Abstract
The ability of hemoglobin to scavenge the potent vasodilator nitric oxide in the blood has been well established as a mechanism of vascular tone homeostasis. In endothelial cells, the alpha chain of hemoglobin (hereafter, alpha globin) and endothelial nitric oxide synthase form a macromolecular complex, providing a sink for nitric oxide directly adjacent to the production source. We have developed an alpha globin mimetic peptide (named HbαX) that displaces endogenous alpha globin and increases bioavailable nitric oxide for vasodilation. Here we show that, in vivo, HbαX administration increases capillary oxygenation and blood flow in arterioles acutely and produces a sustained decrease in systolic blood pressure in normal and angiotensin II-induced hypertensive states. HbαX acts with high specificity and affinity to endothelial nitric oxide synthase, without toxicity to liver, kidney, and no effect on p50 of O2 binding in red blood cells. In human vasculature, HbαX blunts vasoconstrictive response to cumulative doses of phenylephrine, a potent constricting agent. By binding to endothelial nitric oxide synthase and displacing endogenous alpha globin, HbαX modulates important metrics of vascular function, increasing vasodilation and flow in the resistance vasculature.
Keywords: alpha globin, endothelium, mimetic peptide, blood pressure, endothelial nitric oxide synthase
Introduction
Endothelial nitric oxide synthase (NOS3, eNOS) production of nitric oxide (NO) is a critical step in homeostatic regulation of vascular tone and function1, 2. NO is a potent vasodilatory gas, activating soluble guanylyl cyclase in smooth muscle cells to prevent constriction and decrease resistance in vessels from the aorta to arterioles. Intracellularly, eNOS is localized at a subcellular structure unique to the vasculature called the myoendothelial junction (MEJ), where endothelial cells project through the inner elastic lamina to directly contact the surround smooth muscle cells3. Localization of eNOS in the MEJ places signal generation (NO production) directly adjacent to the site of action (smooth muscle cells). Critically, many positive and negative regulators of eNOS are sequestered in the MEJ, including calmodulin and caveolin-1, respectively4–8.
Previously, we published a new paradigm for regulation of NO signaling: expression of the alpha subunit of hemoglobin (alpha globin) in endothelial cells is critical for NO scavenging effects in resistance vasculature9, 10. In the vasculature, alpha globin is preferentially expressed in MEJs where it can bind directly to eNOS9. The formation of a macromolecular complex with alpha globin and eNOS was verified by immunoprecipitation, proximity ligation assay, and immunofluorescence microscopy in both murine arteries and a vascular cell co-culture system9, 10. Since alpha globin is a potent scavenger of NO, we hypothesized that the binding of alpha globin to eNOS was critical for efficient scavenging. To disrupt the macromolecular complex, and thus increase NO availability, we designed a peptide mimicking a species-conserved sequence in alpha globin10. This peptide, termed HbαX, was shown in vivo and in vitro to have vasodilatory properties. The physiological response (blunted vasoconstriction in response to phenylephrine (PE), a norepinephrine analog) to HbαX is similar to alpha globin depletion (via siRNA knockdown) from endothelium of ex vivo murine arterioles9. The decreased constriction is sensitive to an eNOS inhibitor, L-nitroarginine methyl ester (L-NAME), implicating eNOS/NO as the major vasodilator in the absence of alpha globin. Without alpha globin, NO is not efficiently scavenged, increasing diffusion to smooth muscle cells and activation of dilatory pathways.
However, the pharmacologic properties of HbαX, its effect on blood flow, and its possible therapeutic effect in long-term hypertension treatment in humans is still not known. In this study, we measure hemodynamics including flow rate and oxygen saturation in murine resistance vasculature in response to HbαX administration, and show that the mechanism of action (binding of HbαX to eNOS to disrupt complex formation) produces a sustained decrease in blood pressure with little toxicity. Importantly, we demonstrate that this mechanism is translatable to human microvasculature.
Methods
Murine and human subjects declaration
All mouse procedures were performed in accordance with the University of Virginia’s Institutional Animal Care and Use Committee. Human studies were approved by a University of Virginia Institutional Review Board and conformed to the principles of the Declaration of Helsinki and U.S. Federal Regulations.
Photoacoustic microscopy
An established multi-parametric photoacoustic microscopy (PAM) system11, 12 was utilized to acquire oxygen saturation of hemoglobin (sO2) and blood flow speed at the microscopic level in vivo. SKH1-Hrhr- hairless mice were used in this study (n=3). Throughout the experiment, mice were anesthetized with 1.5% vaporized isoflurane, and body temperature was maintained using a heating pad. The ear to be imaged was gently pressed against the base of a water tank heated to 37°C. A bolus (20 mg/kg) of HbαX was administered intraperitoneally without moving the mouse. A 1 mm × 2 mm field of view was imaged prior to the injection and at 5, 60, and 120 minutes post-injection (see Supplementary Figure S1). Quantification of the sO2 and flow in the arteriole and venule of the mice was performed as described previously12.
Blood pressure measurement
Radiotelemetry blood pressure measurement and Angiotensin II (AngII) osmotic mini-pump insertion (Alzet, model #2006) were performed as described10, 13. Briefly, the pumps were loaded with AngII with a constant infusion rate of 1000 ng/kg/min, and a 6 week maximum life span as noted by the manufacturer. Injections of saline or peptides (5 mg/kg) were performed every other day between 16:00–19:00 via IP. Control peptide was a scrambled sequence of HbαX, (FPYFSTKLTT)10. Mice were implanted with radiotelemetry units [Data Sciences International (DSI)]. Briefly, while under isoflurane anesthesia, the catheter of the radiotelemetry unit was placed in the left carotid artery and the radiotransmitter was placed in a subcutaneous pouch on the right flank. Blood pressure was recorded for 5 minutes/hour/day for 70 days.
Monitoring of bone marrow, liver, and kidney toxicities
Submandibular punctures were performed every 15 days and approximately 200 μL of blood was obtained for measurement of liver and kidney function by the University of Virginia blood pathology lab.
Lucigenin assay for measuring superoxide production in tissues
Measurement of tissue superoxide level was performed as previously described14. Briefly, approximately 5 mg of tissue was added to oxygenated KREBS-HEPES buffer (KREBS-HENSELEIT buffer (Sigma, K3753) plus 25 mmol/L NaHCO3, 20 mmol/L Sodium HEPES, 2.5 mmol/L CaCl2, pH= 7.2–7.4) and incubated at 37°C for 30 minutes. Oxygenated buffer and lucigenin reagent (N,N′-Dimethyl-9,9′-biacridinium dinitrate, Sigma, M8010) were added to the wells of a microplate for final lucigenin concentration of 5 μmol/L in the well. After baseline readings, tissue samples were added to wells and luminescence values were recorded. Tissue was then dried at 60–70°C overnight. Values are reported as counts/mg dry tissue.
