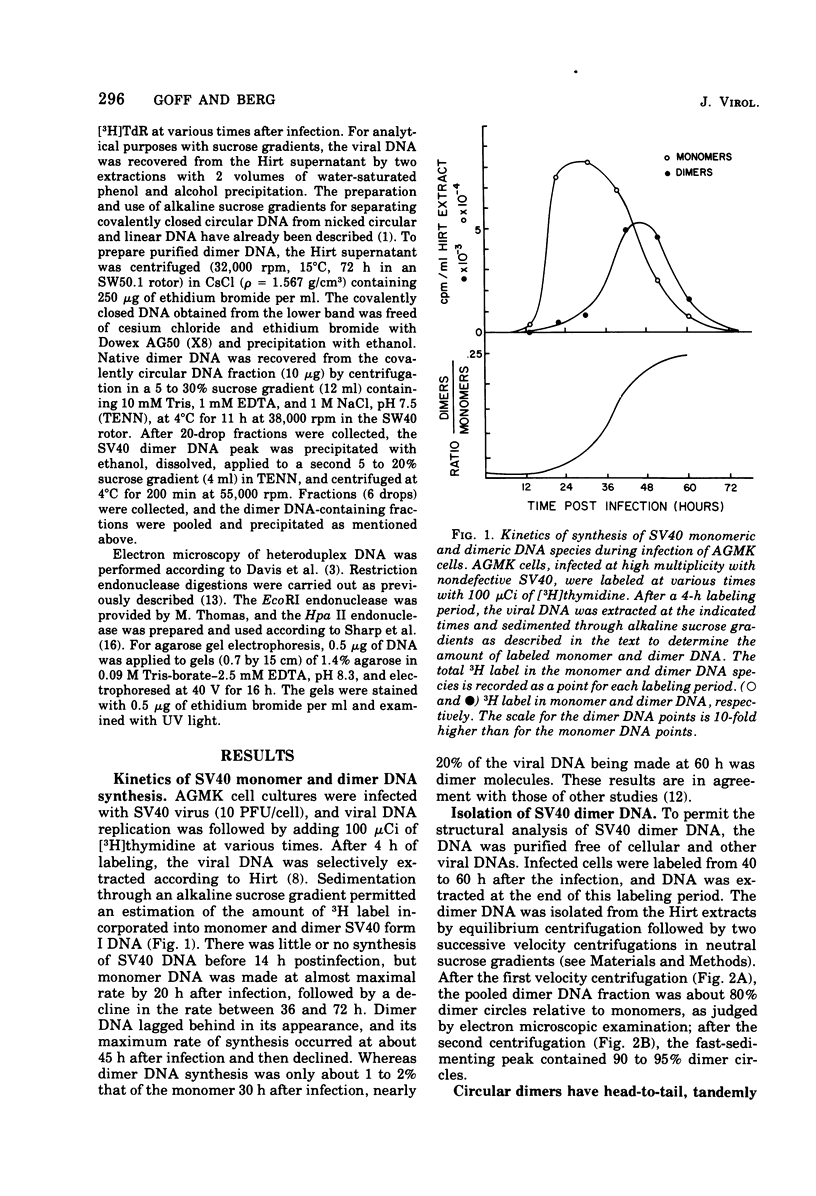
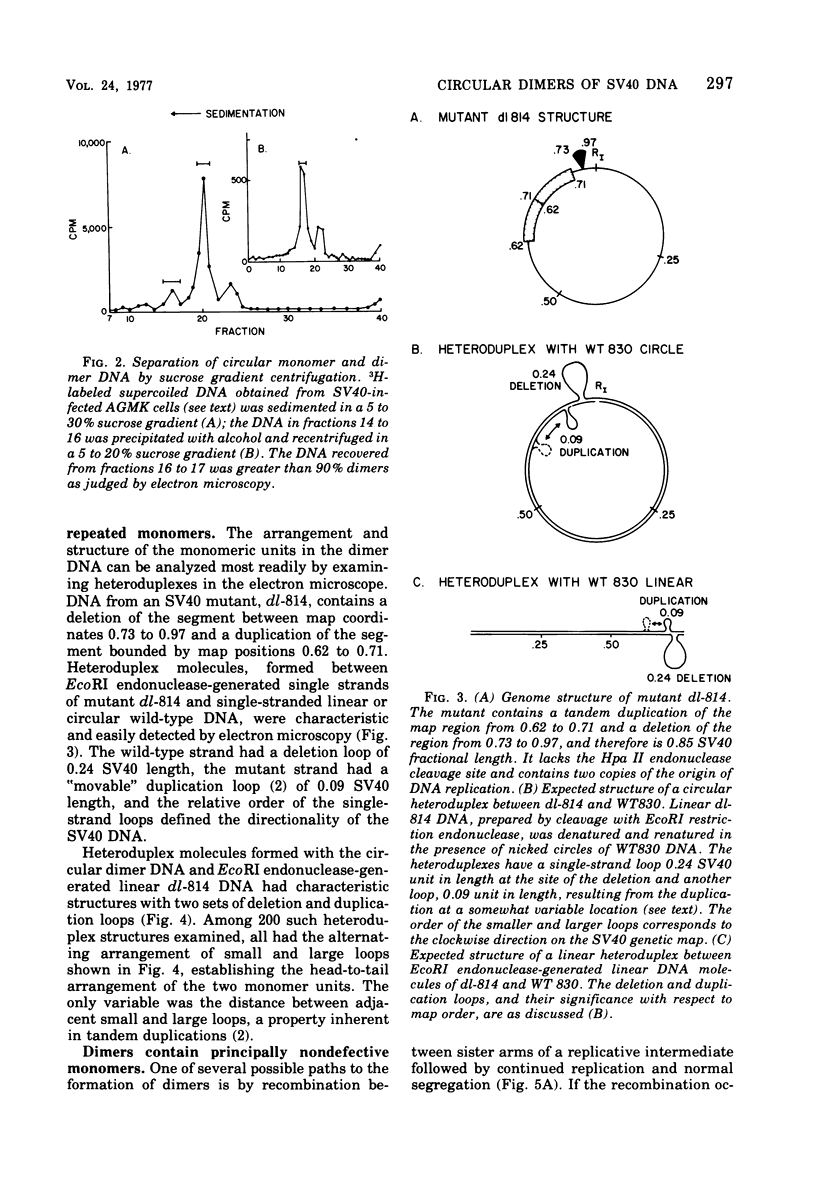
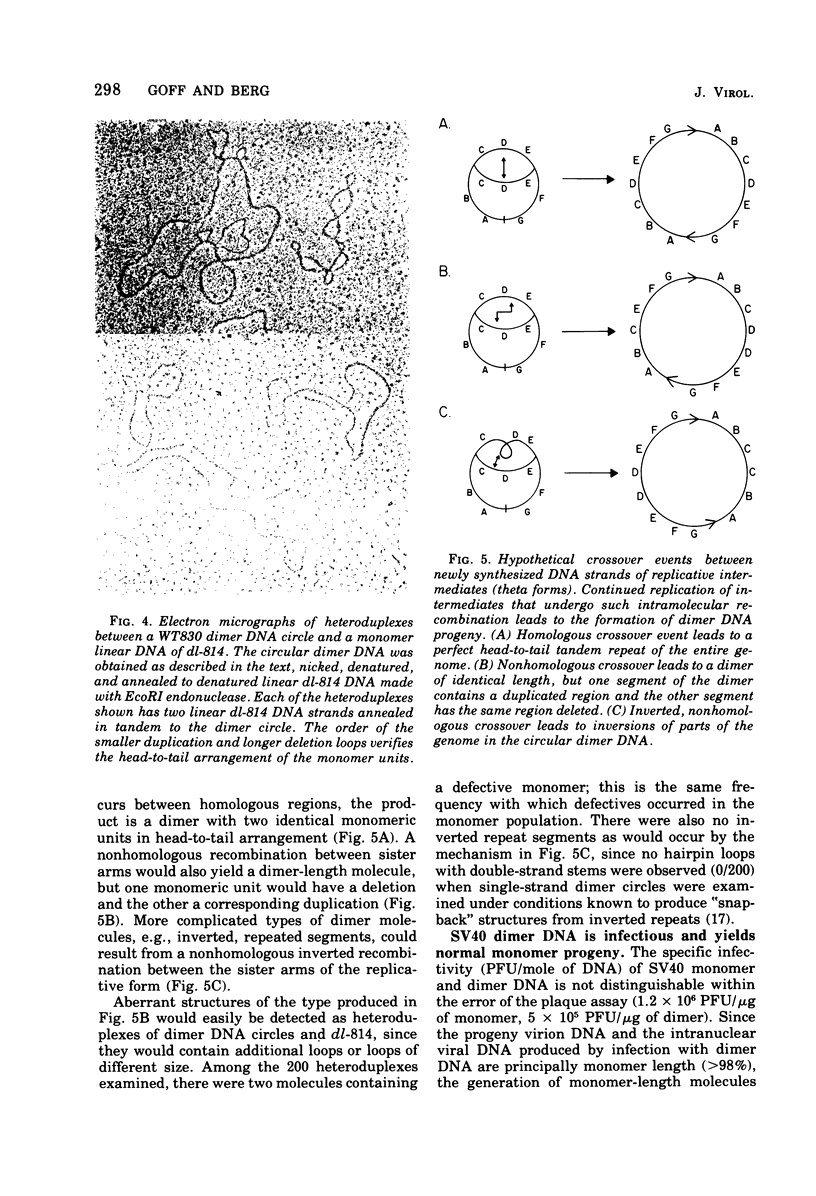
Abstract
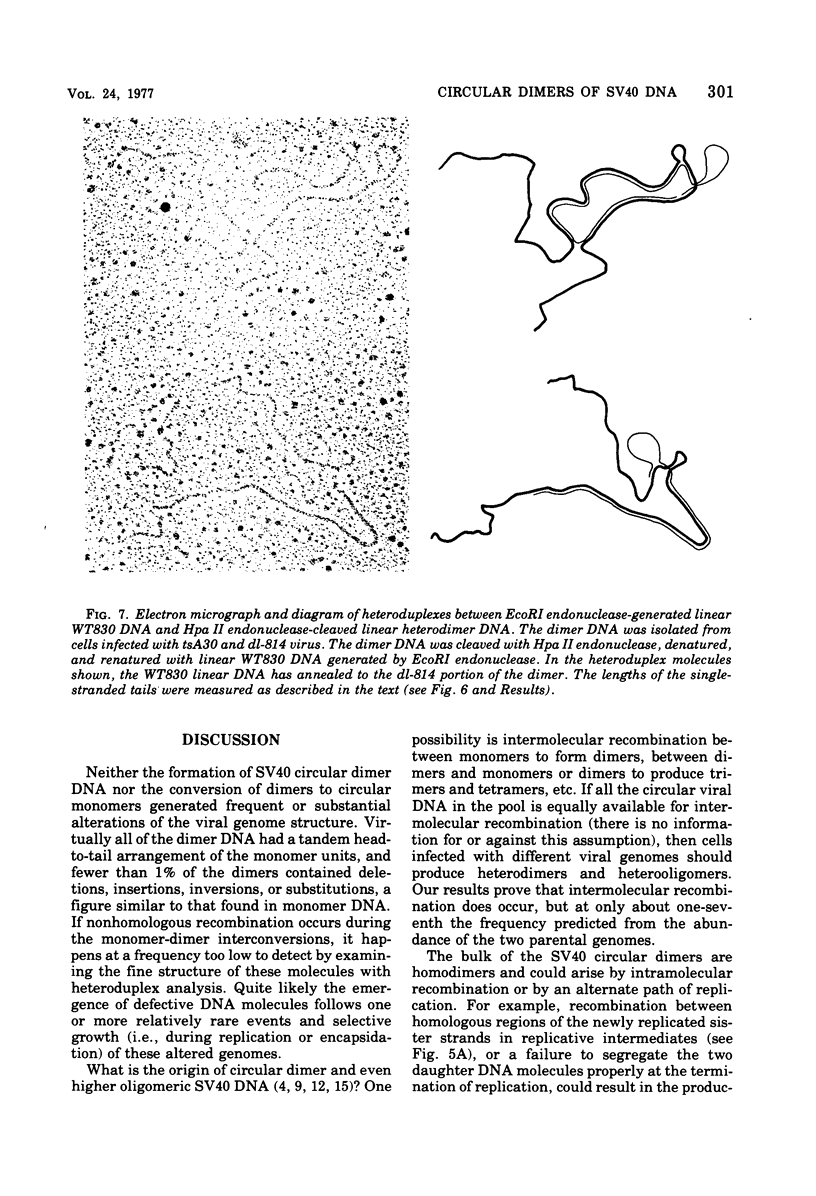
Most of the viral DNA extracted from simian virus 40 (SV40)-infected African green monkey kidney cells consists of circular molecules about 5.3 kilobases in contour length. However, about 1% of the viral DNA was found to occur as closed circular dimers that appeared to be formed, preferentially, late in infection. The monomeric units of dimers were organized in a head-to-tail, tandem arrangement; moreover, the monomeric units were not defective; i.e., they lacked deletions or other rearrangements. After infections with dimer DNA, nondefective monomers were formed. These findings suggest that dimers are not intermediates in the production of defective SV40 genomes. The majority of the dimers formed in mixed infections with two mutants were homodimers, but about 5% of the circular dimers were heterodimers and must have arisen by intermolecular recombination.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beard P., Morrow J. F., Berg P. Cleavage of circular, superhelical simian virus 40 DNA to a linear duplex by S1 nuclease. J Virol. 