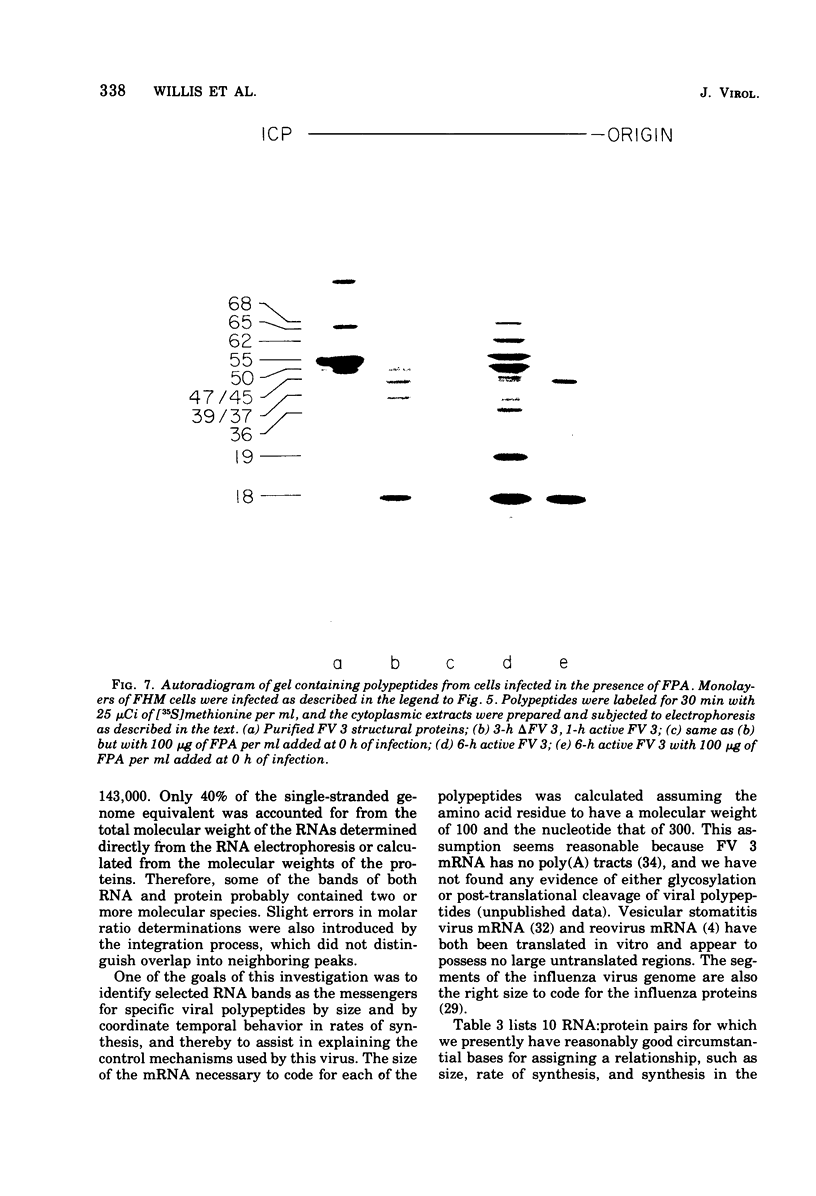
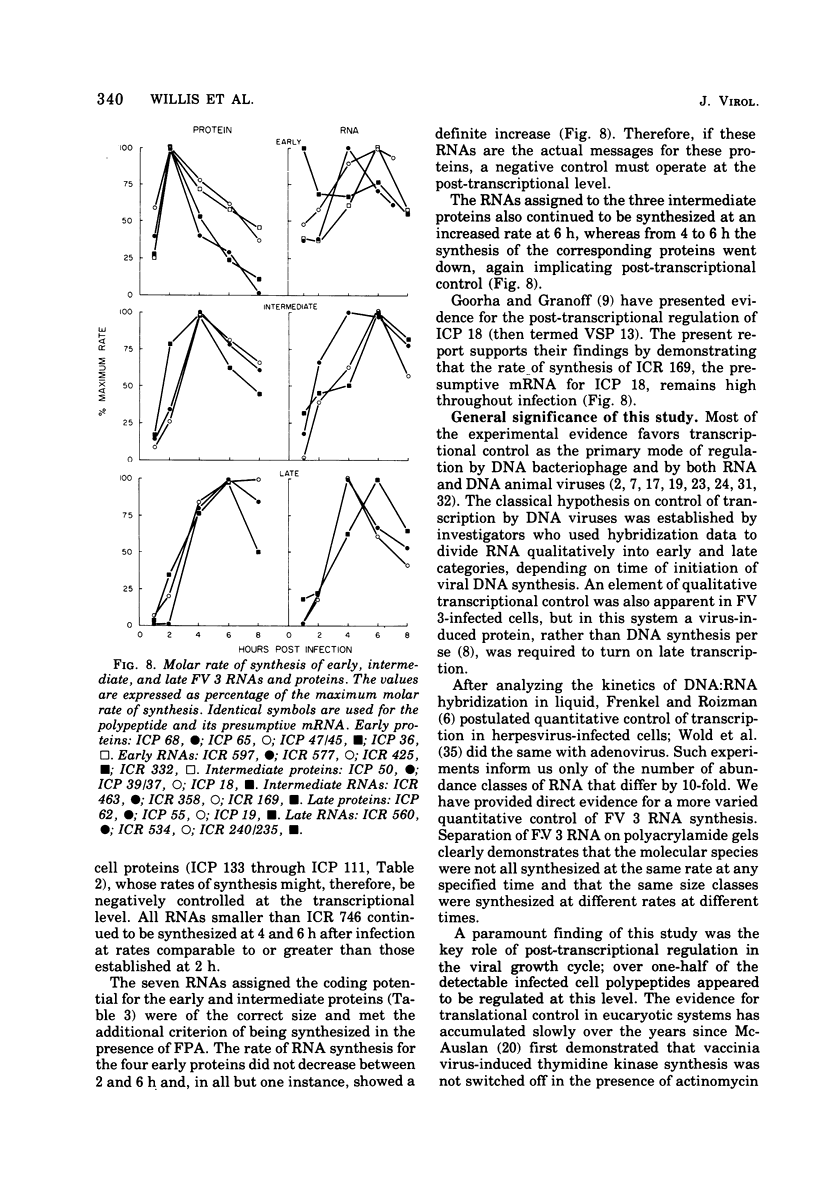
Abstract
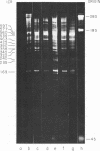
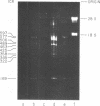
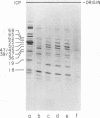
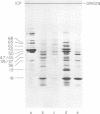
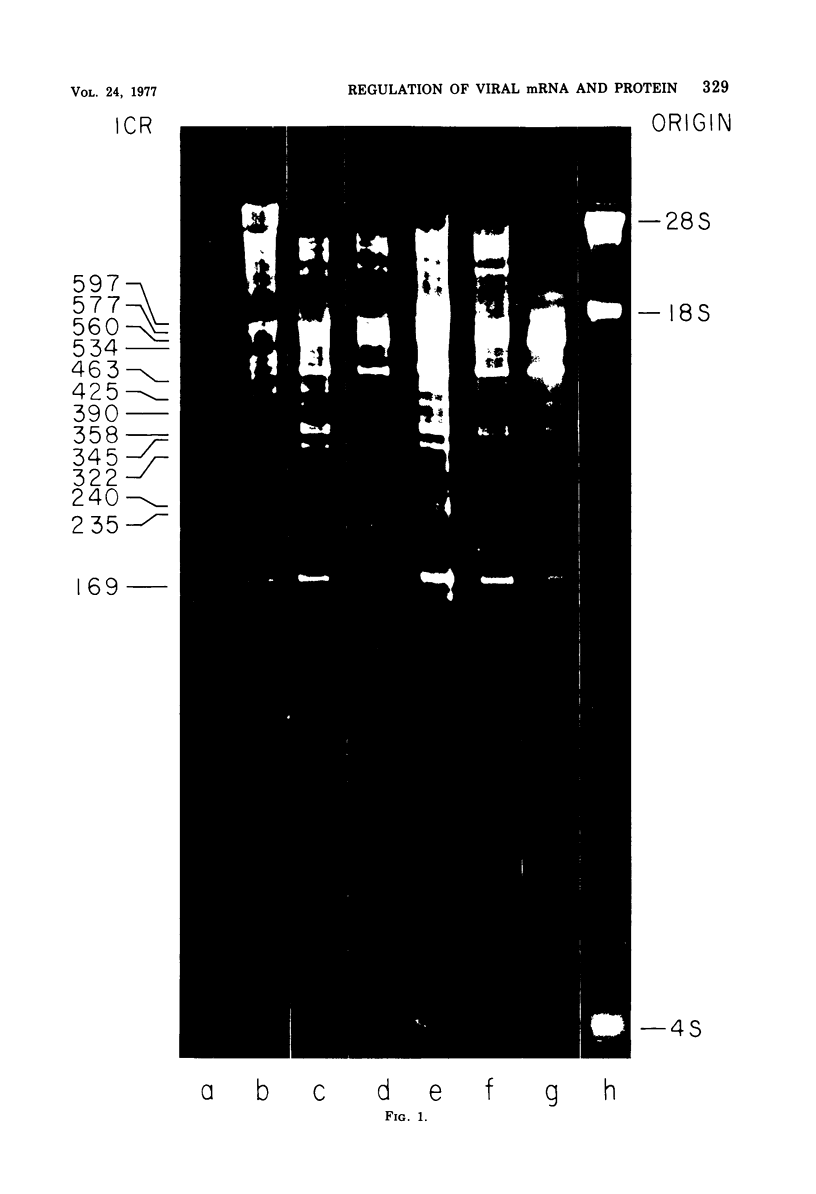
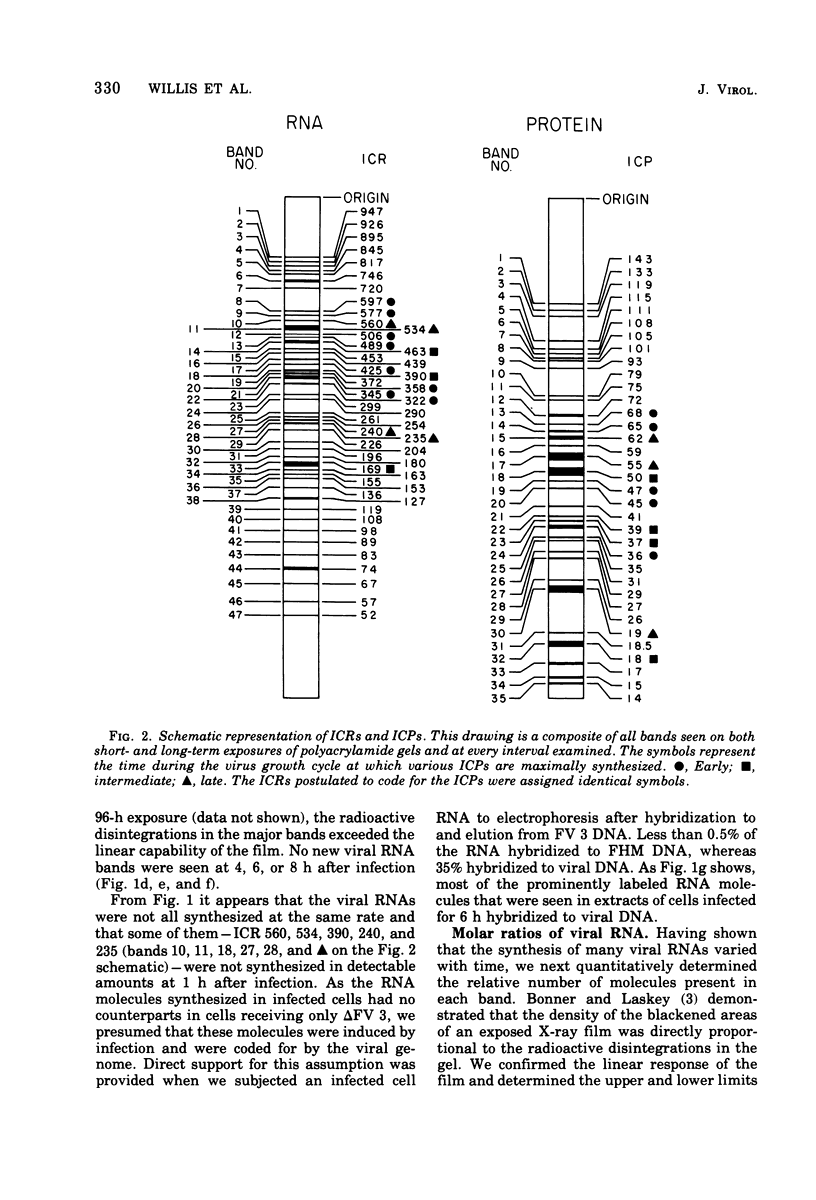
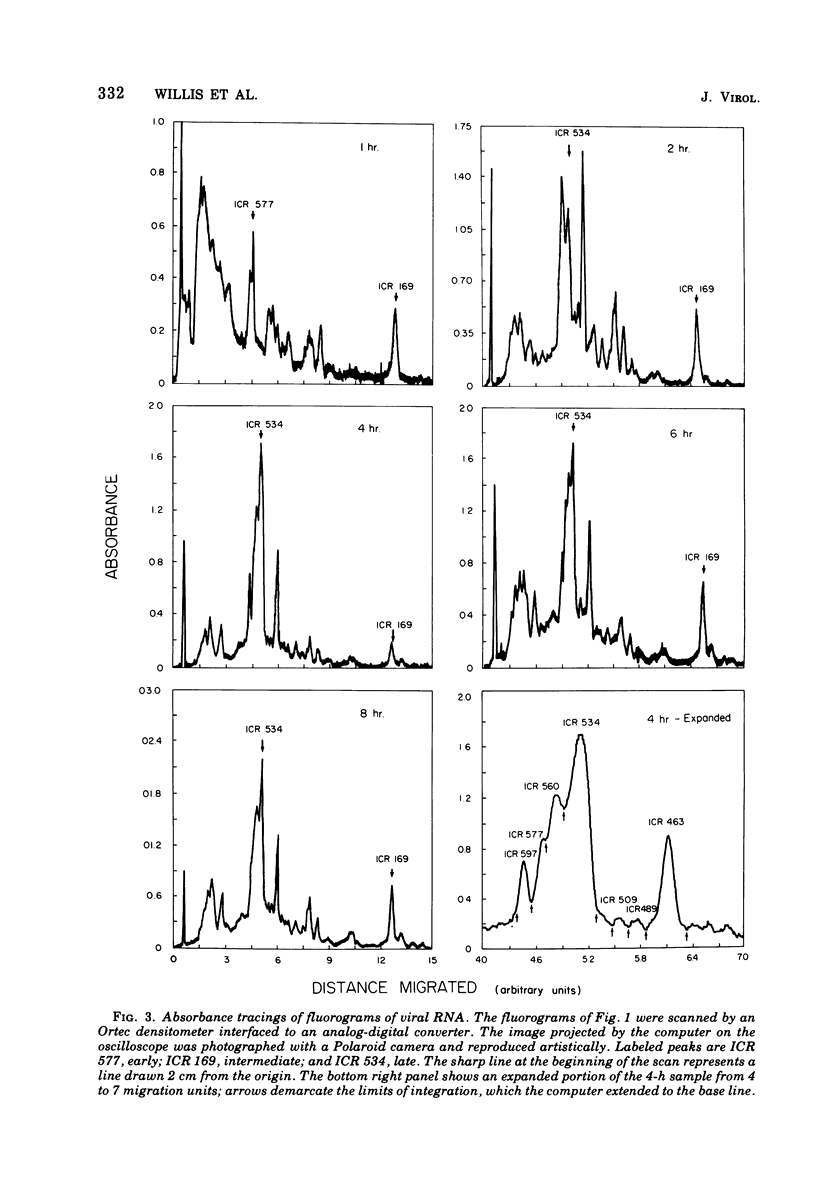
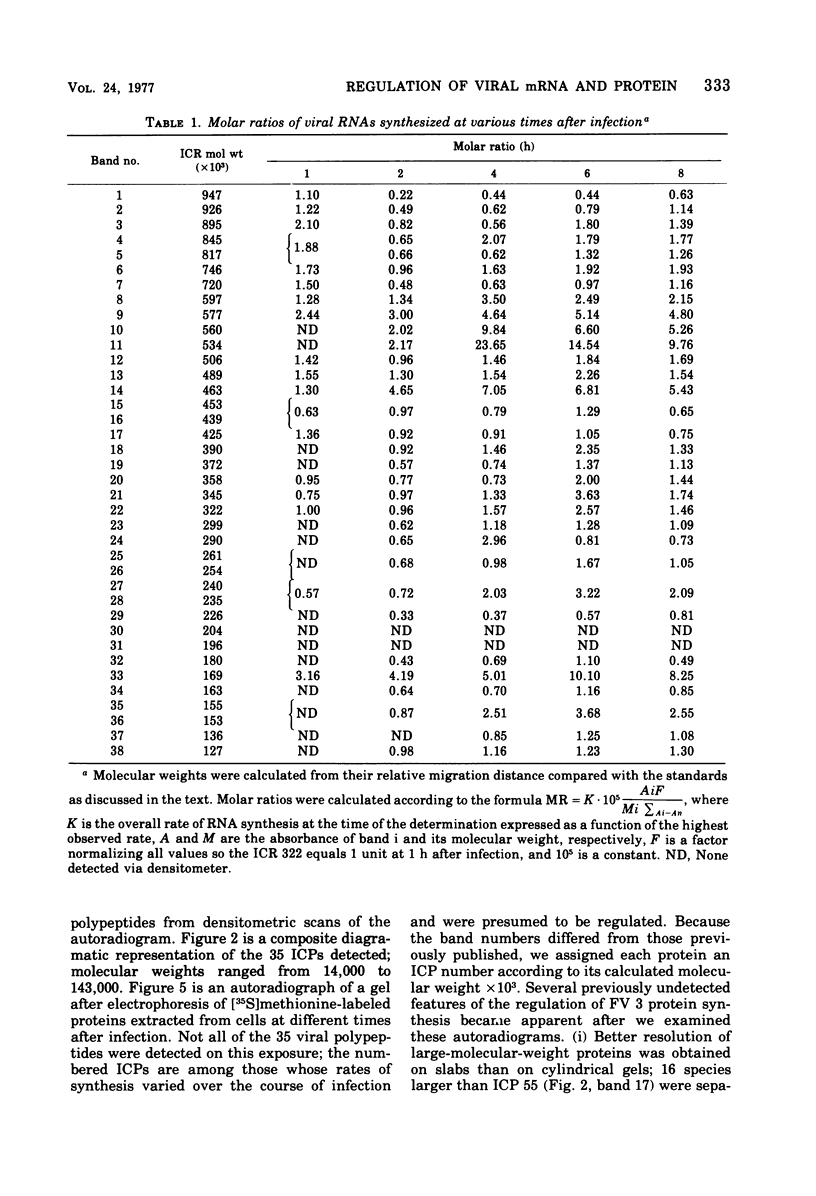
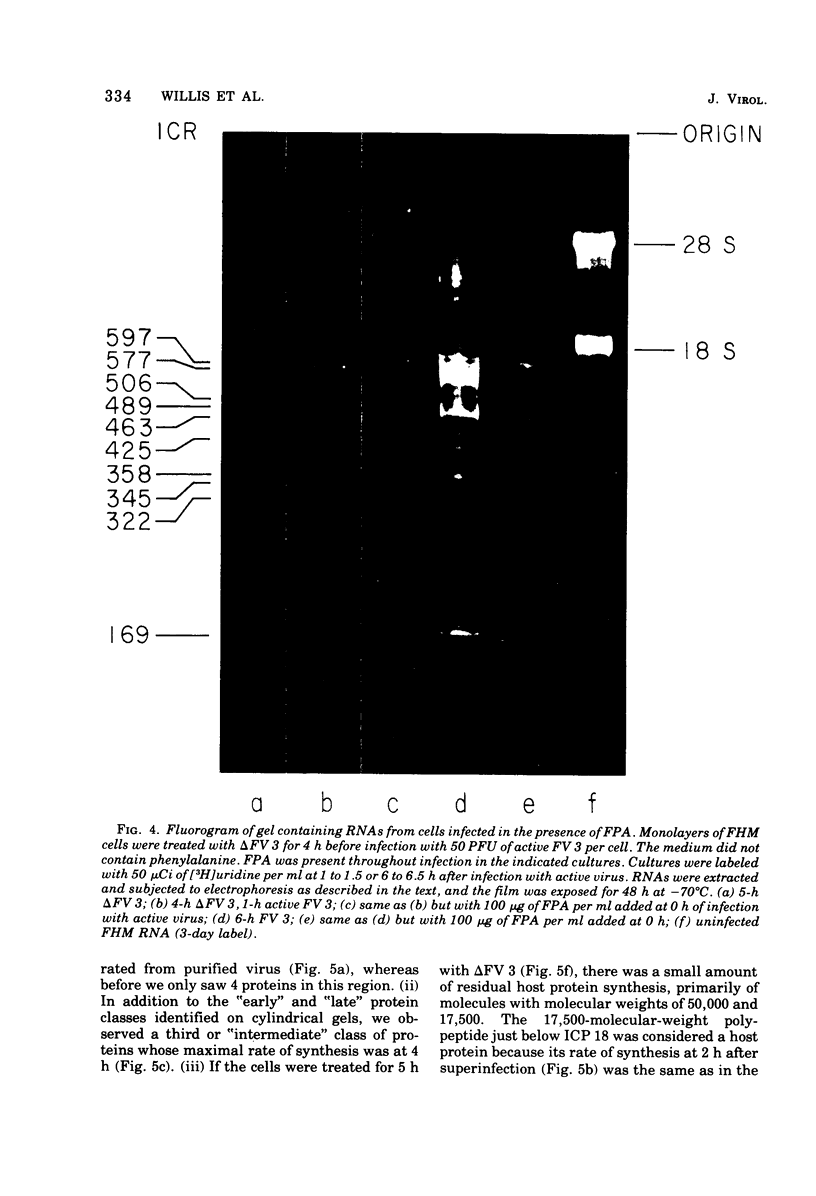
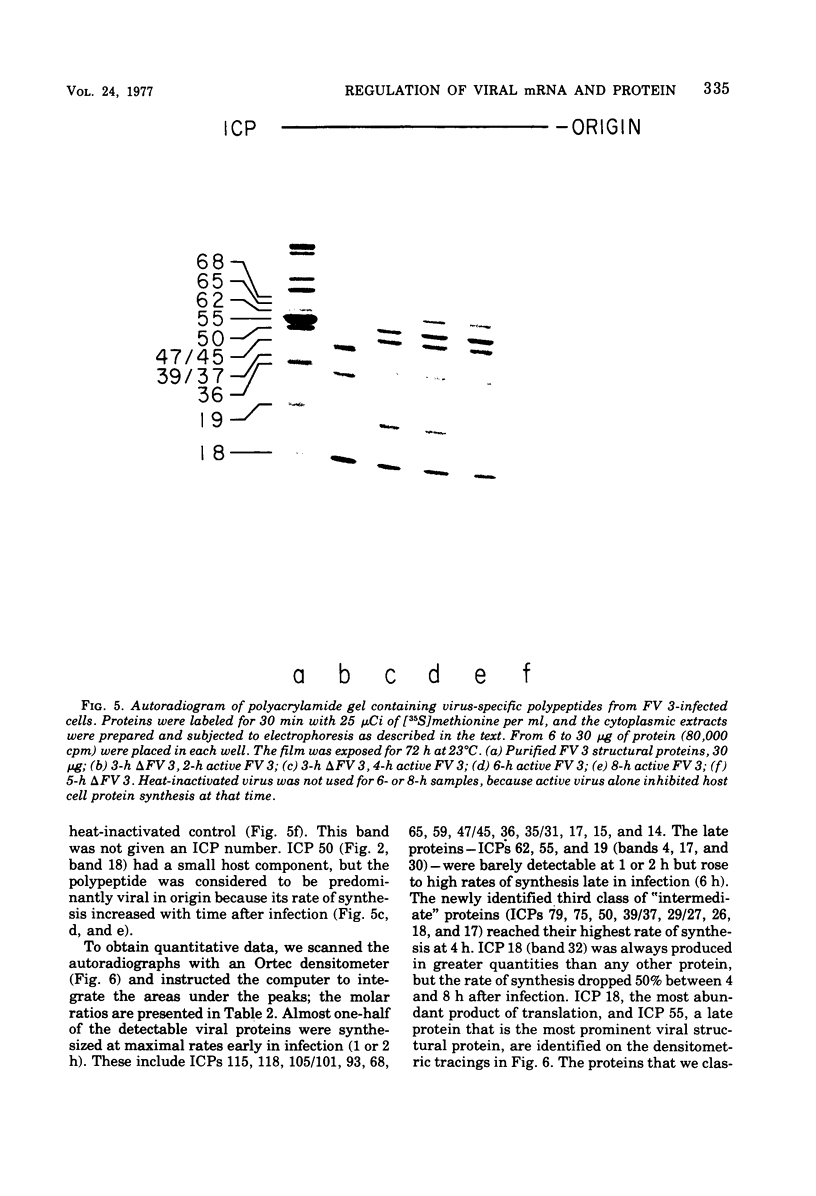
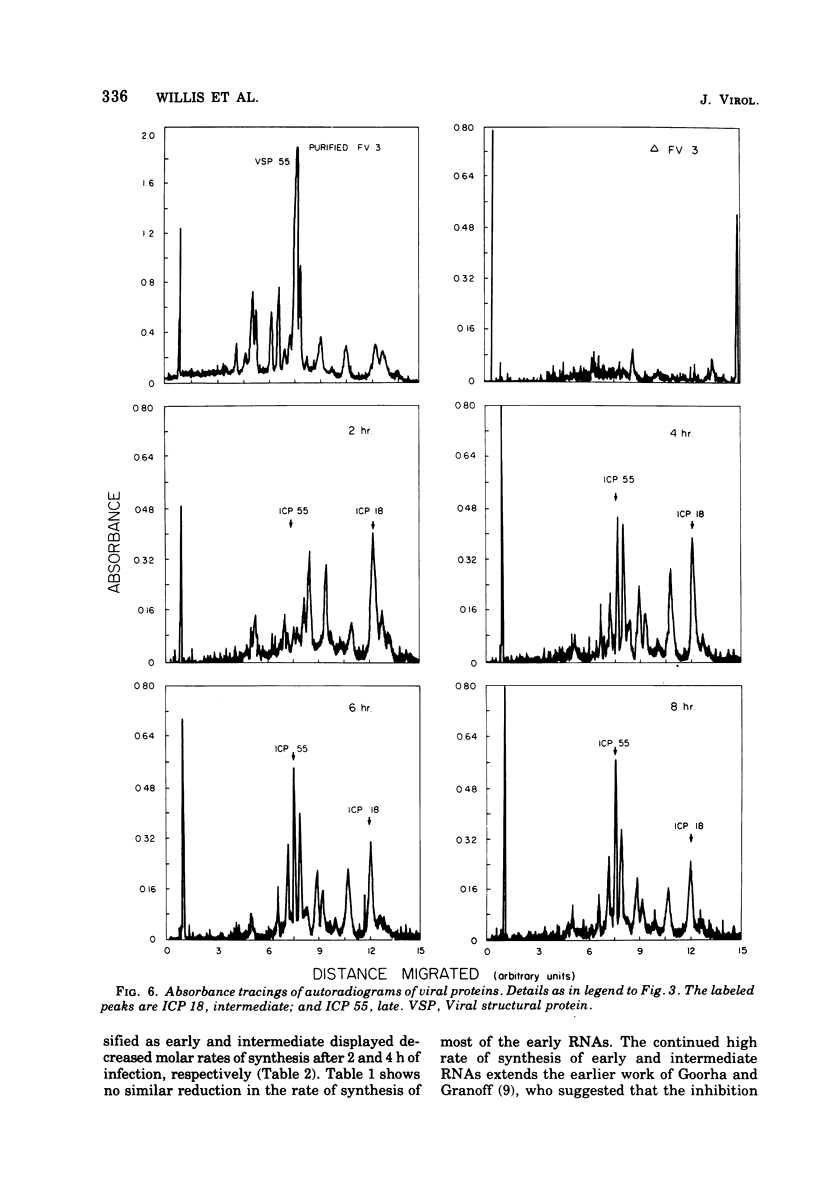
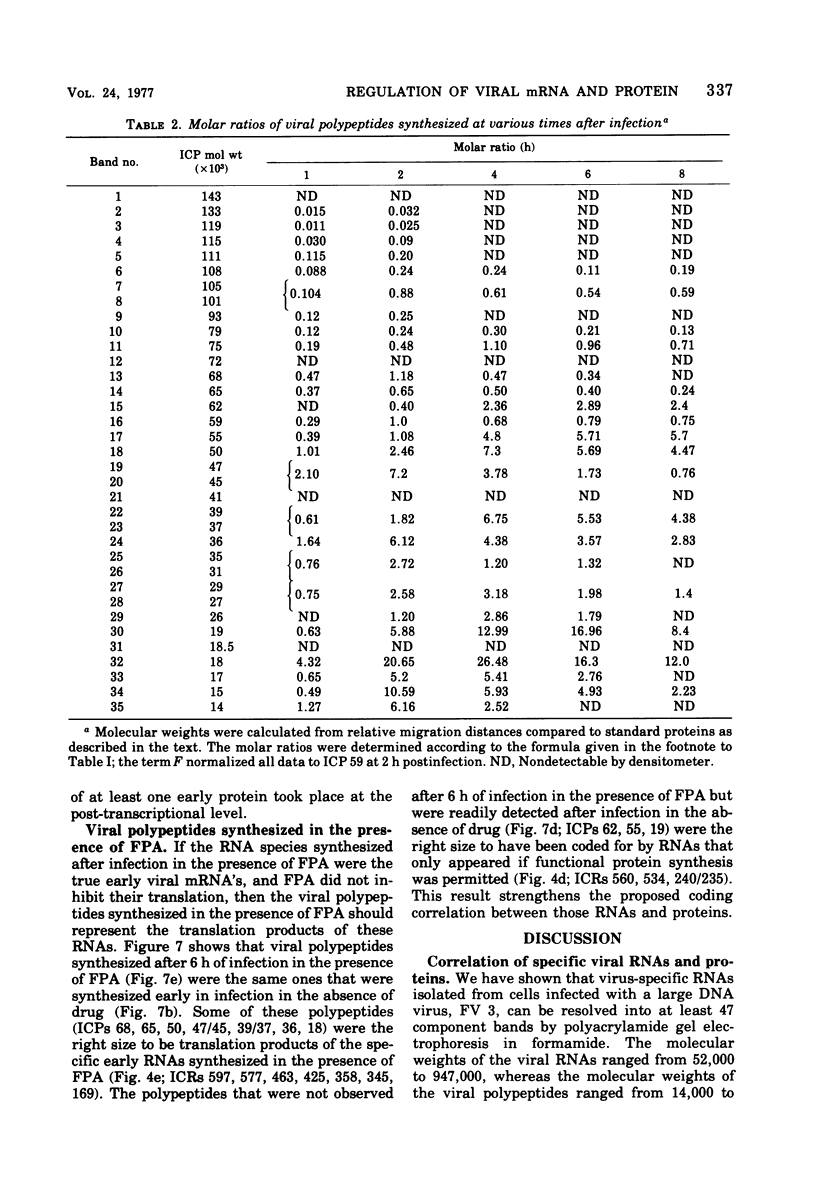
We have used improved techniques for separating individual species of RNA and protein to study the mechanisms that control gene expression by frog virus 3, a eucaryotic DNA virus. Forty-seven species of viral RNA and 35 viral polypeptide species were resolved by polyacrylamide gel electrophoresis. The relative molar ratios of virus-specific polypeptides synthesized at various times after infection were determined by computer planimetry and were compared with the molar ratios of appropriate-sized viral RNAs to code for each polypeptide. Viral polypeptides were classified according to the time during the growth cycle at which their maximal rate of synthesis