Co-immunoprecipitation of eNOS and HbαX peptide
An eNOS/HbαX complex was visualized by immunoprecipitation and Western blotting. A full description of this protocol is available in the supplemental methods.
Red blood cell oxygen
Measurement of red blood cell oxygenation was performed as described previously15. Blood was collected from n=3 healthy human volunteers and each sample was tested in each treatment condition.
Recombinant protein expression and purification
Protocol for eNOS protein isolation adapted from Fischmann et al.16. See supplemental methods for a full description.
Fluorescence polarization binding assays
FITC-labeled HbαX was dissolved in a small amount of water and diluted to 5 nmol/L in 5 mL of the above purification buffer. Recombinant eNOS is titrated into the wells of a 96-well plate from a maximum of 1 μmol/L. A full description of the method is available in the supplement.
Far Western blotting
Far Western blotting followed the protocol of Wu, Li, and Chen17. However, our biotin-HbαX peptide was incubated directly on the membrane (in lieu of a target protein antibody) in combination with an anti-eNOS primary antibody. Secondary antibodies were used as described above for detection.
Human ex vivo vasoreactivity
Under IRB-HSR Study 17194, human volunteers with hypertension (elevated blood pressure above 140 mm Hg systolic) had an approximate 40 mm x 20 mm biopsy of adipose tissue removed from the abdomen, which was immediately placed in ice-cold KREBS buffer, and arterioles dissected manually within 30 minutes of removal. The arterioles (approximately 50–90 μm) were cannulated, pressurized, and subject to phenylephrine cumulative dose–response curves as extensively described by us10, 18, 19.
Statistical analyses
All results are presented as mean ± SEM. Statistical analysis was performed by one-way ANOVA unless otherwise noted in the figure legends. P-values < 0.05 were considered significant and are denoted by *.
Results
An alpha globin mimetic peptide increases flow in resistance arteries
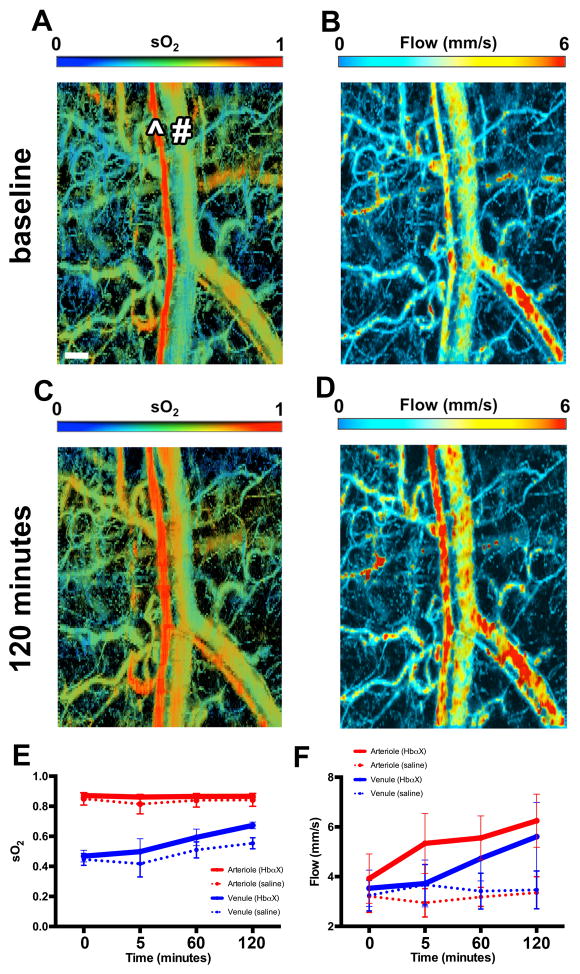
Previously10 we have shown that HbαX blunts vascular constriction to PE and decreases murine mean arterial pressure acutely. However, we were unable to determine if the peptide had a corresponding effect on blood flow. Using photoacoustic microscopy (PAM), we investigated hemoglobin concentration oxygen saturation (sO2), and blood flow (Figure 1) in the ear microvascular bed of SKH1 hairless mice. The abnormal strain and imaging location was used so that high-resolution images and precision measurement of these hemodynamic parameters in individual vessel segments could be obtained. Although there is an increase in sO2 in the venule after HbαX injection, we could not detect a change in arteriole sO2 (Figure 1E). However, as a result of HbαX injection, we could detect increased blood flow in both arteriole and venule (Figure 1F). Additional images at different time points are available in the online data supplement (Supplement Figure S1).
Figure 1. Acute increased O2 saturation and blood flow in microvasculature with single injection of HbαX in vivo.
(A–D) Photoacoustic microscopy images of O2 saturation (sO2) and blood flow from microvasculature of murine ear at baseline (A–B) and 120 minutes post HbαX injection (C–D). All images are from the same portion of the ear. The ^ symbol denotes an arteriole and # denotes a venule. Qualitatively, sO2 increases after HbαX injection shown by warmer colors in the regions of capillary beds. Blood flow is shown in both arterioles and venules with red coloring denoting regions of highest flow. Quantification of sO2 (E) and average flow velocity (F) from mice receiving HbαX injection (solid lines, n=3) and saline injection (dotted lines, n=3); red denotes arteriolar value, blue denotes venular. Scale bar in A is 100 μm.
