Abstract
A recent surge of interest in tuft cells, which are chemosensory intestinal epithelial cells, has uncovered new functional roles for these cells in colorectal cancer, metabolic signaling, and type 2 immunity. Here, we explore emerging evidence suggesting that tuft cells are critical for protection during enteric infections and inflammatory responses.
A Spotlight on Intestinal Tuft Cells
The gastrointestinal (GI) tract is lined by a constantly changing population of intestinal epithelial cells (IECs). Developing IECs transit up the crypt-villus axis and differentiate into seven distinct types of highly specialized cells that work synergistically to absorb nutrients, regulate gut motility, and maintain the mucosal barrier. Recent studies have revealed that tuft cells, a rare and poorly characterized type of IEC, detect helminth infections and orchestrate a type 2 immune response. In this forum, we discuss some of the most recent advances in tuft cells as important mediators of host defense during enteric infection and inflammation.
Characteristics and Functions of Tuft Cells
Tuft cells (also known as brush cells) were first identified over 60 years ago by their unique fusiform shape and their distinctive apical “tuft” of microvilli tubules. These cells have been found in epithelial tissues throughout mice and humans, in organs such as the pancreas, lungs and GI tract, where they comprise approximately 0.4–2% of IECs under homeostatic conditions[1–3].
Several molecular markers are specific to tuft cells in the intestinal epithelium. The most common marker is doublecortin-like kinase 1 (DCLK1). Tuft cells can also be identified by POU domain class 2, transcription factor 3 (Pou2f3, also known as Skn-1), the surface marker Siglec-F, and by the expression of cyclooxygenases COX-1 and COX-2, which are critical enzymes in prostaglandin synthesis[1–4].
Chemosensing
Perhaps one of the most unique and versatile hallmarks of tuft cells is their expression of taste receptors and related signaling proteins including α-gustducin, transient receptor potential cation channel subfamily M member 5 (Trpm5), and Pou2f3. The G-protein α-gustducin relays signals from taste receptors, while Trpm5 is a cation channel that transduces signals from sweet, bitter, and umami (savory) stimuli. Pou2f3 is a transcription factor required for the development of taste receptor cells (including tuft cells) in mice,[5].
All three of these proteins are essential for tuft cell function. Mice lacking α-gustducin, Pou2f3, or Trpm5 are unable to mount an immune response against helminths[1, 2]. Moreover, Pou2f3 knockout (KO) mice cannot develop tuft cells and possess several metabolic abnormalities (decreased weight, increased energy, and increased dopamine levels) when compared to littermate controls[5].
Epithelial Cell Proliferation
Another important role of tuft cells is to promote IEC proliferation[6–8]. Depleting DCLK1+ IECs in mice significantly reduces the number of replicating epithelial cells in the colon relative to control mice[6]. How tuft cells increase cell division is unclear, but DCLK1 expression down-regulates several microRNAs associated with decreased cell proliferation and tumor suppression[9].
In addition, tuft cells can act as a reserve stem cell population[6]. Lineage tracing of tuft cells in Dclk1-LacZ transgenic reporter mice indicates that these cells are able to dedifferentiate and reconstitute entire intestinal crypts[6]. In these experiments, all cellular descendants of DCLK1+ IECs could synthesize the reporter LacZ (marking long-lived epithelial tufts cells). Using intestinal organoids derived from these mice, it was shown that certain intestinal crypts (although rare) could stain positive for LacZ. This indicated that tuft cells could give rise to an entire crypt[6].
Tuft cells also enhance epithelial integrity. IEC-specific Dclk1-KO mice present compromised gut barrier integrity at homeostasis, as well as increased injury following DSS- induced colitis or full body irradiation[7, 8]. This underscores the functional importance of DCLK1 in tuft cells. These results (and others) suggest that tuft cells play a critical role in regulating IEC proliferation, and in mitigating intestinal damage.
Activation of immune responses
There is a growing body of evidence demonstrating that tuft cells initiate type 2 immune responses. Type 2 immunity is generally associated with ensuring protection against helminths in a host, as well as causing allergic inflammation. However, recent studies suggest that type 2 immune responses are also protective in certain forms of enteric colitis.
Indeed, tuft cells are the predominant IEC-derived source of the cytokine interleukin 25 (IL-25), which recruits eosinophils and initiates a type 2 immune response via type 2 innate lymphoid cells (ILC2s)[3]. Two cytokines secreted by ILC2s in response to IL-25 are IL-4 and IL-13, which in addition, can induce tuft and goblet cell hyperplasia, as well as enhance tuft cell signaling, and possibly, mucus production[1, 3].
