NARRATIVE ABSTRACT
Although female contraceptives are very effective at preventing unintended pregnancy, some women cannot use them due to health conditions or side effects, leaving some couples without effective contraceptive options. In addition, many men wish to take active responsibility for family planning. Thus, there is a great need for male contraceptives to prevent unintended pregnancy, of which 80–90 million occur annually. At present, effective male contraceptive options are condoms and vasectomy, which are not ideal for all men. Therefore, efforts are under way to develop novel male contraceptives. This paper will briefly review the advantages and disadvantages of condoms and vasectomies, and then discuss the research directed towards the development of novel methods of male contraception.
Keywords: Male Contraception, spermatogenesis, unintended pregnancy, testosterone, vasectomy
OVERVIEW OF MALE REPRODUCTIVE PHYSIOLOGY
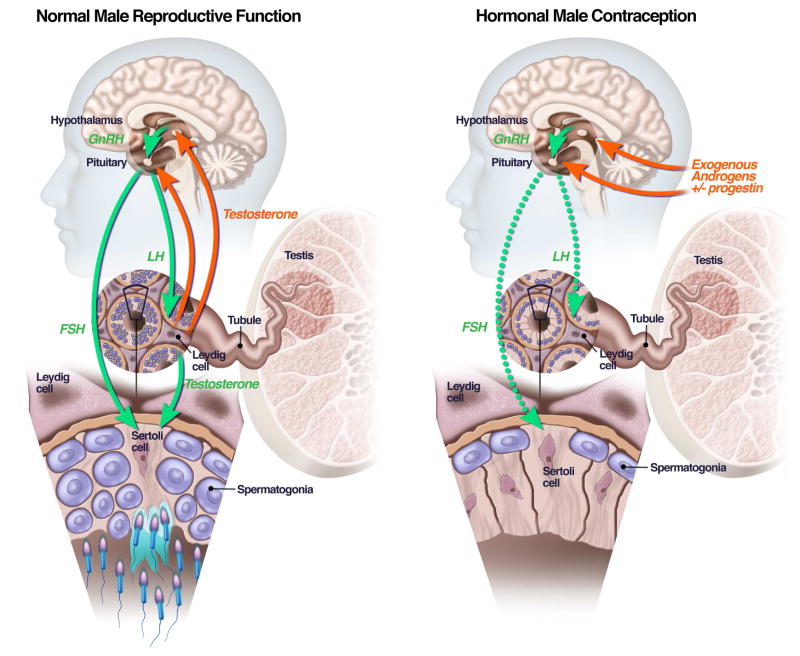
Production of mature sperm in the human testes takes approximately 72 days (1). After puberty, sperm production is continuous and occurs in four distinct phases: (1) a mitotic phase in which the stem cells, the spermatogonia, give rise to diploid spermatocytes; (2) a meiotic phase in which spermatocytes double their chromosome complement and undergo two cycles of cell division resulting in haploid spermatids; (3) spermiogenesis, which involves spermatid nuclear condensation and flagellum formation; and (4) spermiation, which involves release of the spermatozoa into the tubular lumen (2). Storage and further maturation of sperm take place in the epididymis. Sperm aspirated from the cauda epididymis are capable of fertilizing an egg in vitro (3). The testes also synthesize testosterone, a steroid hormone. Testosterone is necessary for sperm production and also maintains sexual function as well as muscle and bone mass (4). Testosterone is produced by Leydig cells in the interstitium of the testes, when stimulated by luteinizing hormone (LH). Sperm production occurs in the seminiferous tubules, where the sperm are nurtured by Sertoli cells stimulated by follicle-stimulating hormone (FSH) and high concentrations of intra-testicular testosterone (Figure-left panel) (5). Given the physiology of sperm production, a male contraceptive can work in one of several ways:
Figure 1.
Left-hand panel: normal function of the hypothalamic-pituitary-testicular axis with FSH and LH stimulating the testes to produce sperm and testosterone, and testosterone providing feedback regulation of FSH and LH production in the pituitary. Right-hand panel: suppression of FSH and LH production from exogenously administered androgens and progestins, which lower FSH and LH concentrations, depriving the testes of the signals required for sperm production, leading to contraception.
By preventing sperm from reaching the egg by physical barriers (condoms, vasectomy, and experimental vas occlusion methods
By preventing sperm production (experimental hormonal and non-hormonal methods), or
By killing or inhibiting the function of sperm or the sperm’s ability to bind the egg after ejaculation (spermicides, experimental anti-motility agents).
This last category of contraceptives, such as spermicides, are usually intended to be used intravaginally by the female, and are therefore more properly considered female contraceptives, so will not be discussed further in this review. Instead, this article will describe the efficacy of existing methods of male contraception and then discuss the research directed towards the development of novel methods of male contraception that function either by inhibiting sperm production or by preventing sperm from reaching the female reproductive tract.
CURRENTLY AVAILABLE MALE CONTRACEPTIVE METHODS
Vasectomy
Vasectomy is a simple, outpatient surgery performed under local anaesthesia in which the vas deferens is surgically interrupted bilaterally through a small scrotal incision. There are approximately 500,000 vasectomies performed in the United States yearly and worldwide over 50 million men have undergone the procedure (6), although there are significant cultural differences in the acceptability of this procedure in different settings. Vasectomies are highly effective with a failure rate under 1% and a low rate of complications (Table) (7,8). The “no-scalpel technique,” developed in China (9) that relies on a single midline puncture in the scrotal raphe using scissors, had been widely adopted (10,11). Drawbacks to vasectomy include a delay in the onset of azoospermia of 3–4 months, post-operative pain, and rare infections. Although most postoperative pain resolves quickly, 10–15% of men experience chronic testicular discomfort (12). In one study of such men, 27 of 33 had relief of their discomfort with reversal of the vasectomy (13).
Table 1.