An alpha globin mimetic peptide can sustain low blood pressure
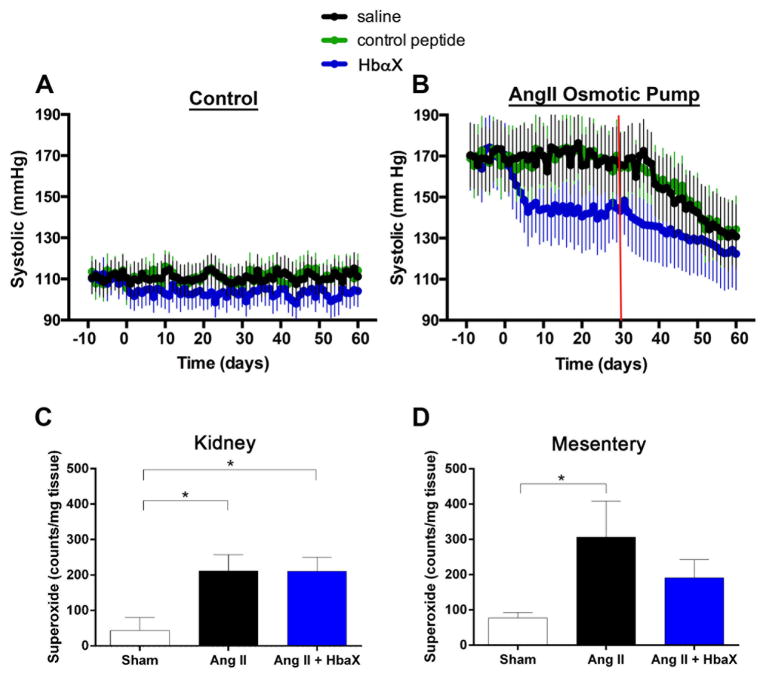
Although the summed acute effects of an alpha globin mimetic appear to indicate a possible effect to lower blood pressure, its long-term effect on blood pressure in a chronic model of hypertension had not been determined. For this reason, we measured blood pressure over time when mice were injected every other day with 5 mg/kg HbαX. During baseline condition, (Figure 2A), mice were normotensive, but injections of HbαX decreased blood pressure after two days. This shows that HbαX has an effect in non-pathological situations. During AngII-induced hypertension, the blood pressure lowering effects of HbαX are more pronounced. The mice had AngII-induced hypertension for ten days prior to injection of HbαX. As shown in Figure 2B, chronic AngII administration resulted in hypertension, with increase systolic blood pressure from a baseline of 110 mmHg to ~170 mmHg. Injection of HbαX resulted in a significant decrease of systolic blood pressure by ~30 mmHg. It has been proposed that prolonged AngII exposure can cause superoxide (O2•−) production via eNOS dimer uncoupling, thus contributing to hypertension by causing endothelial dysfunction20. In a separate group of mice, AngII was delivered via osmotic mini-pumps at 1000 ng/kg/min and HbαX or saline was injected as described above. Since AngII induces superoxide generation in the kidney and vasculature, kidney and mesenteric tissues were obtained to determine superoxide levels using a lucigenin based chemiluminescence assay. Indeed, superoxide generation was increased in both kidney (Figure 2C) and mesenteric tissues (Figure 2D) with AngII administration compared to sham. HbαX attenuated superoxide generation in the mesenteric circulation, but not in whole kidney tissues.
Figure 2. Sustained decrease in systolic blood pressure with HbαX.
Either normotensive mice (A: N=4) or mice with osmotic pumps loaded with AngII (B: N=4) were injected every other day starting at day 0 with saline (black), a scrambled control peptide (green; 5 mg/kg) or HbαX (blue; 5 mg/kg) and systolic blood pressure via radiotelemetery was obtained daily. A baseline was measured for 10 days prior to the start of injections. Red line in B denotes approximate end of AngII infusion due osmotic pump lifetime. Superoxide production from kidney (C) and mesenteric (D) tissues was assayed from mice with AngII pumps and HbαX injections (n=3) or saline injections (n=3), or receiving sham surgery (n=4).
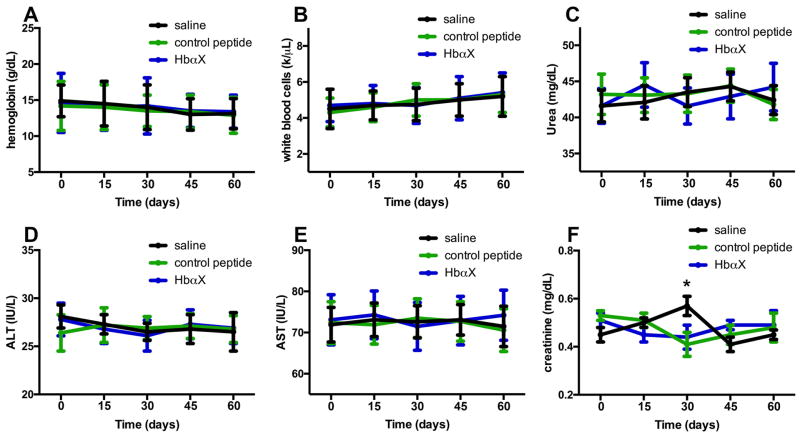
To test toxicity of an alpha globin peptide over such a sustained period of time, mouse blood was drawn every 15 days during the control blood pressure measurements in Figure 2A and several blood-based organ function parameters were measured. The level of hemoglobin (Figure 3A) and number of white blood cells (Figure 3B) were unchanged, suggesting that HbαX had no effect on bone marrow function. Next, we determined kidney and liver parameters and found no difference in blood urea nitrogen (BUN) (Figure 3C), serum creatinine (Figure 3F), alanine and aspartate aminotransferase enzymatic activity (ALT, Figure 3D, and AST, Figure 3E) between HbαX and control treated groups. The sum of these results demonstrate that the HbαX peptide itself is a potent modulator of long-term blood pressure and has no detectable kidney or liver toxicities in the 60 day period during which the peptide was administered.
Figure 3. Prolonged injection of the alpha globin mimetic peptide does not lead to murine toxicity.
Approximately 200 μL of blood was drawn via submandibular puncture every 15 days, starting prior to the first injections on day 0 of saline (black), control peptide (green) or HbαX (blue). The blood was subject to blood pathology laboratories at the University of Virginia to test for basic parameters that could indicate toxicity. These include a change in levels of hemoglobin (A), white blood cells in aggregate (B), urea (C), ALT (D), AST (E), and creatinine (F). Although some variability was observed, there was little significant difference using two-way ANOVA and an alpha level of either p<0.05 or p<0.10. N=3 mice per condition.
An alpha globin mimetic peptide and eNOS have strong affinity
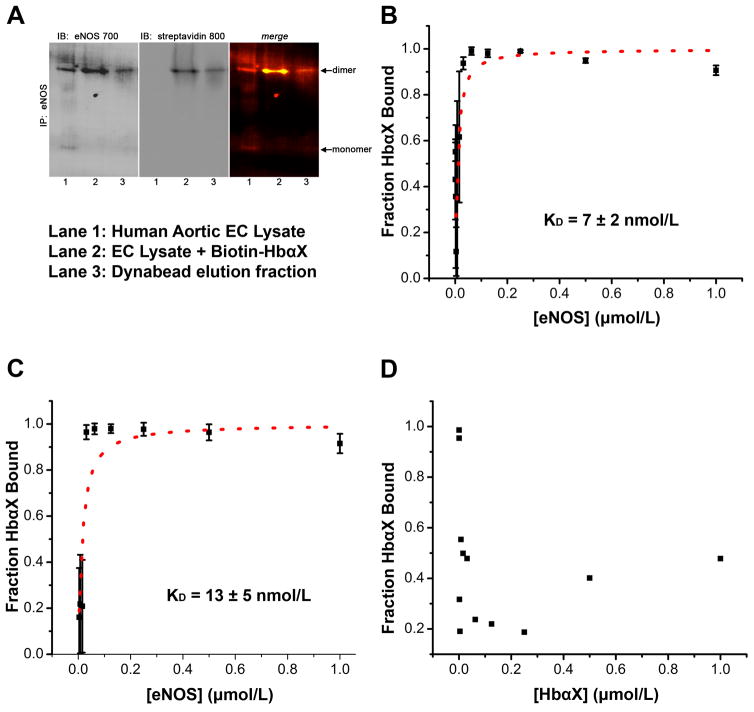
Because eNOS requires homodimerization for full activity, the ability of the peptide to bind eNOS dimers was assayed with non-denaturing gel electrophoresis. In a monolayer of cultured human aortic endothelial cells (hAoECs), the majority of eNOS exists as a dimeric species (Figure 4A, Left, lane 1). Incubating a biotin-tagged HbαX peptide allowed for immunodetection of the peptide with a streptavidin-conjugated antibody, as seen in Figure 4A, middle. The colocalization of the eNOS dimer and biotin bands (Figure 4A, right) shows that eNOS and HbαX are able to form a complex when eNOS is in its active form. In order to quantify HbαX/eNOS association, we show that the peptide also binds to eNOS in vitro using recombinant protein. Figure 4B shows the binding of HbαX to the oxygenase domain of eNOS (residues 64 – 492). Serial dilution of the larger protein eNOS (~50 kDa) from 1 μmol/L with a constant background of 5 nmol/L FITC-labeled HbαX (~1.5 kDa) allows us to look at the binding affinity using fluorescence polarization measurement on a microplate reader. A ligand depleted model was used to fit the data points to calculate a KD21 (red dashed line, Figure 4B and 4C). To confirm the binding affinity of 7 ± 2 nmol/L, the same assay was run with 2.5 nmol/L labeled and 2.5 nmol/L unlabeled HbαX. The competition for binding site is theoretically observed as a doubling of the binding affinity because only half of the ligand is observable using the wavelength for FITC fluorophore emission, and is seen experimentally as an increase to KD = 13 ± 5 nmol/L (Figure 4C). As a control, HbαX self-oligomerization was tested by serially diluting unlabeled HbαX from 1 μmol/L in a solution containing 5 nmol/L FITC-labeled HbαX. The lack of a hyperbolic curve in Figure 4D shows that there are no observable effects of peptide oligomerization in the sample.