Moreover, IL-25 significantly increases the expression of angiogenin-4, an antimicrobial peptide secreted by mouse Paneth and goblet cells[10]. And, it has been known for some time that angiogenin-4 can inhibit the growth of certain bacterial species such as Listeria monocytogenes and Enterococcus faecalis[11]. Thus, tuft cells appear to influence the composition of the microbiome through IL-25-mediated induction of angiogenin-4.
While the role of tuft cells in many diseases is still under investigation, changes in IL-25 levels correlate with disease symptoms in inflammatory bowel disease and C. difficile infections, as discussed below. Consequently, even when tuft cell involvement is not clearly known, changes in IL-25 may be potentially indicative of altered tuft cell numbers or function.
Tuft cells in disease
Helminth Infections
Recently, tuft cells were shown to use taste receptors to detect several different helminth species, in addition to the symbiotic protozoa Tritrichomonas muris. Mice that lacked tuft cells (Pou2f3 KO) or taste receptor function (Trpm5 or α-gustducin KO) exhibited severely impaired helminth clearance relative to wildtype mice, indicating that tuft cells were protective during helminth infections[1–3]. This protective response was deemed to be mediated by tuft cell-derived IL-25, initiating type 2 immune responses[1–3] (Figure 1).
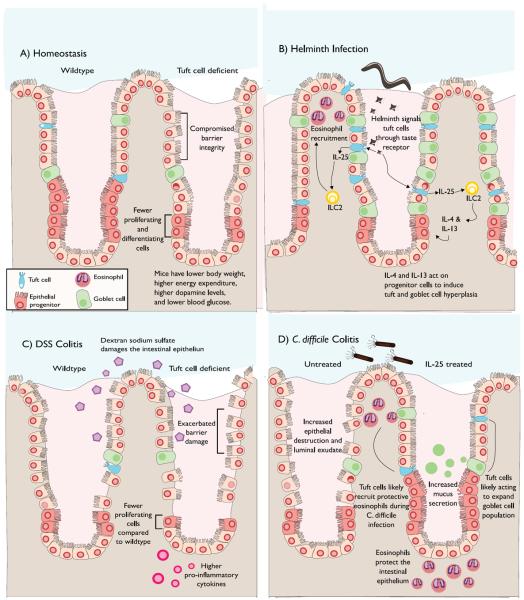
Figure 1. Tuft Cells in the Murine GI Tract.
A) Under homeostatic conditions in the gut, tuft cells can increase the number of dividing IECs and contribute to gut barrier maintenance. Tuft cells are also able to alter metabolic signaling by altering catecholamine levels (dopamine). B) Tuft cells recognize helminth infections via unknown signal(s). Tuft cells induce the secretion of IL-4 and IL-13 from innate lymphoid cell 2 (ILC2s) via IL-25 signaling. IL-4 and IL-13 can then trigger signals that result in an increased number of tuft and goblet cells. C) Relative to controls, IEC-specific Dclk1-KO mice present fewer IEC proliferating cells, increased gut permeability and higher IL-1β and IL-17 levels upon DSS-induced colitis. D) Exogenous IL-25 drastically improves mouse survival following Clostridium difficile infection via an eosinophil-mediated mechanism. Increases in IL-25 levels result in goblet and tuft cell hyperplasia. Exogenous IL-25 also increases mucus secretion and protects the epithelium from damage during C. difficile infection, potentially by increasing the proliferation of these cell types.
Inflammatory Bowel Disease
The direct effects of tuft cells during human inflammatory bowel disease (IBD) are presently unknown, but tuft cells have been found to be protective in mouse DSS-induced colitis, a model for IBD[8] (Figure 1). Indeed, IEC-specific Dclk1-KO mice were reported to present fewer proliferating (Ki67+) IECs following DSS-induced colitis. In addition, these mice exhibited increased gut permeability and higher IL-1β and IL-17 levels in the colon relative to wild type mice[8]. These results suggest that tuft cells increase epithelial reconstitution in response to this type of intestinal injury.
Furthermore, patients with either ulcerative colitis or Crohn's disease possess fewer total IL-25 expressing cells in their intestinal mucosa, with IL-25 levels being substantially lower during active disease when compared to remission[11]. These findings indicate that patients with active IBD may have fewer tuft cells than healthy controls. If the DSS model does translate to human disease, then, increasing tuft cell numbers might hypothetically dampen the pro-inflammatory response to IBD and possibly reduce the severity of intestinal injury.
