6.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly D. C., Vance D. E. The lipid content of two iridescent viruses. J Gen Virol. 1973 Nov;21(2):417–423. doi: 10.1099/0022-1317-21-2-417. [DOI] [PubMed] [Google Scholar]
- LACKS S. Molecular fate of DNA in genetic transformation of Pneumococcus. J Mol Biol. 1962 Jul;5:119–131. doi: 10.1016/s0022-2836(62)80067-9. [DOI] [PubMed] [Google Scholar]
- Lopez R., Tapia A., Portoles A. The influence of glutamic acid and arginine on the competence of Bacillus subtilis growing in a chemostat. Mol Gen Genet. 1975;136(1):87–94. doi: 10.1007/BF00275451. [DOI] [PubMed] [Google Scholar]
- MIZUTANI H., MIZUTANI H. ACTION OF PHOSPHOLIPASE C ON INFLUENZA VIRUS. Nature. 1964 Nov 21;204:781–782. doi: 10.1038/204781b0. [DOI] [PubMed] [Google Scholar]
- Mahony D. E., Easterbrook K. B. Intracellular development of a bacteriophage of Clostridium perfringens. Can J Microbiol. 1970 Oct;16(10):983–988. doi: 10.1139/m70-167. [DOI] [PubMed] [Google Scholar]
- Makula R. A., Finnerty W. R. Microbial assimilation of hydrocarbons: identification of phospholipids. J Bacteriol. 1970 Aug;103(2):348–355. doi: 10.1128/jb.103.2.348-355.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcdonnell M., Lain R., Tomasz A. "Diplophage": a bacteriophage of Diplococcus pneumoniae. Virology. 1975 Feb;63(2):577–582. doi: 10.1016/0042-6822(75)90329-3. [DOI] [PubMed] [Google Scholar]
- Porter R. D., Guild W. R. Characterization of some pneumococcal bacteriophages. J Virol. 1976 Aug;19(2):659–667. doi: 10.1128/jvi.19.2.659-667.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronda-Lain C., Lopez R., Tapia A., Tomasz A. Role of the pneumococcal autolysin (murein hydrolase) in the release of progeny bacteriophage and in the bacteriophage-induced lysis of the host cells. J Virol. 1977 Jan;21(1):366–374. doi: 10.1128/jvi.21.1.366-374.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sands J. A. The phospholipid composition of bacteriophage phi6. Biochem Biophys Res Commun. 1973 Nov 1;55(1):111–116. doi: 10.1016/s0006-291x(73)80066-x. [DOI] [PubMed] [Google Scholar]
- Schaefer R., Hinnen R., Franklin R. M. Further observations on the structure of the lipid-containing bacteriophage PM2. Nature. 1974 Apr 19;248(5450):681–682. doi: 10.1038/248681a0. [DOI] [PubMed] [Google Scholar]
- Schäfer R., Hinnen R., Franklin R. M. Structure and synthesis of a lipid-containing bacteriophage. Properties of the structural proteins and distribution of the phospholipid. Eur J Biochem. 1974 Dec 16;50(1):15–27. doi: 10.1111/j.1432-1033.1974.tb03868.x. [DOI] [PubMed] [Google Scholar]
- TOMASZ A., HOTCHKISS R. D. REGULATION OF THE TRANSFORMABILITY OF PHEUMOCOCCAL CULTURES BY MACROMOLECULAR CELL PRODUCTS. Proc Natl Acad Sci U S A. 1964 Mar;51:480–487. doi: 10.1073/pnas.51.3.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiraby J. G., Tiraby E., Fox M. S. Pneumococcal bacteriophages. Virology. 1975 Dec;68(2):566–569. doi: 10.1016/0042-6822(75)90300-1. [DOI] [PubMed] [Google Scholar]
- Tomasz A., Waks S. Mechanism of action of penicillin: triggering of the pneumococcal autolytic enzyme by inhibitors of cell wall synthesis. Proc Natl Acad Sci U S A. 1975 Oct;72(10):4162–4166. doi: 10.1073/pnas.72.10.4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truden J. L., Franklin R. M. Structure and synthesis of a lipid-containing bacteriophage. IX. Serological disparity between bacteriophage PM2 and its host cell components. Virology. 1971 Dec;46(3):808–816. doi: 10.1016/0042-6822(71)90082-1. [DOI] [PubMed] [Google Scholar]
- Vidaver A. K., Koski R. K., Van Etten J. L. Bacteriophage phi6: a Lipid-Containing Virus of Pseudomonas phaseolicola. J Virol. 1973 May;11(5):799–805. doi: 10.1128/jvi.11.5.799-805.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Pringle J. R., Osborn M. Measurement of molecular weights by electrophoresis on SDS-acrylamide gel. Methods Enzymol. 1972;26:3–27. doi: 10.1016/s0076-6879(72)26003-7. [DOI] [PubMed] [Google Scholar]