Figure 4. An alpha globin mimetic peptide and eNOS dimers bind with high affinity.
(A) Co-immunoprecipitation of eNOS and biotin-labeled HbαX from hAoECs shows that HbαX binds to the dimeric form of eNOS. Non-denaturing IP and electrophoresis conditions were used to preserve the conformational state of eNOS. Lane 1 shows EC lysate as a control for eNOS species. Lane 2 is from beads incubated with EC Lysate and biotin-conjugated HbαΧ peptide. Lane 3 is sample eluted from dynabeads with low pH. (B) Fluorescence polarization assays show nanomolar binding affinity between fluorescein isothiocyanate (FITC) – labeled HbαX and the oxygenase domain of eNOS. The concentration of FITC-HbαX is 5 nmol/L in (B). The total concentration of peptide in (C) was held constant, but only half (2.5 nmol/L) was fluorescently tagged. This effectively will double the observed binding affinity because of the 1:1 competition for binding site. (D) shows no curve because no binding was observed between HbαX peptides. The concentration of labeled peptide was held constant (5 nmol/L) and was used to serially dilute unlabeled HbαX. In B–D, the points represent mean and bars indicate standard deviation, samples were run in triplicate in one experimental preparation.
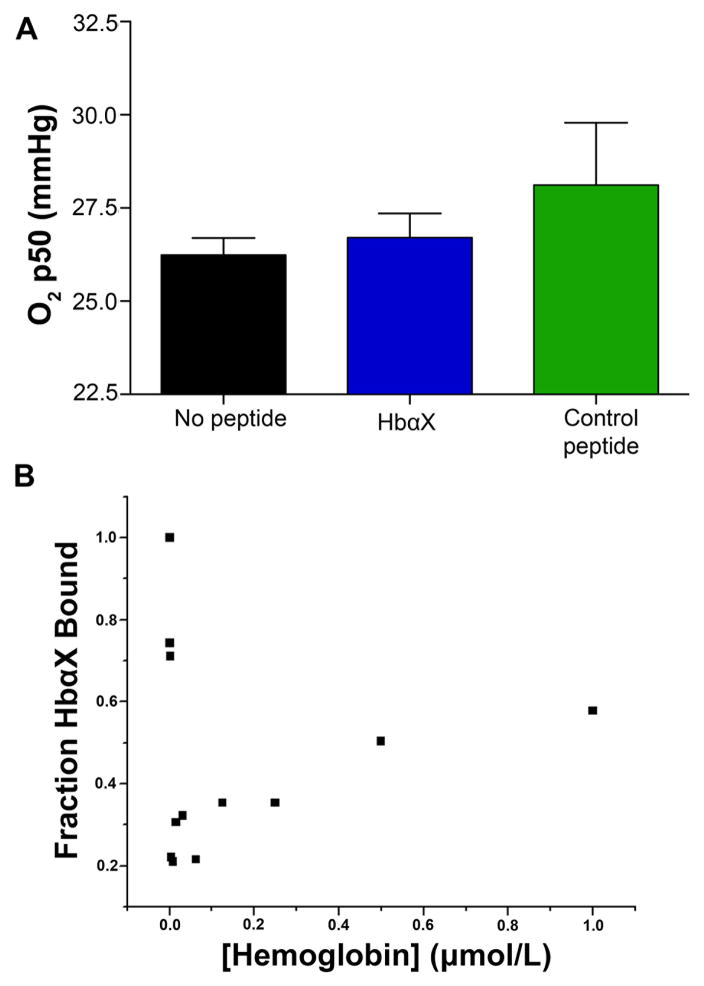
Another test of the specificity of HbαX for eNOS is to determine if there are possible effects on the other component of the endothelial NO regulatory system, hemoglobin. Because HbαX is distributed through the circulation, we investigated the effects of the peptide on hemoglobin in red blood cells (RBCs) to confirm that there is no disruption of O2 carrying ability. To show that HbαX was not inducing hypoxia and initiating a vasodilatory signaling cascade upstream of eNOS, we checked the partial pressure of oxygen needed for 50% saturation of hemoglobin. Using freshly isolated human blood, the addition of HbαX or a control peptide did not significantly change the oxygen p50 of hemoglobin in RBCs (Figure 5A). To verify the effect of HbαX in endothelium, bovine hemoglobin was serially diluted in a constant 5 nmol/L FITC-HbαX solution and fluorescence polarization was measured. The lack of a hyperbolic curve in Figure 5B shows that there is no binding of HbαX to hemoglobin in vitro. Taken together, these results show that HbαX is predominantly binding to eNOS, and we propose that this binding is resulting in increased NO bioavailability due to reduced scavenging by alpha globin.
Figure 5. An alpha globin mimetic peptide does not alter the O2 binding parameters of hemoglobin.
(A) The oxygen p50 of whole human blood was measured and was found not to be altered by incubation for 5 minutes with HbαX (blue) or a scrambled control peptide (green) (n=3 experiments for each condition). (B) Fluorescence polarization of 5 nmol/L FITC-labeled HbαX incubated with bovine hemoglobin shows no concentration-dependent binding of the molecules due to the lack of a hyperbolic shape.