Clostridium difficile
The protective role of tuft cells in C. difficile infections is underscored by the importance of IL-25 in drastically reducing disease symptoms. Immunohistochemistry indicates that patients infected with C. difficile produce less IL-25 in colonic biopsies, a phenotype also observed in a mouse model of C. difficile-mediated colitis[12]. In this study, restoring IL-25 levels in mice increased survival via an unanticipated mechanism of eosinophil-dependent protection of the gut barrier (Figure 1). Moreover, treating mice with an anti-Siglec-F antibody ablated the protective phenotype of IL-25[12]. Together, these data suggest that IL-25-mediated induction of eosinophils protects the host from C. difficile-induced disease.
Interestingly, the deadly epidemic strain of C. difficile, ribotype 027, produces the toxin CDT (C. difficile transferase), which intoxicates mice via a toll-like receptor-2 (TLR-2)-dependent mechanism of eosinophil killing[13]. These data suggest that part of the virulence strategy for this specific C. difficile strain may be to overcome eosinophil-mediated host responses.
In this study, IL-25 blocked the cytotoxic effects of C. difficile without decreasing bacterial burden or toxin levels, indicating that IL-25 could protect the host exclusively by limiting and/or repairing bacterial-induced damage. Additionally, intestinal eosinophilia was observed even in the absence of exogenously administered IL-25, suggesting that tuft cell regulated type 2 immune responses were part of the innate immune responses against C. difficile- induced colitis[13].
Colorectal Cancer
Tuft cells can protect the host from enteric infections by enhancing IEC regeneration, but this phenotype may exacerbate colorectal cancer. DCLK1+ cells are over-represented in approximately 75% of human primary colorectal cancers and colorectal adenocarcinomas[14]. Exceptionally high levels of DCLK1+ cells have significantly correlated with lower patient survival following cancer resection[14]. In mice, DCLK1+ cells seem to be the predominant tumor stem cell and destroying DCLK1+ cells has been reported to halt tumor growth[15]. The precise origin of DCLK1+ cells is controversial, but tuft cells are the likely culprit. In addition to being the only IECs to express DCLK1 at homeostasis, tuft cells are a reserve stem cell population; a subset of these cells are DCLK1+ quiescent and long-lived tuft cells that upon genetic insult could be prompted to proliferate and become “cancer-initiating”[6].
Concluding Remarks
Tuft cells are a key epithelial cell type increasing IEC proliferation, gut barrier integrity and mounting IL-25-associated immune responses against helminths, and quite likely, other enteric pathogens. Indeed, intestinal IL-25 secretion can leads to goblet cell hyperplasia, angiogenin-4 production, and changes in gut motility (stretching/contractions). Tuft cells can also affect metabolic signaling[5], presumably by responding to nutritional or metabolite signals such as changes in carbohydrates, lipids, or amino acid levels in the lumen. As such, tuft cells have the machinery to detect changes in the lumen and to initiate host responses. We hypothesize that the primary function of tuft cells is to respond to metabolic shifts in the lumen, which enables the host to monitor the microbiota and expel detrimental microflora such as luminal pathogens.
With this forum piece, we've aimed to raise awareness about the growing body of evidence supporting the importance of tufts cells in the enteric environment. An increased understanding of tuft cell biology may ultimately contribute to prevention or treatment strategies against colitis or cancer.
Acknowledgements
This work was supported by grants from the National Institute of Health (R21 AI114734 and R01 AI124214).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest WAP is a consultant for Seres Therapeutics and TechLab, Inc. that make therapeutic and diagnostic products for C. difficile infection.