1973 Dec;12(6):1303–1313. doi: 10.1128/jvi.12.6.1303-1313.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busse H. G., Baldwin R. L. Tandem genetic duplications in a derivative of phage lambda. II. Evidence for structure and location of endpoints. J Mol Biol. 1972 Apr 14;65(3):401–412. doi: 10.1016/0022-2836(72)90197-0. [DOI] [PubMed] [Google Scholar]
- Dubbs D. R., Kit S., Jaenisch R., Levine A. J. Isolation of simian virus 40 recombinants from cells infected with oligomeric forms of simian virus 40 deoxyribonucleic acid. J Virol. 1972 Apr;9(4):717–719. doi: 10.1128/jvi.9.4.717-719.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubbs D. R., Rachmeler M., Kit S. Recombination between temperature-sensitive mutants of simian virus 40. Virology. 1974 Jan;57(1):161–174. doi: 10.1016/0042-6822(74)90117-2. [DOI] [PubMed] [Google Scholar]
- Ganem D., Nussbaum A. L., Davoli D., Fareed G. C. Isolation, propagation and characterization of replication requirements of reiteration mutants of Simian Virus 40. J Mol Biol. 1976 Feb 15;101(1):57–83. doi: 10.1016/0022-2836(76)90066-8. [DOI] [PubMed] [Google Scholar]