Effects of the alpha globin mimetic peptide in human arterioles
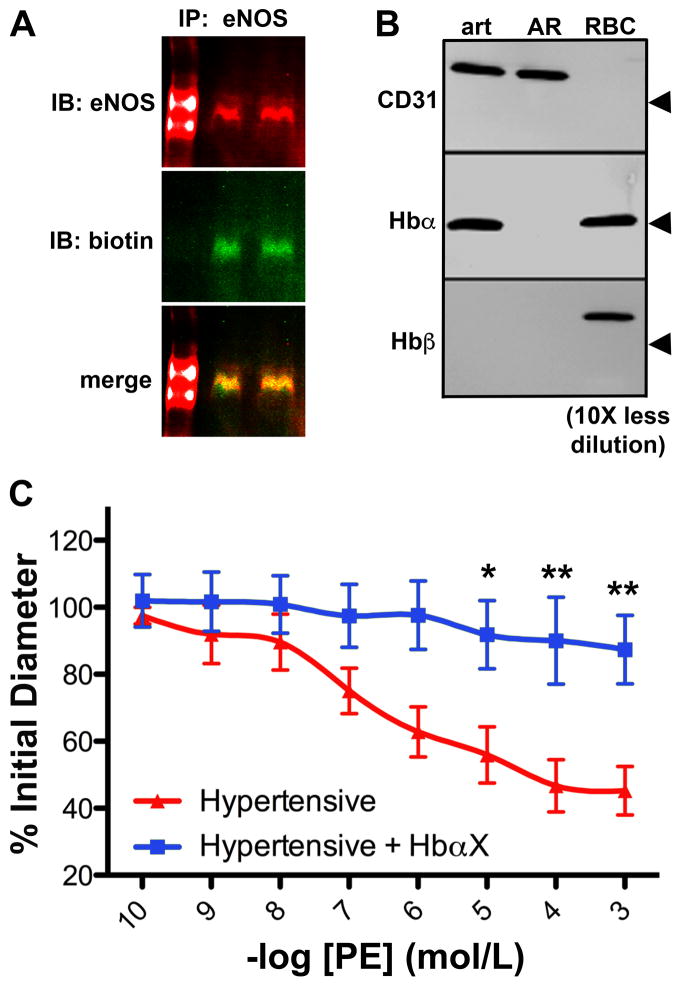
Finally, to demonstrate the translational potential of HbαX, we tested the peptide in vitro (using recombinant human eNOS, described above) and ex vivo (measuring vasoreactivity of human arterioles excised from adipose biopsy samples). Figure 6A shows a Far Western blot of eNOS using HbαX as a probe. Briefly, purified recombinant human eNOS was run in a non-denaturing, low temperature polyacrylamide gel and transferred to a PVDF membrane for blotting with biotin-HbαX and anti-eNOS antibodies. Fluorescent secondary antibodies show the overlap of eNOS and biotin bands, indicating that HbαX is able to bind to human eNOS. To confirm that disruption of the alpha globin/eNOS complex is relevant to human physiology, we assayed the distribution of alpha globin in the human vasculature. As previously demonstrated in mice, alpha globin is observed in the biopsied human arterioles, but not beta globin (Figure 6B). Finally, the effect of peptide administration was assayed via vasoreactivity of human arterioles excised from adipose biopsies obtained from patients with hypertension (see Supplementary Table S1). The arterioles excised from these patients were responsive to the vasoconstricting agent phenylephrine (PE). However, when HbαX is incubated with the arteriole (5 nmol/L), the PE dose response is significantly blunted (Figure 6C). This is identical to the observed effect in peptide-treated C57BL/6 mice10, which was restored with a NOS inhibitor. This data provides initial evidence that HbαX may alter human peripheral resistance. When summed with the murine data above, the evidence supports a potential therapeutic value of HbαX in hypertension.
Figure 6. An alpha globin mimetic peptide significantly reduces hyperconstriction in arterioles from human patients with hypertension.
(A) Far-Western blotting for recombinant human eNOS oxygenase domain on a non-denaturing gel using anti-eNOS and biotin-HbαX probes and corresponding secondary antibodies for fluorescent detection. Left lane serves as a molecular weight marker, but it is not used to estimate molecular weight due to the non-denaturing condition of the gel. The samples were run in duplicate. (B) Western blot of the expression of alpha globin without its beta globin partner is confirmed in human arterioles, and a 10-fold dilution of RBC samples is used as a positive control for alpha and beta globin chains. Note the lack of alpha globin in aorta. Arrowheads for CD31 indicate 100 kDa, and alpha and beta globin indicate 10 kDa. (C) Resistance arterioles (approximately 100 μm in diameter) isolated from human adipose tissue biopsies were cannulated and subject to phenylephrine cumulative dose-response curves in the absence (black) or presence (blue) of 5 μmol/L HbαX. (n=4). * indicates p<0.05, ** indicates p<0.01 using two-way ANOVA and a Bonferroni post-test.
Discussion
Here, we report hemodynamic changes in mice and humans after injection of the alpha globin mimetic peptide HbαX. We hypothesize that HbαX acts as a vasodilator by displacing alpha globin from eNOS and disrupting its ability to scavenge NO at the source.
The result of increased NO levels by inhibiting scavenging can be seen using PAM in Figure 1. Using the dual optical absorbance wavelengths of oxy- and deoxyhemoglobin, total hemoglobin concentration and sO2 is measured in live tissue12. Oxygen saturation of hemoglobin in the vessels is described in Figure 1. A difference exists in the sO2 of arteries and veins at baseline, as expected. Upon injection of the HbαX peptide, arterial sO2 is unchanged (red trace) and venous sO2 increases (blue trace) after 60 minutes. The vessel-type dependent response is consistent with physiological response to increased blood flow. Arterial oxygenation is not changed because HbαX does not change hemoglobin oxygenation dynamics as shown in Figure 5. Venous oxygenation increases due to total increases in oxygen concentration in the tissue, so that low-O2 induced diffusion is decreased in capillary beds. This is the result of increased blood flow in the tissues, as seen in Figure 1. Administration of HbαX increased flow in both arterioles and venules after 60 minutes. The result of higher flow is increased O2 distribution in the tissue surrounding capillary beds. Although the effects were only studied in ear tissue, similar effects are expected across all resistance vasculature. Additionally, NO does not provide a majority of the vasodilatory signals in resistance vasculature under normal physiology, but with relative overproduction, the impact is likely increased.