References
- 1.Gerbe F, Sidot E, Smyth DJ, Ohmoto M, Matsumoto I, Dardalhon V, Cesses P, Garnier L, Pouzolles M, Brulin B, Bruschi M, Harcus Y, Zimmermann VS, Taylor N, Maizels RM, Jay P. Intestinal epithelial tuft cells initiate type 2 mucosal immunity to helminth parasites. Nature. 2016;529(7585):226–230. doi: 10.1038/nature16527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Howitt MR, Lavoie S, Michaud M, Blum AM, Tran SV, Weinstock JV, Gallini CA, Redding K, Margolskee RF, Osborne LC, Artis D, Garrett WS. Tuft cells, taste-chemosensory cells, orchestrate parasite type 2 immunity in the gut. Science. 2016;351(6279):1329–1333. doi: 10.1126/science.aaf1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.von Moltke J, Ji M, Liang HE, Locksley RM. Tuft-cell-derived IL-25 regulates an intestinal ILC2-epithelial response circuit. Nature. 2016;529(7585):221–225. doi: 10.1038/nature16161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gerbe F, van Es JH, Makrini L, Brulin B, Mellitzer G, Robine S, Romagnolo B, Shroyer NF, Bourgaux JF, Pignodel C, Clevers H, Jay P. Distinct ATOH1 and Neurog3 requirements define tuft cells as a new secretory cell type in the intestinal epithelium. J Cell Biol. 2011;192(5):767–780. doi: 10.1083/jcb.201010127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ushiama S, Ishimaru Y, Narukawa M, Yoshioka M, Kozuka C, Watanabe N, Tsunoda M, Osakabe N, Asakura T, Masuzaki H, Abe K. Catecholamines facilitate fuel expenditure and protect against obesity via a novel network of the gut-brain axis in transcription factor skn-1-deficient mice. EBioMedicine. 2016;8:60–71. doi: 10.1016/j.ebiom.2016.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Westphalen CB, Asfaha S, Hayakawa Y, Takemoto Y, Lukin DJ, Nuber AH, Brandtner A, Setlik W, Remotti H, Muley A, Chen X, May R, Houchen CW, Fox JG, Gershon MD, Quante M, Wang TC. Long-lived intestinal tuft cells serve as colon cancer-initiating cells. J Clin Invest. 2014;124(3):1283–1295. doi: 10.1172/JCI73434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.May R, Qu D, Weygant N, Chandrakesan P, Ali N, Lightfoot SA, Li L, Sureban SM, Houchen CW. Brief report: Dclk1 deletion in tuft cells results in impaired epithelial repair after radiation injury. Stem Cells. 2014;32(3):822–827. doi: 10.1002/stem.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qu D, Weygant N, May R, Chandrakesan P, Madhoun M, Ali N, Sureban SM, An G, Schlosser MJ, Houchen CW. Ablation of doublecortin-like kinase 1 in the colonic epithelium exacerbates dextran sulfate sodium-induced colitis. PLoS One. 2015;10(8):e0134212. doi: 10.1371/journal.pone.0134212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sureban SM, May R, Qu D, Weygant N, Chandrakesan P, Ali N, Lightfoot SA, Pantazis P, Rao CV, Postier RG, Houchen CW. DCLK1 regulates pluripotency and angiogenic factors via microRNA-dependent mechanisms in pancreatic cancer. PLoS One. 2013;8(9):e73940. doi: 10.1371/journal.pone.0073940. doi:10.1371/journal.pone.0073940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Noor Z, Burgess SL, Watanabe K, Petri WA., Jr. Interleukin-25 mediated induction of angiogenin-4 is interleukin-13 dependent. PLoS One. 2016;11(4):e0153572. doi: 10.1371/journal.pone.0153572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Su J, Chen T, Ji XY, Liu C, Yadav PK, Wu R, Yang P, Liu Z. IL-25 downregulates Th1/Th17 immune response in an IL-10-dependent manner in inflammatory bowel disease. Inflamm Bowel Dis. 2013;19(4):720–728. doi: 10.1097/MIB.0b013e3182802a76. [DOI] [PubMed] [Google Scholar]
- 12.Buonomo EL, Cowardin CA, Wilson MG, Saleh MM, Pramoonjago P, Petri WA., Jr. Microbiota-regulated IL-25 increases eosinophil number to provide protection during clostridium difficile infection. Cell Rep. 2016;16(2):432–443. doi: 10.1016/j.celrep.2016.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cowardin CA, Buonomo EL, Saleh MM, Wilson MG, Burgess SL, Kuehne SA, Schwan C, Eichhoff AM, Koch-Nolte F, Lyras D, Aktories K, Minton NP, Petri WA. The binary toxin CDT enhances clostridium difficile virulence by suppressing protective colonic eosinophilia. Nature Microbiology. 2016;1:16108. doi: 10.1038/nmicrobiol.2016.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gagliardi G, Goswami M, Passera R, Bellows CF. DCLK1 immunoreactivity in colorectal neoplasia. Clin Exp Gastroenterol. 2012;5:35–42. doi: 10.2147/CEG.S30281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakanishi Y, Seno H, Fukuoka A, Ueo T, Yamaga Y, Maruno T, Nakanishi N, Kanda K, Komekado H, Kawada M, Isomura A, Kawada K, Sakai Y, Yanagita M, Kageyama R, Kawaguchi Y, Taketo MM, Yonehara S, Chiba T. Dclk1 distinguishes between tumor and normal stem cells in the intestine. Nat Genet. 2013;45(1):98–103. doi: 10.1038/ng.2481. [DOI] [PubMed] [Google Scholar]