Percent of couples using a male contraceptive and efficacy of these methods in the prevention of unintended pregnancy (from references 20 & 21).
| Method | Year | Unintended Pregnancy Rate per year (%) | |||
|---|---|---|---|---|---|
| 1992 | 1995 | 2002 | 2008 | ||
| Vasectomy | 11 | 11 | 9 | 10 | 0.1 |
| Condoms | 12 | 20 | 18 | 16 | 15–20 |
| Withdrawal | 2 | 3 | 4 | 5 | 25–30 |
| Total Male | 25 | 34 | 31 | 31 | |
Vasectomies are most appropriate for men who do not wish any future fertility. However, 3–5% of men with vasectomies do eventually request reversal, usually due to remarriage (14). For this reason, some urologists recommend freezing a semen sample prior to the procedure. Vasectomy reversal, or vasovasostomy, restores fertility in most cases; however, rates of pregnancy vary from 50–75% depending on the length of time between the vasectomy and the vasovasostomy for two reasons. In some of men, vasovasostomy is unable to restore patency of the vas, especially if more than 8 years have elapsed since the original vasectomy (15). Secondly, 20–30% of men remain infertile despite restored patency of the vas as documented by imaging techniques probably due to the presence of anti-sperm antibodies (16). For these reasons, vasectomy cannot be recommended as a truly reversible method of contraception. Vasectomy is safe in terms of overall male health. Reports of associations between vasectomy and cardiovascular disease and prostate cancer first reported in the 1980s have proven incorrect (17, 18). In summary, vasectomy is highly effective and very safe. The major drawbacks are chronic testicular discomfort in some men and the inability of vasovasostomy to reliably restore fertility in all cases when desired.
Condoms
Condoms, originally made of animal intestine, have been used contraception, and as protection against sexually transmitted infections for several hundred years. Since 1920, most condoms have been made of latex. Latex condoms are unique among contraceptives in that they protect against many sexually transmitted diseases including the human immunodeficiency virus. Condoms are relatively free from side effects. The main drawback to condoms is their marginal contraceptive efficacy, which results mostly from improper or inconsistent usage, or breakage, which occurs in up to 4% of cases (19). Pregnancy rates for couples using condoms as their sole means of contraception approach 15–20% per year (20), although failure rates are likely higher in young couples with high spontaneous fertility (21). In addition, some men dislike condoms because they feel that condoms either diminish sexual pleasure or are difficult to use (22). Lastly, some men and women develop allergic reactions to the latex, which is derived from rubber trees, causing skin irritation and, rarely, anaphylaxis (23). Polyurethane condoms are available for couples in whom one of the partners has a latex allergy. These condoms are slightly less effective than latex condoms; however, mainly due to slippage from their looser fit (24–26).
EXPERIMENTAL MALE CONTRACEPTIVES
Hormonal Male Contraceptives
Because of the need for improved methods of male contraception, efforts have been made to develop a hormonally-derived contraceptive analogous to estrogen-progesterone birth control pill for women. A male hormonal contraceptive has the potential to be easy to use and reversible. Testosterone functions as a male contraceptive by suppressing the secretion of LH and FSH from the pituitary via negative feedback (Figure, right panel). Low concentrations of LH and FSH deprive the testis of the signals needed for spermatogenesis. This leads to markedly decreased sperm counts in most, but not all, men after 3–4 months of testosterone administration. Sperm counts return to pre-treatment levels 3–6 months after discontinuing testosterone administration in most men (27). Surveys conducted in several countries suggest that a hormonally-derived male contraceptive would be welcomed by a large percentage of men and, importantly, most women would trust their partner to use it (28, 29).
In normal men, sperm concentrations in the ejaculate range from 15–150 million sperm per milliliter of ejaculate. The absence of spermatozoa in the ejaculate, a condition termed azoospermia, makes fertilization impossible and is therefore the ultimate goal of male hormonal contraceptives—a goal that has yet to be reached. In all studies of this approach to contraception, a subset of men experience a reduction of their sperm counts below 15 million per ml, called oligospermia, without becoming azoospermic (30). Severe oligospermia (concentrations of less than 1 million sperm per milliliter) appears to decrease the chances of conception to less than 1% per year (31). Therefore, suppression of 100% of treated men to sperm concentrations under 1 million sperm/ml is considered a reasonable goal for male contraceptive research (32).
Interestingly, there are ethnic differences in the response to male hormonal contraception. Study volunteers in Asia are more susceptible to testosterone-induced suppression of spermatogenesis, with rates of azoospermia in the 90–100% range, whereas men studied in Europe, North America, and Australia have rates of azoospermia closer to 60–80% on the same regimens (30, 31). There is no clear explanation for this difference. Nevertheless, it is important in the interpretation of trial results and complicates extrapolation of rates of suppression between trials performed in different geographical areas.
Administration of un-esterified testosterone orally or parenterally is ineffective because it is quickly degraded by the liver. Therefore, most hormonal contraceptive regimens have used longer-acting injectable testosterone esters such as testosterone enanthate (TE) administered by intramuscular injection on a weekly basis. Two multi-center trials of weekly TE injections as a male contraceptive were conducted by the World Health Organization (WHO). The first study enrolled 271 subjects who were dosed with 200 mg TE intramuscularly weekly (30). Sixty percent of these men became azoospermic, and an additional 30% became severely oligospermic. One hundred and nineteen of the men who became azoospermic discontinued other birth control and continued on TE injections using the injections as their sole method of contraception for one year. During that period, only 1 pregnancy occurred, demonstrating testosterone-induced azoospermia was a highly effective contraceptive.
The second WHO study examined contraceptive efficacy of TE injections in men who became azoospermic or oligospermic to less than 3 million sperm per ml of ejaculate with TE injections (31). Three hundred and ninety-nine mostly Asian men were enrolled in this study. Three-hundred ninety-one (98%) of the men became oligospermic or azoospermic. There were no pregnancies caused by the men who became azoospermic, and in men who became oligospermic, fertility was reduced to eight pregnancies per 100 person-years. The overall failure rate (including the eight men who failed to suppress their sperm counts) was 3.4%, for an overall contraceptive efficacy of 96.6%. In both groups, sperm counts returned to normal after the cessation of testosterone injections, and there were no major side effects.