Injections every other day were enough to decrease systolic pressure in mice at baseline and mice made hypertensive by constant infusion of AngII. Even without elevated blood pressure, the administration of HbαX decreased systolic pressure by approximately 5 mmHg (Figure 2A). This shows that increasing NO has an effect in non-pathological contexts because there is constant regulation of NO availability by alpha globin, and that disrupting the macromolecular context permits increased NO signaling in smooth muscle cells. In an AngII model of hypertension, administration of HbαX resulted in a ~30 mmHg decrease in blood pressure compared to saline and control peptide treatment that was constant until the end of pump lifetime (red line, Figure 2B). AngII has been shown to increase damaging superoxide radical formation in the tissues due to activation of NADH and NADPH oxidase through its type 1 receptor22, 23. In order to address whether the large decrease in blood pressure in HbαX treated-treated mice in AngII-hypertension is in part due to a reduction in superoxide levels, lucigenin was used to assay tissue production of O2•− in the kidney (a major target organ of AngII) and the resistance mesenteric vessels. In whole kidney tissues, AngII-induced superoxide generation was not attenuated by HbαX injection. This could be explained by lack of sensitivity of the assay due to the presence of many other cell types, including epithelial cells that do not express eNOS; thus, any effect of HbαX on eNOS would be difficult to detect when whole renal tissue is assessed. However, there were lower superoxide levels in the mesenteric tissue after treatment with HbαX. This could be due to a scavenging effect of superoxide by NO, forming peroxynitrite and other nitroxide species24. Increased NO availability by disruption of alpha globin/eNOS binding could lead to slightly lower superoxide levels in the resistance vasculature. Although the damage of free oxygen radicals produced by AngII-activated oxidases is not completely mitigated by this mechanism, some radical scavenging is likely beneficial to endothelial function. Although the decrease in blood pressure with HbαX did not return to 110 mmHg as seen in the basal condition, a decrease in systolic blood pressure of 30 mmHg is clinically significant and desirable in treatment. Other mechanisms of hypertension, including aldosterone dis-regulation or increased sympathetic drive, may also be sensitive to increases in NO production because the constrictive signaling pathways are distinct from those involved in NO action. The interplay of increased dilatory signals among the constrictive signals might be enough to shift the balance towards lower overall blood pressure.
Because HbαX was administered as a peptide injection, the toxicity readouts are important before considering human treatments. There are no detectable effects on liver or kidney function when the peptide is injected for up to 60 days. Because HbαX is a peptide chain, the breakdown products (amino acids) should be relatively inert, and cleared intracellularly or from blood using endogenous protein breakdown machinery. Critically, the injection of the mimetic peptide did not increase white blood cell count, indicating no inflammatory response to the peptide (Figure 3B). Because the sequence is found in endogenous hemoglobin, it is unlikely that antibodies would be developed against the sequence. Measures of liver and kidney function (assayed by urea, ALT, AST, and plasma creatinine levels, Figure 3, C–F) showed no difference between saline, control peptide, or HbαX treated mice, illustrating no detectable toxicity and its potential as a novel therapeutic agent in the treatment of hypertension by increasing NO availability in endothelial cells.
To confirm the proposed mechanism of HbαX on the disruption of the alpha globin/eNOS complex10, we assayed the binding of HbαX and eNOS in vitro. In cultured ECs, HbαX immunoprecipitates with dimeric eNOS protein (Figure 4A), showing that eNOS can remain in its active oligomeric state when the peptide is bound. An active eNOS enzyme is critical for increased NO availability with HbαΧ treatment, as the peptide works to disrupt the formation of an alpha globin/eNOS macromolecular complex. Our model suggests the alpha globin mimetic peptide HbαX competes with the full alpha globin chain for a binding pocket on the oxygenase domain of eNOS, and previous work has shown fluorescently labeled HbαX colocalized with alpha globin in the MEJ, where a functional pool of eNOS is located in ECs3, 9, 10. These results suggest proximity of the functional parts, but do not directly show binding. We use FP assays to prove that HbαX binds to eNOS and determine the binding affinity. In our experiment, we determined that HbαX and eNOS bind with low nanomolar affinity (Figure 4B). As validation of this result, half of the fluorescently labeled HbαX in the ligand buffer was replaced with unlabeled HbαX. This competition experiment effectively doubles the observed binding affinity because only labeled peptide (now at half the concentration) is able to be measured and is competing for the same number of binding sites. Roughly, a doubling of the binding affinity is observed in this mode (Figure 4C). This is not an effect attributable to HbαX oligomerization, because there is no observable binding curve from data in Figure 4D.
There is considerable evidence that NO can also be modulated by RBCs as well as the endothelium. It has been elegantly demonstrated that NO production in erythrocytes can occur either by nitrite conversion or by eNOS in the RBCs25–30. It is possible that free NO availability in the circulating cells is regulated by a similar mechanism as in ECs due to the vast amounts of hemoglobin present directly adjacent to a membrane-bound NOS enzyme. Overproduction of NO in RBCs might lead to higher levels of methemoglobin within the cells and negatively affect the O2 carrying capacity of the red blood cells. A reduced O2 capacity for RBCs might trigger a hypoxic response leading to the observed vasodilation in vivo. To determine if HbαX application could increase NO production and higher p50 values for isolated RBCs, freshly isolated human blood was incubated with concentrations of HbαX much higher (30 μmol/L, Figure 5) than would be encountered with therapeutic administration. Even with this level, there was no effect on the p50 of RBC hemoglobin in intact RBCs. With this result, we believe that the effects on RBC eNOS are not the major contributor to the vasodilatory response seen in our system. However, the systems for NO bioavailablity in RBCs may be inherently different in terms of a signaling mechanism, or the peptide may not have been able to penetrate the membrane of the RBC.
The translational capacity of HbαX to disrupt the alpha globin/eNOS complex to increase NO production is shown in Figure 6. HbαX is able to bind to the oxygenase domain of eNOS, and this interaction is physiologically relevant in human vasculature. The expression of alpha globin in arteriole ECs (but not in the aortic root) shows that disruption of the alpha globin/eNOS interaction predominantly occurs in resistance vasculature, where blood pressure is controlled. The effect of HbαX can be observed ex vivo on individual arterioles using pressure myography. After incubation with HbαX, blunted vasoconstriction to PE is observed in arterioles excised from humans with hypertension (Supplementary Table S1). Thus, in the context of systemic high blood pressure, increasing local NO signaling in the resistance vasculature might provide therapeutic benefit. This result provides a base for novel treatments of hypertension by increasing endogenous NO production.
Other strategies exist for increasing production of NO in vivo by removing an inhibitory pathway for eNOS. One well-characterized inhibitory binding partner for eNOS is caveolin-1, the main coat protein of caveolae31–34. Interestingly, a peptide mimicking the eNOS-binding sequence of caveolin, Cav16–8, and HbαX share some sequence similarity. Supplementary Table S2 shows the sequences of the Cav16 peptide and HbαX10 for comparison. Of note, the peptides are composed of both polar and hydrophobic residues, which indicate both buried and solvent-exposed side chains in the binding sequence. The intracellular eNOS-binding interface of caveolin interacts with the calmodulin-binding motif between the oxygenase and reductase domains of eNOS to prevent activation and NO production34. The similarity of the threonine-rich sequences with surrounding hydrophobic residues may be coincidental, but provides a direction for further study of the binding location for alpha globin/HbαX on eNOS.