occurred - early, 2 to 2.5 h; intermediate, 4 to 4.5 h; and late, 6 to 6.5 h. The viral RNAs, which were assumed to be mRNA's, could not be classified according to time of maximum synthesis; once their synthesis had begun, most of the RNAs continued to be synthesized at the same or higher rates. However, only 10 of the 47 viral RNA bands were plainly visible after electrophoresis of extracts from cells labeled from 1 to 1.5 h after infection; these 10 RNAs were designated “early” RNA. The early pattern of both RNA and polypeptide synthesis was maintained for at least 6 h in the presence of the amino acid analog fluorophenylalanine, which indicates that a functional viral polypeptide was required for “late” transcription and translation. The presumptive mRNA's for late polypeptides did not appear until 2 h after infection, but two of these “late” RNAs became the major products of transcription by 4 h into the infectious cycle. In contrast to the declining rate of synthesis of the early proteins, corresponding early RNA species continued to be synthesized at the same or higher rates throughout the replicative cycle. Although the synthesis of late virus-specific proteins appeared to be regulated at the level of transcription, our results suggest that the synthesis of both early and intermediate proteins was regulated at the post-transcriptional level.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aloni Y., Winocour E., Sachs L. Characterization of the simian virus 40-specific RNA in virus-yielding and transformed cells. J Mol Biol. 1968 Feb 14;31(3):415–429. doi: 10.1016/0022-2836(68)90418-x. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Both G. W., Lavi S., Shatkin A. J. Synthesis of all the gene products of the reovirus genome in vivo and in vitro. Cell. 1975 Feb;4(2):173–180. doi: 10.1016/0092-8674(75)90124-5. [DOI] [PubMed] [Google Scholar]
- Bradley D. E., Pitt T. L. Pilus-dependence of four Pseudomonas aeruginosa bacteriophages with non-contractile tails. J Gen Virol. 1974 Jul;24(1):1–15. doi: 10.1099/0022-1317-24-1-1. [DOI] [PubMed] [Google Scholar]
- Duesberg P. H., Vogt P. K. Gel electrophoresis of avian leukosis and sarcoma viral RNA in formamide: comparison with other viral and cellular RNA species. J Virol. 1973 Sep;12(3):594–599. doi: 10.1128/jvi.12.3.594-599.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenkel N., Roizman B. Ribonucleic acid synthesis in cells infected with herpes simplex virus: controls of transcription and of RNA abundance. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2654–2658. doi: 10.1073/pnas.69.9.2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage L. P., Geiduschek E. P. Repression of early messenger transcription in the development of a bacteriophage. J Mol Biol. 1967 Dec 14;30(2):435–440. [PubMed] [Google Scholar]
- Goorha R., Granoff A. Macromolecular synthesis in cells infected by frog virus 3. I. Virus-specific protein synthesis and its regulation. Virology. 1974 Jul;60(1):237–250. doi: 10.1016/0042-6822(74)90381-x. [DOI] [PubMed] [Google Scholar]
- Goorha R., Granoff A. Macromolecular synthesis in cells infected by frog virus 3. II. Evidence for post-transcriptional control of a viral structural protein. Virology. 1974 Jul;60(1):251–259. doi: 10.1016/0042-6822(74)90382-1. [DOI] [PubMed] [Google Scholar]
- Goorha R., Naegele R. F., Purifoy D., Granoff A. Macromolecular synthesis in cells infected with frog virus 3. III. Virus-specific protein synthesis by temperature-sensitive mutants. Virology. 1975 Aug;66(2):428–439. doi: 10.1016/0042-6822(75)90215-9. [DOI] [PubMed] [Google Scholar]
- Goorha R., Willis D. B., Granoff A. Macromolecular synthesis in cells infected by frog virus 3. VI. Frog virus 3 replication is dependent on the cell nucleus. J Virol. 1977 Feb;21(2):802–805. doi: 10.1128/jvi.21.2.802-805.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granoff A., Came P. E., Breeze D. C. Viruses and renal carcinoma of Rana pipiens. I. The isolation and properties of virus from normal and tumor tissue. Virology. 1966 May;29(1):133–148. doi: 10.1016/0042-6822(66)90203-0. [DOI] [PubMed] [Google Scholar]
- Honess R. W., Roizman B. Proteins specified by herpes simplex virus. XI. Identification and relative molar rates of synthesis of structural and nonstructural herpes virus polypeptides in the infected cell. J Virol. 1973 Dec;12(6):1347–1365. doi: 10.1128/jvi.12.6.1347-1365.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honess R. W., Roizman B. Regulation of herpesvirus macromolecular synthesis. I. Cascade regulation of the synthesis of three groups of viral proteins. J Virol. 1974 Jul;14(1):8–19. doi: 10.1128/jvi.14.1.8-19.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honess R. W., Roizman B. Regulation of herpesvirus macromolecular synthesis: sequential transition of polypeptide synthesis requires functional viral polypeptides. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1276–1280. doi: 10.1073/pnas.72.4.