These studies demonstrated that testosterone injections are effective as a contraceptive in most men; however, a proportion of men fail to suppress below 3 million sperm per milliliter and therefore remain potentially fertile. In addition, the necessity of weekly intramuscular injections was unpopular with subjects, twelve per cent of whom discontinued involvement due to dislike of the injection schedule. Side effect were fairly minimal, but notably, high-dose TE deceases serum HDL cholesterol, which might impact the development of atherosclerosis (33,34); however, as is the case with any male hormonal contraceptive, the long-term effects on prostate and liver health as well as mood and behaviour are unknown.
Testosterone undecanoate (TU) is a long-chain ester that normalize serum T concentrations in hypogonadal men for 6–12 weeks after injection (35–37). A large trial of TU injections for male contraception was conducted in China (38). Volunteers received monthly injections of 500 or 1000 mg TU. Ninety percent of the men had sperm concentrations below 1 million sperm/ml and used the injections as their sole method of contraception for one year. Only a few pregnancies were reported in treated men and side effects were minimal, however, the method was not approved for use for unclear reasons.
In the hopes of achieving greater rates of azoospermia, several male contraceptive studies have combined testosterone administration with administration of progestins, which additively suppress FSH and LH from the pituitary and may have direct anti-sperm effects on the testes (39). Combinations of testosterone and depot injections of medroxyprogesterone acetate (DMPA) induced azoospermia in half of study subjects with some degree of oligozoospermia in most others. The contraceptive efficacy of these combinations, however, was poor, with several couples conceiving while receiving therapy despite simultaneous use of other contraceptives (40).
Several male contraceptive studies of the potent oral progestin, levonorgestrel (LNG) have been performed. For example, in one study LNG (500 mcg orally daily) was combined with TE (100 mg intramuscularly per week) for six months. The LNG-TE combination was superior to TE alone in terms of azoospermia (67% versus 33%) and 94% of men has sperm concentrations of less than 1 million per ml compared with 61% of the TE-alone group (41). Drawbacks to the LNG-TE regimen included greater weight gain and decreases in HDL cholesterol when compared with the TE-alone group. Other progestins, such as desogestrel, have been tested in male contraceptive regimens with similar results, but without causing weight gain or large reductions HDL cholesterol (42). One large industry sponsored study using testosterone decanoate injections coupled with etonogestrel implants suppressed 80–90% of men below a sperm concentration of 1 million/ml over one year (43). A follow-up study, one of the only placebo-controlled studies in the field, combined etonorgestrel with testosterone Undecanoate with similar results in terms of suppression of spermatogenesis (44). Unfortunately, the companies sponsoring these two studies have not pursued this work further. Lastly, only one study with 52 couples testing androgen-progestin combinations for pregnancy prevention has been published (45). A larger trial testing testosterone undecanoate and noresthisterone enanthate to prevent pregnancy has been performed but has not been published. Cyproterone acetate is a unique progestin with anti-androgenic properties that has been studied in combination with weekly injections of testosterone enanthate with very high efficacy, but only in small trials of 10–15 men (46,47).
Nestorone is a 19-norprogesterone-derived progestin which can be applied as a transdermal gel (48). A combination of Nestorone gel and testosterone transdermal gels were studied for gonadotropin suppression (49) and in a 6-month male contraceptive trial (50). In this latter study, 89% of men achieved suppression of their sperm concentration down to less than or equal to 1 million sperm/ml. Importantly, a majority of subjects on this regimen were very satisfied with the regimen, stating they would be likely to use it if it were commercially available (51). Ongoing studies are working to simplify the regimen into a single combination gel for Phase II testing at six international sites beginning in 2017.
Dimethandrolone undecanoate (DMAU) is a potent synthetic 19-norandrogen that acts as a ligand at both androgen and progesterone receptors making DMAU a potential “single-agent” contraceptive (52). Studies in rodents and rabbits have shown both reversible suppression of gonadotropins and sperm with orally administered DMAU (53, 54). Phase I testing in humans has demonstrated short term safety and tolerability with reversible suppression of gonadotropins (55) and Phase II testing of this compound is underway. Interestingly, DMAU can be administered either orally or by intramuscular injection, making it a very exciting compound for male hormonal contraceptive development.
Non-Hormonal Male Contraceptives
Several groups are examining approaches to “non-hormonal” male contraception, although to date, none of the current generation of candidates has been tested in men. Non-hormonal male contraception doesn’t involve the administration of hormones or compounds that block hormone secretion or hormone action. Non-hormonal contraception may be more appealing to men as it avoids any impact on testosterone concentrations or sexual function. In addition, the use of testosterone or another anabolic steroid could lead to sports disqualification. Lastly, non-hormonal contraceptives may be more easily dosed orally than steroid preparations due to rapid “first pass” metabolism of testosterone in the liver.
An early example of a non-hormonal contraceptive candidate is Adjudin. Adjudin is an anti-sperm compound that disrupts the adhesion of spermatids to Sertoli cells, causing premature spermiation and infertility. Administration of two doses of 50 mg/kg of adjudin weekly induced 100% infertility after 5 weeks of treatment in adult rats without changes in serum testosterone, FSH, or LH concentrations (56). Because there was some liver inflammation observed in a 29-day study of adjudin administration (57), researchers conjugated adjudin to a FSHβ mutant specifically targeting it to Sertoli cells, thereby significantly reducing the dose necessary for contraception (58). Unfortunately, the cost of this approach and the possibility of developing anti-FSH autoantibodies has stalled progress to human studies.