- Gilbert W., Dressler D. DNA replication: the rolling circle model. Cold Spring Harb Symp Quant Biol. 1968;33:473–484. doi: 10.1101/sqb.1968.033.01.055. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Jaenisch R., Levine A. J. Infection of primary African green monkey cells with SV40 monomeric and dimeric DNA. J Mol Biol. 1971 Nov 14;61(3):735–738. doi: 10.1016/0022-2836(71)90076-3. [DOI] [PubMed] [Google Scholar]
- Jaenisch R., Levine A. DNA replication in SV40-infected cells. V. Circular and catenated oligomers of SV40 DNA. Virology. 1971 Jun;44(3):480–493. doi: 10.1016/0042-6822(71)90361-8. [DOI] [PubMed] [Google Scholar]
- Kelly T. J., Jr, Lewis A. M., Jr, Levine A. S., Siegel S. Structure of two adenovirus-simian virus 40 hybrids which contain the entire SV40 genome. J Mol Biol. 1974 Oct 15;89(1):113–126. doi: 10.1016/0022-2836(74)90165-x. [DOI] [PubMed] [Google Scholar]
- Martin M. A., Howley P. M., Byrne J. C., Garon C. F. Characterization of supercoiled oligomeric SV40 DNA molecules in productively infected cells. Virology. 1976 May;71(1):28–40. doi: 10.1016/0042-6822(76)90091-x. [DOI] [PubMed] [Google Scholar]