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Lucas J. J., Ginsberg H. S. Synthesis of virus-specific ribonucleic acid in KB cells infected with type 2 adenovirus. J Virol. 1971 Aug;8(2):203–214. doi: 10.1128/jvi.8.2.203-214.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCAUSLAN B. R. THE INDUCTION AND REPRESSION OF THYMIDINE KINASE IN THE POXVIRUS-INFECTED HELA CELL. Virology. 1963 Nov;21:383–389. doi: 10.1016/0042-6822(63)90199-5. [DOI] [PubMed] [Google Scholar]
- Milcarek C., Price R., Penman S. The metabolism of a poly(A) minus mRNA fraction in HeLa cells. Cell. 1974 Sep;3(1):1–10. doi: 10.1016/0092-8674(74)90030-0. [DOI] [PubMed] [Google Scholar]
- Naegele R. F., Granoff A. Viruses and renal carcinoma of Rana pipiens. XI. Isolation of frog virus 3 temperature-sensitive mutants; complementation and genetic recombination. Virology. 1971 May;44(2):286–295. doi: 10.1016/0042-6822(71)90260-1. [DOI] [PubMed] [Google Scholar]
- Nonoyama M., Millward S., Graham A. F. Control of transcription of the reovirus genome. Nucleic Acids Res. 1974 Mar;1(3):373–385. doi: 10.1093/nar/1.3.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda K. I., Joklik W. K. Hybridization and sedimentation studies on "early" and "late" vaccinia messenger RNA. J Mol Biol. 1967 Aug 14;27(3):395–419. doi: 10.1016/0022-2836(67)90047-2. [DOI] [PubMed] [Google Scholar]