H2-Gamendazole is an anti-sperm compound that impairs the function of the apical ectoplasmic specialization (59). All male rats who received a single oral dose of gamendazole at 6 mg/kg were infertile, but only 57% regained fertility (60). In terms of toxicology, three out of five rats died after receiving a dose of 200 mg/kg of H2-gamendazole; however, no observable abnormalities including liver inflammation, necrosis or hemorrhage was detected at dosages lower than 200 mg/kg. These investigators are hoping to move into human testing at lower doses to minimize the risk of toxicity.
Two other noteworthy approaches to non-hormonal male contraception have been published. The first study demonstrated that seven of nine male non-human primates could be immunized against the semen protein Eppin were unable to father pregnancies and the effect was reversible when the immunizations were stopped (61). This group is now working to develop small molecular inhibitors of Eppin binding as male contraceptives. A second high-profile paper showed the small molecule JQ1 could reversibly suppress spermatogenesis in mice by inhibiting the function of the protein BRDT (62). Unfortunately, this compound also inhibits other similar proteins. Therefore, this group is attempting to develop a BRDT specific inhibitor.
It has been known since 1925 that vitamin-A (retinol) is required for normal spermatogenesis (63). Vitamin-A and its active metabolite retinoic acid is required at puberty for the initiation of spermatogenesis and for the maintenance of spermatogenesis in adults (64, 65). Retinoic acid produced from retinol in situ binds one of several retinoic acid receptors (RARs), which regulate gene expression. Because male RAR knockout animals are sterile due to various problems in spermatogenesis (66–69), blockade of retinoic acid function or biosynthesis is an appealing approach to male non-hormonal contraceptive development.
BMS-189453 is an orally active retinoic acid receptor pan-antagonist. At daily oral doses of 15, 60, or 240 mg/kg for 1 month, BMS-189453 produced marked testicular degeneration in rats, but also lead to increases in leukocyte counts, alkaline phosphatase and alanine aminotransferase levels (70). One group has explored whether a lower dose of BMS-189453 might function as a contraceptive without the toxicity seen at higher doses (71). Two groups of 30 mice each were given BMS-189453 in oral dose of 5 mg/kg for 2 weeks and 2.5 mg/kg for 4 weeks. The study showed that the mice were completely sterile by 4 weeks after a dosing regimen of 5 mg/kg and by the end of treatment with a dose of 2.5 mg/kg for 4 weeks. By 12 weeks after treatment, fertility was completely restored in all males. This compound, or a more specific retinoic acid-alpha antagonist (72,73), hold promise for non-hormonal contraception.
Over fifty years ago, the oral administration of WIN 18,446 was shown to completely and reversibly inhibit spermatogenesis in man (74–76). Unfortunately, subjects taking WIN 18,446 experienced a “disulfiram reaction” consisting of nausea, vomiting, palpitations and sweating, when they took WIN 18,446 and drank alcohol. Because of this, further development of WIN 18,446 was abandoned without an understanding of its mechanism of action. Our group demonstrated that WIN 18,446 suppresses spermatogenesis by inhibiting testicular retinoic acid biosynthesis, via inhibition of the testes-specific aldehyde dehydrogenase ALDH1A2 (77, 78). Using a rabbit model, we observed that oral administration of WIN 18,446 induced reversible azoospermia, and reductions in spermatogenesis were preceded by a reduction in intra-testicular retinoic acid. These findings suggest that inhibition of the testicular retinoic acid biosynthesis is a promising target for male contraceptive development. Our group is focusing on the development of novel, specific compounds that inhibit testicular retinoic acid biosynthesis via ALDH1A2 without interfering with alcohol metabolism. Hopefully, this work will result in compounds that reversibly inhibit spermatogenesis without significant side effects.
Vas Occlusion Methods
Since the early 1990s, efforts have been underway in India and China to develop a temporary plug for the vas deferens, which could theoretically be removed or dissolved by an injection at a later date to provide reversibility. The Indian vas occlusion device is called RISUG for “reversible inhibition of sperm under guidance.” Using ultrasound guidance, sterile styrene maleic anhydrate is instilled into the vas bilaterally occluding it and preventing the passage of sperm. Several small clinical trials in men have been performed using this technique (79, 80), showing excellent contraceptive efficacy over periods of up to one year. The procedure has been shown to be reversible in some animal models (81) but data on efficacy and reversibility from large-scale clinical trials are not available (82). Similar vas occlusion devices using medical-grade silicone and polyurethane plugs were studied in China (83, 84). Unfortunately, both compounds had problems with time to sperm suppression and recovery of sperm counts after reversal, leading these investigators to abandon this approach.
Conclusions
Contraception is essential for the prevention of unintended pregnancy. Approximately 30% of couples currently rely on male contraceptive methods, specifically condoms and vasectomy. Shortcomings of these methods have led to efforts to develop new types of male contraceptives. Hormonal-based male contraceptive regimens have undergone extensive clinical testing, but suffer from incomplete suppression of spermatogenesis in all men and unknown long-term side effect profiles. Non-hormonal methods in development appear promising in pre-clinical studies, but extensive testing of these approaches will be required before human studies can be performed to determine their efficacy for the prevention of unintended pregnancy. Interest in the research community for these efforts is high as demonstrated by the recent creation of an International Consortium for Male Contraception to promote this work.
Acknowledgments
This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, a division of the National Institute of Health through cooperative agreement U54 HD04245 and K24 HD082231 (JKA).