There are further questions raised by these results. Our previous work has used an eNOS inhibitor, L-nitroarginine methyl ester (L-NAME), to show that blunted response to PE is a NO-dependent phenomenon9, 10. A lack of human volunteers for biopsy prevented tests of L-NAME sensitivity in human arterioles, a limitation of using human samples in research. The dynamics of the alpha globin/eNOS interaction is also of importance. The binding affinity, kon, and koff rates for the full alpha globin chain binding to eNOS are yet to be determined and will provide evidence for a model of static or transient interaction in the scavenging of NO by alpha globin. Having a model of the alpha globin/eNOS interaction that includes dynamics will be useful in further modification of the HbαX peptide for better pharmacokinetics.
In conclusion, this study provides evidence for modulation of blood flow and decreasing blood pressure using an alpha globin mimetic peptide to prevent NO scavenging. The peptide, HbαX, disrupts an alpha globin/eNOS macromolecular complex by binding to eNOS and increases blood flow and oxygen saturation in vivo. HbαX does not appear to affect bone marrow, liver or kidney function. The target specificity of the peptide prevents negative interactions with endogenous hemoglobin, and enhances NO signaling, leading to vasodilatory effects to lower blood pressure.
Perspectives
This work characterizes an alpha globin mimetic peptide and its role in decreasing vascular resistance and increasing blood flow. By increasing NO bioavailability, HbαX is a potent therapeutic both for acute hypertensive crises (by increasing tissue oxygenation and blood flow in resistance arteries) and long-term treatment of hypertension (due to its low toxicity and sustained decrease in blood pressure).
Supplementary Material
Novelty and Significance.
What is new?
This is the first study of the effects of modulating hemodynamic factors using an alpha globin mimetic peptide to increase NO availability.
What is relevant?
These results provide evidence that an alpha mimetic peptide may be a candidate for a long-term therapeutic for blood pressure pathologies.
Summary
Hypertension affects a growing number of worldwide adults, and treatment resistant hypertension is becoming more prevalent with no definitive therapy. By increasing nitric oxide availability, we are able to induce a sustained reduction in blood pressure and increase vascular flow in animal models and blunt hyperreactive vasoconstriction in human arterioles
Acknowledgments
The authors thank Adam C Straub (University of Pittsburgh) for helpful discussion and Linda Jahn and Lee Hartline (University of Virginia) for coordinating patient enrollment.
Sources of Funding
This work was supported by the Ivy Translational Fund, University of Virginia Patent Foundation (BEI and LC), NIH 088554 (BEI), NIH 087828 and the Cottrell Award from the Research Corporation for the Advancement of Science (LC), NIH T32GM007055 and American Heart Association Grant 16PRE31180040 (TCSK), The Hartwell Foundation and NIH EY022063 (SMP), R01GM113838 (AD), NIH DK094907 (THL), AHA 15SDG25960005 and NIH EB020843 (SH).
Footnotes
Conflict of Interest
BEI and LC are co-authors on a patent titled “Composition and methods for modulating arterial tone.” The authors declare no other conflict of interest.
References
- 1.Ignarro LJ, Cirino G, Casini A, Napoli C. Nitric oxide as a signaling molecule in the vascular system: an overview. Journal of Cardiovascular Pharmacology. 1999;34:879–886. doi: 10.1097/00005344-199912000-00016. [DOI] [PubMed] [Google Scholar]
- 2.Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 3.Looft-Wilson RC, Billaud M, Johnstone SR, Straub AC, Isakson BE. Interaction between nitric oxide signaling and gap junctions: effects on vascular function. Biochimica et Biophysica Acta. 2012;1818:1895–1902. doi: 10.1016/j.bbamem.2011.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garcia-Cardena G, Fan R, Stern DF, Liu J, Sessa WC. Endothelial Nitric Oxide Synthase is Regulated by Tyrosine Phosphorylation and Interacts with Caveolin-1. The Journal of Biological Chemistry. 1996;271:27237–27240. doi: 10.1074/jbc.271.44.27237. [DOI] [PubMed] [Google Scholar]
- 5.Fulton D, Gratton JP, Sessa WC. Post-Translational Control of Endothelial Nitric Oxide Synthase: Why Isn't Calcium/Calmodulin Enough? The Journal of Pharmacology and Experimental Therapeutics. 2001;299:818–824. [PubMed] [Google Scholar]
- 6.Trane AE, Pavlov D, Sharma A, Saqib U, Lau K, van Petegem F, Minshall RD, Roman LJ, Bernatchez PN. Deciphering the binding of caveolin-1 to client protein endothelial nitric-oxide synthase (eNOS): scaffolding subdomain identification, interaction modeling, and biological significance. The Journal of Biological Chemistry. 2014;289:13273–13283. doi: 10.1074/jbc.M113.528695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bernatchez PN, Bauer PM, Yu J, Prendergast JS, He P, Sessa WC. Dissecting the molecular control of endothelial NO synthase by caveolin-1 using cell-permeable peptides. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:761–766. doi: 10.1073/pnas.0407224102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bernatchez P, Sharma A, Bauer PM, Marin E, Sessa WC. A noninhibitory mutant of the caveolin-1 scaffolding domain enhances eNOS-derived NO synthesis and vasodilation in mice. The Journal of Clinical Investigation. 2011;121:3747–3755. doi: 10.1172/JCI44778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Straub AC, Lohman AW, Billaud M, Johnstone SR, Dwyer ST, Lee MY, Bortz PS, Best AK, Columbus L, Gaston B, Isakson BE. Endothelial cell expression of haemoglobin alpha regulates nitric oxide signalling. Nature. 2012;491:473–477. doi: 10.1038/nature11626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Straub AC, Butcher JT, Billaud M, Mutchler SM, Artamonov MV, Nguyen AT, Johnson T, Best AK, Miller MP, Palmer LA, Columbus L, Somlyo AV, Le TH, Isakson BE. Hemoglobin alpha/eNOS coupling at myoendothelial junctions is required for nitric oxide scavenging during vasoconstriction. Arteriosclerosis, Thrombosis, and Vascular Biology. 2014;34:2594–2600. doi: 10.1161/ATVBAHA.114.303974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ning B, Sun N, Cao R, Chen R, Kirk Shung K, Hossack JA, Lee JM, Zhou Q, Hu S. Ultrasound-aided Multi-parametric Photoacoustic Microscopy of the Mouse Brain. Scientific Reports. 2015;5:18775. doi: 10.1038/srep18775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ning B, Kennedy MJ, Dixon AJ, Sun N, Cao R, Soetikno BT, Chen R, Zhou Q, Kirk Shung K, Hossack JA, Hu S. Simultaneous photoacoustic microscopy of microvascular anatomy, oxygen saturation, and blood flow. Optics Letters. 2015;40:910–913. doi: 10.1364/OL.40.000910. [DOI] [PubMed] [Google Scholar]