- Mertz J. E., Berg P. Defective simian virus 40 genomes: isolation and growth of individual clones. Virology. 1974 Nov;62(1):112–124. doi: 10.1016/0042-6822(74)90307-9. [DOI] [PubMed] [Google Scholar]
- Mertz J. E., Berg P. Viable deletion mutants of simian virus 40: selective isolation by means of a restriction endonuclease from Hemophilus parainfluenzae. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4879–4883. doi: 10.1073/pnas.71.12.4879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush M. G., Eason R., Vinograd J. Identification and properties of complex forms of SV40 DNA isolated from SV40-infected African Green monkey (BSC-1) cells. Biochim Biophys Acta. 1971 Feb 11;228(3):585–594. doi: 10.1016/0005-2787(71)90723-4. [DOI] [PubMed] [Google Scholar]
- Sharp P. A., Sugden B., Sambrook J. Detection of two restriction endonuclease activities in Haemophilus parainfluenzae using analytical agarose--ethidium bromide electrophoresis. Biochemistry. 1973 Jul 31;12(16):3055–3063. doi: 10.1021/bi00740a018. [DOI] [PubMed] [Google Scholar]
- Wilson D. A., Thomas C. A., Jr Palindromes in chromosomes. J Mol Biol. 1974 Mar 25;84(1):115–138. doi: 10.1016/0022-2836(74)90216-2. [DOI] [PubMed] [Google Scholar]