Footnotes
Disclosure Statement: The author has received research funding from Clarus Therapeutics
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Heller CG, Clermont Y. Kinetics of the germinal epithelium in man. Recent Prog Horm Res. 1964;20:545–71. [PubMed] [Google Scholar]
- 2.DeKretser DM. Morphology and physiology of the testis. In: Becker KL, editor. Principles and Practice of Endocrinology and Metabolism. 2. Philadelphia: JB Lippincott; 1995. p. 1032. [Google Scholar]
- 3.Silber SJ, Ord T, Balmaceda J, Patrizio P, Asch RH. Congenital absence of the vas deferensl. The fertilizing capacity of human epididymal sperm. N Engl J Med. 1990;323:1788–92. doi: 10.1056/NEJM199012273232602. [DOI] [PubMed] [Google Scholar]
- 4.Matsumoto AM. Testosterone administration in older men. Endocrinol Metab Clin North Am. 2013;42:271–86. doi: 10.1016/j.ecl.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 5.Roth MY, Page ST, Lin K, et al. Dose-dependent increase in intratesticular testosterone by very low-dose human chorionic gonadotropin in normal men with experimental gonadotropin deficiency. J Clin Endocrinol Metab. 2010;95:3806–13. doi: 10.1210/jc.2010-0360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haws JM, Morgan GT, Pollack AE, et al. Clinical aspects of vasectomies performed in the United States in 1995. Urology. 1998;52:685–91. doi: 10.1016/s0090-4295(98)00274-x. [DOI] [PubMed] [Google Scholar]
- 7.Schmidt SS. Vasectomy by section, luminal fulguration and fascial interposition: Results from 6248 cases. Br J Urol. 1995;76:373–4. [PubMed] [Google Scholar]
- 8.Philp T, Guillebaud J, Budd D. Complications of vasectomy: review of 16,000 patients. Br J Urol. 1984;56:745–8. doi: 10.1111/j.1464-410x.1984.tb06161.x. [DOI] [PubMed] [Google Scholar]
- 9.Li S-Q, Goltein M, Shu J, Huber D. The no-scalpel vasectomy. J Urol. 1991;145:341–4. doi: 10.1016/s0022-5347(17)38334-9. [DOI] [PubMed] [Google Scholar]
- 10.Nirapathpongporn A, Huber DJ, Krieger JN. No scalpel vasectomy at the King’s birthday vasectomy festival. Lancet. 1990;335:894–5. doi: 10.1016/0140-6736(90)90487-p. [DOI] [PubMed] [Google Scholar]
- 11.Skriver M, Skovsgaard F, Miskowiak J. Conventional or Li vasectomy: A questionnaire study. Br J Urol. 1997;79:596–8. doi: 10.1046/j.1464-410x.1997.00390.x. [DOI] [PubMed] [Google Scholar]
- 12.McMahon AJ, Buckley J, Taylor A, et al. Chronic testicular pain following vasectomy. Br J Urol. 61992(9):188–90. doi: 10.1111/j.1464-410x.1992.tb15494.x. [DOI] [PubMed] [Google Scholar]
- 13.Myers SA, Mershon CE, Fuchs EF. Vasectomy reversal for treatment of the post-vasectomy pain syndrome. J Urol. 1997;157:518–20. [PubMed] [Google Scholar]
- 14.Jequier AM. Vasectomy related infertility: A major and costly medical problem. Hum Reprod. 1998;13:1757–9. doi: 10.1093/humrep/13.7.1757. [DOI] [PubMed] [Google Scholar]
- 15.Belker AM, Thomas AJ, Fuchs EF, et al. Results of 1,469 microsurgical vasectomy reversals by the Vasovasostomy Study Group. J Urol. 1991;145:505–11. doi: 10.1016/s0022-5347(17)38381-7. [DOI] [PubMed] [Google Scholar]
- 16.Heidenreich A, Bonfig R, Wilbert DM, et al. Risk factors for antisperm antibodies in infertile men. Am J Reprod Immunol. 1994;31:69–76. doi: 10.1111/j.1600-0897.1994.tb00849.x. [DOI] [PubMed] [Google Scholar]
- 17.Peterson HB, Howards SS. Vasectomy and prostate cancer: The evidence to date. Fertil Steril. 1998;70:201–203. doi: 10.1016/s0015-0282(98)00139-3. [DOI] [PubMed] [Google Scholar]
- 18.Manson JE, Ridker PM, Spelsberg A, Ajani U, Lotufo PA, Hennekens CH. Vasectomy and subsequent cardiovascular disease in US physicians. Contraception. 1999;59:181–6. doi: 10.1016/s0010-7824(99)00020-7. [DOI] [PubMed] [Google Scholar]
- 19.D’Anna LH, Korosteleva O, Warner L, Douglas J, Paul S, Metcalf C, McIlvaine E, Malotte CK. Factors associated with condom use problems during vaginal sex with main and non-main partners. Sex Transm Dis. 2012;39:687–9. doi: 10.1097/OLQ.0b013e31825ef325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fu H, Darroch JE, Haas T, et al. Contraceptive failure rates: New estimates from the 1995 National Survey of Family Growth. Fam Plann Perspect. 1999;31:56–84. [PubMed] [Google Scholar]
- 21.Martinez GM, Chandra A, Abma JC, Jones J, Mosher WD. Fertility, contraception, and fatherhood: data on men and women from cycle 6 (2002) of the 2002 National Survey of Family Growth. Vital Health Stat 23. 2006;26:1–142. [PubMed] [Google Scholar]
- 22.Newby KV, Brown KE, French DP, Wallace LM. Which outcome expectancies are important in determining young adults’ intentions to use condoms with casual sexual partners?: a cross-sectional study. BMC Public Health. 2013;13:133–141. doi: 10.1186/1471-2458-13-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levy DA, Khouader S, Leynadier F. Allergy to latex condoms. Allergy. 1998;53:110–112. doi: 10.1111/j.1398-9995.1998.tb03827.x. [DOI] [PubMed] [Google Scholar]
- 24.Steiner MJ, Dominik R, Rountree RW, et al. Contraceptive effectiveness of a polyurethane condom and a latex condom: a randomized controlled trial. Obstet Gynecol. 2003;101:539–47. doi: 10.1016/s0029-7844(02)02732-1. [DOI] [PubMed] [Google Scholar]