- 13.Billaud M, Chiu YH, Lohman AW, et al. A molecular signature in the pannexin1 intracellular loop confers channel activation by the α1 adrenoreceptor in smooth muscle cells. Science Signalling. 2015;8ra17:1–12. doi: 10.1126/scisignal.2005824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cechova S, Zeng Q, Billaud M, Mutchler S, Rudy CK, Straub AC, Chi L, Chan FR, Hu J, Griffiths R. Loss of collectrin, an angiotensin-converting enzyme 2 homolog, uncouples endothelial nitric oxide synthase and causes hypertension and vascular dysfunction. Circulation. 2013;128:1770–1780. doi: 10.1161/CIRCULATIONAHA.113.003301. [DOI] [PubMed] [Google Scholar]
- 15.Doctor A, Platt R, Sheram ML, Eischeid A, McMahon T, Maxey T, Doherty J, Axelrod M, Kline J, Gurka M, Gow A, Gaston B. Hemoglobin conformation couples erythrocyte S-nitrosothiol content to O2 gradients. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:5709–5714. doi: 10.1073/pnas.0407490102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fischmann TO, Hruza A, Niu XD, Fossetta JD, Lunn CA, Dolphin E, Prongay AJ, Reichert P, Lundell DJ, Narula SK, Weber PC. Structural characterization of nitric oxide synthase isoforms reveals striking active-site conservation. Nature Structural & Molecular Biology. 1999;6:233–242. doi: 10.1038/6675. [DOI] [PubMed] [Google Scholar]
- 17.Wu Y, Li Q, Chen X-Z. Detecting protein–protein interactions by far western blotting. Nature Protocols. 2007;2:3278–3284. doi: 10.1038/nprot.2007.459. [DOI] [PubMed] [Google Scholar]
- 18.Billaud M, Lohman AW, Straub AC, Looft-Wilson R, Johnstone SR, Araj CA, Best AK, Chekeni FB, Ravichandran KS, Penuela S, Laird DW, Isakson BE. Pannexin1 regulates α1-adrenergic receptor- mediated vasoconstriction. Circulation Research. 2011;109:80–85. doi: 10.1161/CIRCRESAHA.110.237594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang L, Yin J, Nickles HT, et al. Hypoxic pulmonary vasoconstriction requires connexin 40-mediated endothelial signal conduction. The Journal of Clinical Investigation. 2012;122:4218–4230. doi: 10.1172/JCI59176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang YM, Huang A, Kaley G, Sun D. eNOS uncoupling and endothelial dysfunction in aged vessels. American Journal of Physiology: Heart and Circulatory Physiology. 2009;297:H1829–H1836. doi: 10.1152/ajpheart.00230.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hulme EC, Trevethick MA. Ligand binding assays at equilibrium: validation and interpretation. British Journal of Pharmacology. 2010;161:1219–1237. doi: 10.1111/j.1476-5381.2009.00604.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Touyz RM, Schiffrin EL. Increased generation of superoxide by angiotensin II in smooth muscle cells from resistance arteries of hypertensive patients: role of phospholipase D-dependent NAD (P) H oxidase-sensitive pathways. Journal of Hypertension. 2001;19:1245–1254. doi: 10.1097/00004872-200107000-00009. [DOI] [PubMed] [Google Scholar]
- 23.Rajagopalan S, Kurz S, Münzel T, Tarpey M, Freeman BA, Griendling KK, Harrison DG. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. Journal of Clinical Investigation. 1996;97:1916–1923. doi: 10.1172/JCI118623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. American Journal of Physiology: Cell Physiology. 1996;271:C1424–C1437. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- 25.Cortese-Krott MM, Kelm M. Endothelial nitric oxide synthase in red blood cells: key to a new erythrocrine function? Redox Biology. 2014;2:251–258. doi: 10.1016/j.redox.2013.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cosby K, Partovi KS, Crawford JH, Patel RP, Reiter CD, Martyr S, Yang BK, Waclawiw MA, Zalos G, Xu X, Huang KT, Shields H, Kim-Shapiro DB, Schechter AN, Cannon RO, Gladwin MT. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nature Medicine. 2003;9:1498–1505. doi: 10.1038/nm954. [DOI] [PubMed] [Google Scholar]
- 27.Han TH, Hyduke DR, Vaughn MW, Fukuto JM, Liao JC. Nitric oxide reaction with red blood cells and hemoglobin under heterogeneous conditions. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:7763–7768. doi: 10.1073/pnas.122118299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim-Shapiro DB, Schechter AN, Gladwin MT. Unraveling the reactions of nitric oxide, nitrite, and hemoglobin in physiology and therapeutics. Arteriosclerosis, Thrombosis, and Vascular Biology. 2006;26:697–705. doi: 10.1161/01.ATV.0000204350.44226.9a. [DOI] [PubMed] [Google Scholar]
- 29.Kleinbongard P, Schulz R, Rassaf T, et al. Red blood cells express a functional endothelial nitric oxide synthase. Blood. 2006;107:2943–2951. doi: 10.1182/blood-2005-10-3992. [DOI] [PubMed] [Google Scholar]
- 30.Liu C, Wajih N, Liu XB, et al. Mechanisms of human erythrocytic bioactivation of nitrite. The Journal of Biological Chemistry. 2015;290:1281–1294. doi: 10.1074/jbc.M114.609222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sessa WC. Regulation of endothelial derived nitric oxide in health and disease. Memórias do Instituto Oswaldo Cruz. 2005;100:15–18. doi: 10.1590/s0074-02762005000900004. [DOI] [PubMed] [Google Scholar]
- 32.Segal SS, Brett SE, Sessa WC. Codistribution of NOS and caveolin throughout peripheral vasculature and skeletal muscle of hamsters. American Journal of Physiology. 1999;277:1167–1177. doi: 10.1152/ajpheart.1999.277.3.H1167. [DOI] [PubMed] [Google Scholar]
- 33.Murata T, Lin MI, Huang Y, Yu J, Bauer PM, Giordano FJ, Sessa WC. Reexpression of caveolin-1 in endothelium rescues the vascular, cardiac, and pulmonary defects in global caveolin–1 knockout mice. The Journal of Experimental Medicine. 2007;204:2373–2382. doi: 10.1084/jem.20062340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gratton JP, Fontana J, O'Connor DS, Garcia-Cardena G, McCabe TJ, Sessa WC. Reconstitution of an endothelial nitric-oxide synthase (eNOS), hsp90, and caveolin-1 complex in vitro. Evidence that hsp90 facilitates calmodulin stimulated displacement of eNOS from caveolin-1. The Journal of Biological Chemistry. 2000;275:22268–22272. doi: 10.1074/jbc.M001644200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.