- Oppermann H., Koch G. On the regulation of protein synthesis in vaccinia virus infected cells. J Gen Virol. 1976 Aug;32(2):261–273. doi: 10.1099/0022-1317-32-2-261. [DOI] [PubMed] [Google Scholar]
- Peacock A. C., Dingman C. W. Molecular weight estimation and separation of ribonucleic acid by electrophoresis in agarose-acrylamide composite gels. Biochemistry. 1968 Feb;7(2):668–674. doi: 10.1021/bi00842a023. [DOI] [PubMed] [Google Scholar]
- RICHMOND M. H. The effect of amino acid analogues on growth and protein synthesis in microorganisms. Bacteriol Rev. 1962 Dec;26:398–420. doi: 10.1128/br.26.4.398-420.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchey M. B., Palese P., Schulman J. L. Mapping of the influenza virus genome. III. Identification of genes coding for nucleoprotein, membrane protein, and nonstructural protein. J Virol. 1976 Oct;20(1):307–313. doi: 10.1128/jvi.20.1.307-313.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatkin A. J., Sipe J. D., Loh P. Separation of ten reovirus genome segments by polyacrylamide gel electrophoresis. J Virol. 1968 Oct;2(10):986–991. doi: 10.1128/jvi.2.10.986-991.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travers A. A. Bacteriophage sigma factor for RNA polymerase. Nature. 1969 Sep 13;223(5211):1107–1110. doi: 10.1038/2231107a0. [DOI] [PubMed] [Google Scholar]
- Villarreal L. P., Breindl M., Holland J. J. Determination of molar ratios of vesicular stomatitis virus induced RNA species in BHK21 cells. Biochemistry. 1976 Apr 20;15(8):1663–1667. doi: 10.1021/bi00653a012. [DOI] [PubMed] [Google Scholar]
- Willis D. B., Granoff A. Macromolecular synthesis in cells infected by frog virus 3. IV. Regulation of virus-specific RNA synthesis. Virology. 1976 Apr;70(2):399–410. doi: 10.1016/0042-6822(76)90281-6. [DOI] [PubMed] [Google Scholar]
- Willis D. B., Granoff A. Macromolecular synthesis in cells infected with frog virus 3. V. The absence of polyadenylic acid in the majority of frog virus 3-specific mRNA species. Virology. 1976 Sep;73(2):543–547. doi: 10.1016/0042-6822(76)90417-7. [DOI] [PubMed] [Google Scholar]
- Wold W. S., Green M., Brackmann K. H., Cartas M. A., Devine C. Genome expression and mRNA maturation at late stages of productive adenovirus type 2 infection. J Virol. 1976 Nov;20(2):465–477. doi: 10.1128/jvi.20.2.465-477.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]