- 25.Walsh TL1, Frezieres RG, Peacock K, et al. Evaluation of the efficacy of a nonlatex condom: results from a randomized, controlled clinical trial. Perspect Sex Repro Health. 2003;35:79–86. [PubMed] [Google Scholar]
- 26.Gallo MF, Grimes DA, Lopez LM, Schulz KF. Non-latex versus latex male condoms for contraception. Cochrane Database Syst Rev. 2006;1:CD003550. doi: 10.1002/14651858.CD003550.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu PY, Swerdloff RS, Christenson PD, Handelsman DJ, Wang C. Rate, extent, and modifiers of spermatogenic recovery after hormonal male contraception: an integrated analysis. Lancet. 2006;367:1412–20. doi: 10.1016/S0140-6736(06)68614-5. [DOI] [PubMed] [Google Scholar]
- 28.Martin CW, Anderson RA, Cheng L, et al. Potential impact of hormonal male contraception: Cross-cultural implications for development of novel preparations. Hum Reprod. 2000;15:637–640. doi: 10.1093/humrep/15.3.637. [DOI] [PubMed] [Google Scholar]
- 29.Glasier AF, Anakwe R, Everington D, et al. Would women trust their partners to use a male pill? Hum Reprod. 2000;15:646–9. doi: 10.1093/humrep/15.3.646. [DOI] [PubMed] [Google Scholar]
- 30.World Health Organization Task Force on Methods for the Regulation of Male Fertility. Contraceptive efficacy of induced azoospermia and oligozoospermia in normal men. Fertil Steril. 1996;65:821–829. [PubMed] [Google Scholar]
- 31.World Health Organization. Contraceptive efficacy of testosterone-induced azoospermia in normal men. Lancet. 1990;336:995–1002. [PubMed] [Google Scholar]
- 32.Aaltonen P, Amory JK, Anderson R, et al. 10th Summit Meeting consensus: recommendations for regulatory approval for hormonal male contraception. J Androl. 2007;28:362–3. doi: 10.2164/jandrol.106.002311. [DOI] [PubMed] [Google Scholar]
- 33.Bagatell CJ, Heiman JR, Matsumoto AM, et al. Metabolic and behavioral effects of high-dose, exogenous testosterone in healthy men. J Clin Endocrinol Metab. 1994;79:561–6. doi: 10.1210/jcem.79.2.8045977. [DOI] [PubMed] [Google Scholar]
- 34.Meriggiola MC, Marcovina S, Paulsen CA, et al. Testosterone enanthate at the dose 200 mg/week decreases HDL cholesterol levels in healthy men. Int J Androl. 1995;18:237–42. [PubMed] [Google Scholar]
- 35.Zhang GY, Gu YQ, Wang XH, et al. A pharmacokinetic study of injectable testosterone undecanoate in hypogonadal men. J Androl. 1998;19:761–7. [PubMed] [Google Scholar]
- 36.Behre HM, Abshagen K, Oettel M, et al. Intramuscular injection of testosterone undecanoate for the treatment of male hypogonadism: Phase I studies. Eur J Endrocrinol. 1999;140:414–9. doi: 10.1530/eje.0.1400414. [DOI] [PubMed] [Google Scholar]
- 37.Nieschlag E, Buchter D, VonEckardstein S, et al. Repeated intramuscular injections of testosterone undecanoate for substitution therapy in hypogonadal men. Clin Endocrinol (Oxf) 1999;51:757–63. doi: 10.1046/j.1365-2265.1999.00881.x. [DOI] [PubMed] [Google Scholar]
- 38.Gu Y, Liang X, Wu W, et al. Multicenter contraceptive efficacy trial of injectable testosterone undecanoate in Chinese men. J Clin Endocrinol Metab. 2009;94:1901–1915. doi: 10.1210/jc.2008-1846. [DOI] [PubMed] [Google Scholar]
- 39.Meriggiola MC, Bremner WJ. Progestin-androgen combination regimens for male contraception. J Androl. 1997;18:240–4. [PubMed] [Google Scholar]
- 40.Barfield A, Melo J, Coutinho E, et al. Pregnancies associated with sperm concentrations below 10 million/ml in clinical studies of a potential male contraceptive method, monthly depot medroxyprogesterone acetate and testosterone esters. Contraception. 1979;20:121–7. doi: 10.1016/0010-7824(79)90084-2. [DOI] [PubMed] [Google Scholar]
- 41.Bebb RA, Anawalt BD, Christensen RB, et al. Combined administration of levonorgestrel and testosterone induces more rapid and effective suppression of spermatogenesis than testosterone alone: A promising male contraceptive approach. J Clin Endocrinol Metab. 1996;81:757–62. doi: 10.1210/jcem.81.2.8636300. [DOI] [PubMed] [Google Scholar]
- 42.Wu FC, Balasubramanian R, Mulders TIM, et al. Oral progestogen combined with testosterone as a potential male contraceptive: Additive effects between desogestrel and testosterone enanthate in suppression of spermatogenesis, pituitary-testicular axis, and lipid metabolism. J Clin Endocrinol Metab. 1999;84:112–22. doi: 10.1210/jcem.84.1.5412. [DOI] [PubMed] [Google Scholar]
- 43.Brady BM, Amory JK, Perheentupa A, Zitzmann M, Hay CJ, Apter D, Anderson RA, Bremner WJ, Pollanen P, Nieschlag E, Wu FC, Kersemaekers WM. A multicentre study investigating subcutaneous etonogestrel implants with injectable testosterone decanoate as a potential long-acting male contraceptive. Hum Reprod. 2006;21:285–94. doi: 10.1093/humrep/dei300. [DOI] [PubMed] [Google Scholar]
- 44.Mommers E, Kersemaekers WM, Elliesen J, et al. Male hormonal contraception: a double-blind, placebo-controlled study. J Clin Endocrinol Metab. 2008;93:2572–80. doi: 10.1210/jc.2008-0265. [DOI] [PubMed] [Google Scholar]
- 45.Turner L, Conway AJ, Jimenez M, Liu PY, Forbes E, McLachlan RI, Handelsman DJ. Contraceptive efficacy of a depot progestin and androgen combination in men. J Clin Endocrinol Metab. 2003;88:4659–67. doi: 10.1210/jc.2003-030107. [DOI] [PubMed] [Google Scholar]
- 46.Meriggiola MC, Bremner WJ, Paulsen CA, et al. A combined regimen of cyproterone acetate and testosterone enanthate as a potentially highly effective male contraceptive. J Clin Endocrinol Metab. 1996;81:3018–3023. doi: 10.1210/jcem.81.8.8768868. [DOI] [PubMed] [Google Scholar]
- 47.Meriggiola MC, Bremner WJ, Costantino A, Di Cintio G, Flamigni C. Low dose of cyproterone acetate and testosterone enanthate for contraception in men. Hum Reprod. 1998;13:1225–1229. doi: 10.1093/humrep/13.5.1225. [DOI] [PubMed] [Google Scholar]
- 48.Kumar N, Koide SS, Tsong Y, Sundaram K. Nestorone: a progestin with a unique pharmacological profile. Steroids. 2000;65:629–636. doi: 10.1016/s0039-128x(00)00119-7. [DOI] [PubMed] [Google Scholar]
- 49.Mahabadi V, Amory JK, Swerdloff RS, et al. Combined transdermal testosterone gel and the progestin nestorone suppresses serum gonadotropins in men. J Clin Endocrinol Metab. 2009;94:2313–2320. doi: 10.1210/jc.2008-2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ilani N, Roth MY, Amory JK, et al. A new combination of testosterone and nestorone transdermal gels for male hormonal contraception. J Clin Endocrinol Metab. 2012;97:3476–3486. doi: 10.1210/jc.2012-1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Roth MY, Shih G, Ilani N, et al. Acceptability of a transdermal gel-based male hormonal contraceptive in a randomized controlled trial. Contraception. 2014;90:407–412. doi: 10.1016/j.contraception.2014.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Attardi BJ, Hild SA, Reel JR. Dimethandrolone undecanoate: a new potent orally active androgen with progestational activity. Endocrinology. 2006;147:3016–3026. doi: 10.1210/en.2005-1524. [DOI] [PubMed] [Google Scholar]
- 53.Hild SA, Attardi BJ, Koduri S, et al. Development of Dimethandrolone 17B-Undecanoate (DMAU) as an Oral Male Hormonal Contraceptive: Induction of Infertility and Recovery of Fertility in Adult Male Rabbits. J Androl. 2011;32:530–40. doi: 10.2164/jandrol.110.011817. [DOI] [PubMed] [Google Scholar]
- 54.Attardi BJ, Marck BT, Matsumoto AM, Koduri S, Hild SA. Long-term effects of dimethandrolone 17β-undecanoate and 11β-methyl-19-nortestosterone 17β-dodecylcarbonate on body composition, bone mineral density, serum gonadotropins, and androgenic/anabolic activity in castrated male rats. J Androl. 2011;32:183–192. doi: 10.2164/jandrol.110.010371. [DOI] [PubMed] [Google Scholar]
- 55.Surampudi P, Page ST, Swerdloff RS, et al. Single, escalating dose pharmacokinetics, safety and food effects of a new oral androgen dimethandrolone undecanoate in man: a prototype oral male hormonal contraceptive. Andrology. 2014;2:579–587. doi: 10.1111/j.2047-2927.2014.00216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mruk DD, Cheng CY. Testin and actin are key molecular targets of adjudin, an anti-spermatogenic agent, in the testes. Spermatogenesis. 2011;1:137–46. doi: 10.4161/spmg.1.2.16449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mok K-W, Mruk DD, Lie PPY, Lui W-Y, Cheng CY. Adjudin, a potential male contraceptive, exerts its effects locally in the seminiferous epithelium of mammalian testes. Reproduction. 2011;141:571–80. doi: 10.1530/REP-10-0464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mruk DD, Wong CH, Silvestrini B, Cheng CY. A male contraceptive targeting germ cell adhesion. Nature Medicine. 2006;12:1323–8. doi: 10.1038/nm1420. [DOI] [PubMed] [Google Scholar]
- 59.Tash JS, Attardi B, Hild SA, Chakrasali R, Jakkaraj SR, Georg GI. A novel potent indazole carboxylic acid derivative blocks spermatogenesis and is contraceptive in rats after a single oral dose. Biol Reprod. 2008;78:1127–38. doi: 10.1095/biolreprod.106.057810. [DOI] [PubMed] [Google Scholar]
- 60.Tash JS, Chakrasali R, Jakkaraj SR, et al. Gamendazole, an orally active indazole carboxylic acid male contraceptive agent, targets HSP90AB1 and EEF1A1, and stimluates II1a transcription in rat Sertoli cells. Biol Reproduction. 2008;78:1139–1152. doi: 10.1095/biolreprod.107.062679. [DOI] [PubMed] [Google Scholar]
- 61.O’rand MG, Widgren EE, Sivashanmugam P, et al. Reversible immunocontraception in male monkeys immunized with Eppin. Science. 2004;306:1189–90. doi: 10.1126/science.1099743. [DOI] [PubMed] [Google Scholar]
- 62.Matzuk MM, McKeown MR, Filippakopoulos P, et al. Small-molecule inhibition of BRDT for male contraception. Cell. 2012;150:673–684. doi: 10.1016/j.cell.2012.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wolbach SB, Howe PR. Tissue changes following deprivation of fat soluble A Vitamin. J Exp Med. 1925;42:753–777. doi: 10.1084/jem.42.6.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vernet N, Dennefeld C, Rochett-Egly C, Oulad-Abdelghani M, Chambon P, Ghyselinck NB, Mark M. Retinoic acid metabolism and signaling pathways in the adult and developing mouse testis. Endocrinology. 2006;147:96–110. doi: 10.1210/en.2005-0953. [DOI] [PubMed] [Google Scholar]
- 65.Koubova J, Menke D, Zhou Q, Capel B, Griswold MD, Page DC. Retinoic acid regulates sex-specific timing of meiotic initiation in mice. Proc Natl Acad Sci USA. 2006;103:2472–2479. doi: 10.1073/pnas.0510813103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dufour JM, Kim KH. Cellular and subcellular localization of six retinoid receptors in rat testis during postnatal devlopment: identification of potential heterimeric receptors. Biol Reprod. 1999;61:1300–1308. doi: 10.1095/biolreprod61.5.1300. [DOI] [PubMed] [Google Scholar]
- 67.Lufkin T, Lohnes D, Mark M, Dierich A, Gorry P, Gaub MP, LeMeur M, Chambon P. High postnatal lethality and testis degeneration in retinoic acid receptor alpha mutant mice. Proc Natl Acad Sci USA. 1993;90:7225–7229. doi: 10.1073/pnas.90.15.7225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lohnes D, Kastner P, Dierich A, Mark M, LeMeur M, Chambon P. Function of retinoic acid receptor gamma in the mouse. Cell. 1993;73:643–658. doi: 10.1016/0092-8674(93)90246-m. [DOI] [PubMed] [Google Scholar]
- 69.Kastner P, Mark M, Leid M, Gansmuller A, Chin W, Grondona JM, Décimo D, Krezel W, Dierich A, Chambon P. Abnormal spermatogenesis in RXR beta mutant mice. Genes Dev. 1996;10:80–92. doi: 10.1101/gad.10.1.80. [DOI] [PubMed] [Google Scholar]
- 70.Schulze GE, Clay RJ, Mezza LE, Bregman CL, Buroker RA, Frantz JD. BMS-189453, a novel retinoid receptor antagonist, is a potent testicular toxin. Toxicol Sci. 2001;59:297–308. doi: 10.1093/toxsci/59.2.297. [DOI] [PubMed] [Google Scholar]
- 71.Chung SS, Wang X, Roberts SS, Griffey SM, Reczek PR, Wolgemuth DJ. Oral administration of a retinoic Acid receptor antagonist reversibly inhibits spermatogenesis in mice. Endocrinology. 2011;152:2492–502. doi: 10.1210/en.2010-0941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chung SS, Sung W, Wang X, Wolgemuth DJ. Retinoic acid receptor alpha is required for synchronization of spermatogenic cycles and its absence results in progressive breakdown of the spermatogenic process. Dev Dyn. 2004;230:754–66. doi: 10.1002/dvdy.20083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chung SS, Wang X, Wolgemuth DJ. Male sterility in mice lacking retinoic acid receptor alpha involves specific abnormalities in spermiogenesis. Differentiation. 2005;73:188–198. doi: 10.1111/j.1432-0436.2005.00018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Heller CG, Moore DJ, Paulsen CA. Suppression of spermatogenesis and chronic toxicity in men by a new series of Bis (dichloroacetyl) Diamines. Toxicol Appl Pharmacol. 1961;3:1–11. doi: 10.1016/0041-008x(61)90002-3. [DOI] [PubMed] [Google Scholar]
- 75.Coulston F, Beyler AL, Drobeck HP. The biologic actions of a new series of bis(dichloroacetyl) diamines. Toxicol Appl Pharmacol. 1960;2:715–721. doi: 10.1016/0041-008x(60)90088-0. [DOI] [PubMed] [Google Scholar]
- 76.Beyler AL, Potts GO, Coulston F, Surrey AR. The selective testicular effects of certain bis-(dichloroacetyl) diamines. Endocrinology. 1961;69:819–833. [Google Scholar]
- 77.Amory JK, Muller CH, Shimshoni AJ, Isoherranen N, Paik J, Moreb JS, Amory DW, Evanoff R, Goldstein AS, Griswold MD. Suppression of spermatogenesis by bisdichloroacetyldiamines is mediated by inhibition of testicular retinoic acid biosynthesis. J Androl. 2011;32:111–119. doi: 10.2164/jandrol.110.010751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Paik J, Haenisch M, Muller CH, et al. Inhibition of retinoic acid biosynthesis by the bisdichloroacetyldiamine WIN 18,446 markedly suppresses spermatogenesis and alters retinoid metabolism in mice. J Biol Chem. 2014;289:15104–117. doi: 10.1074/jbc.M113.540211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Guha SK, Singh G, Anand S, et al. Phase I clinical trial of an injectable contraceptive for the male. Contraception. 1993;48:367–375. doi: 10.1016/0010-7824(93)90082-i. [DOI] [PubMed] [Google Scholar]
- 80.Guha SK, Singh G, Ansari S, et al. Phase II clinical trial of a vas deferens injectable contraceptive for the male. Contraception. 1997;56:245–250. doi: 10.1016/s0010-7824(97)00142-x. [DOI] [PubMed] [Google Scholar]
- 81.Waller D, Bolick D, Lissner E, Premanandan C, Gamerman C. Azoospermia in the rabbit following intravasal injection of Vasalgel. Basic Clin Androl. 2016;26:6. doi: 10.1186/s12610-016-0033-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lohiya NK, Alam I, Hussain M, Khan SR, Ansari AS. RISUG: an intravasal injectable male contraceptive. Indian J Med Res. 2014;140(Suppl):S63–72. [PMC free article] [PubMed] [Google Scholar]
- 83.Zhao SC, Zhang SP, Yu RC. Intravasal injection of formed-in-place silicone rubber as a method of vas occlusion. Int J Androl. 1992;15:460–464. doi: 10.1111/j.1365-2605.1992.tb01138.x. [DOI] [PubMed] [Google Scholar]
- 84.Zhao SC, Lian YH, Yu RC, Zhang SP. Recovery of fertility after removal of polyurethane plugs from the human vas deferens occluded for up to 5 years. Int J Androl. 1992;15:465–7. doi: 10.1111/j.1365-2605.1992.tb01139.x. [DOI] [PubMed] [Google Scholar]