Abstract
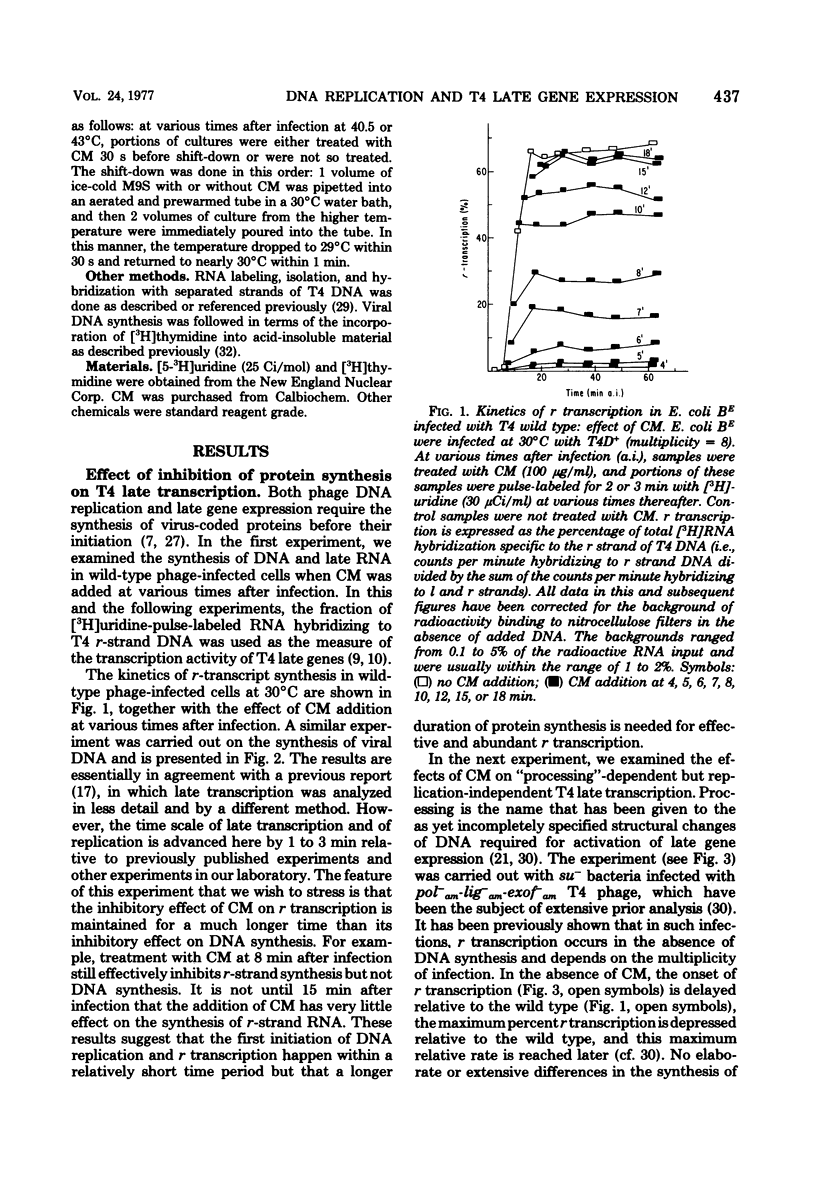
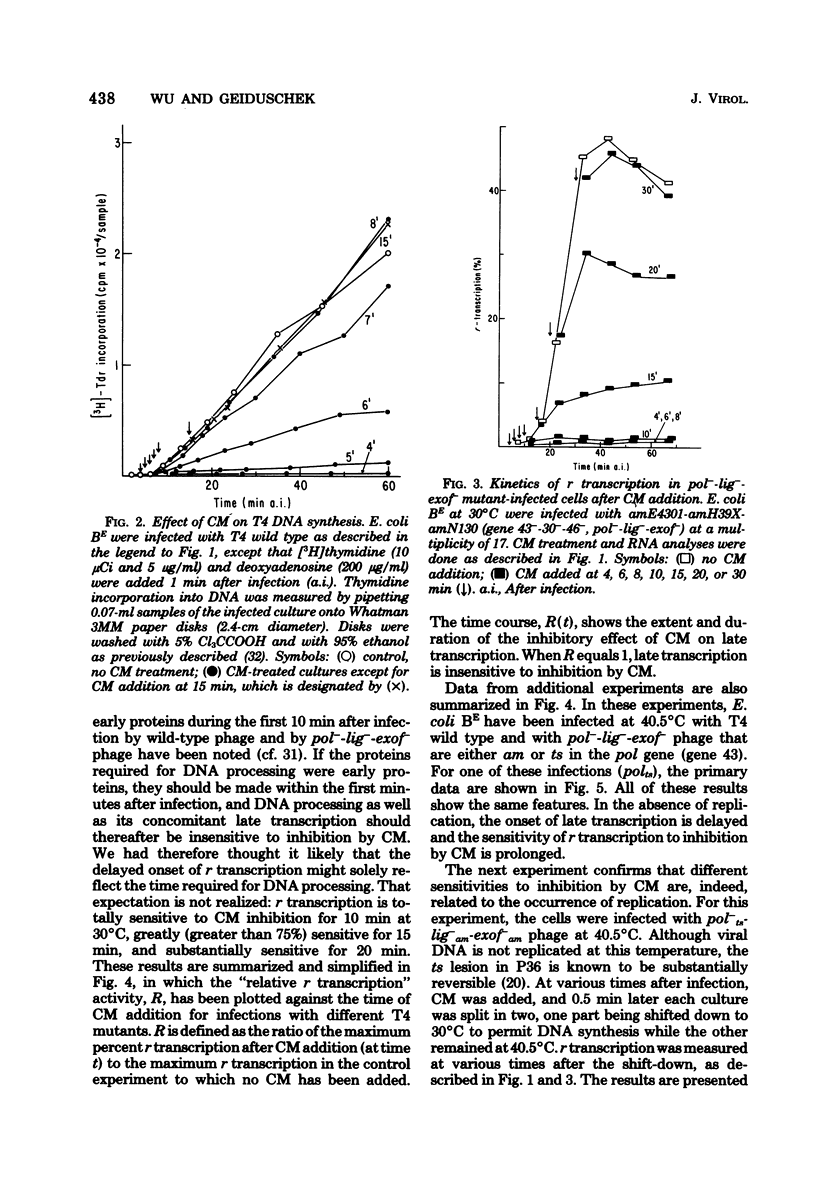
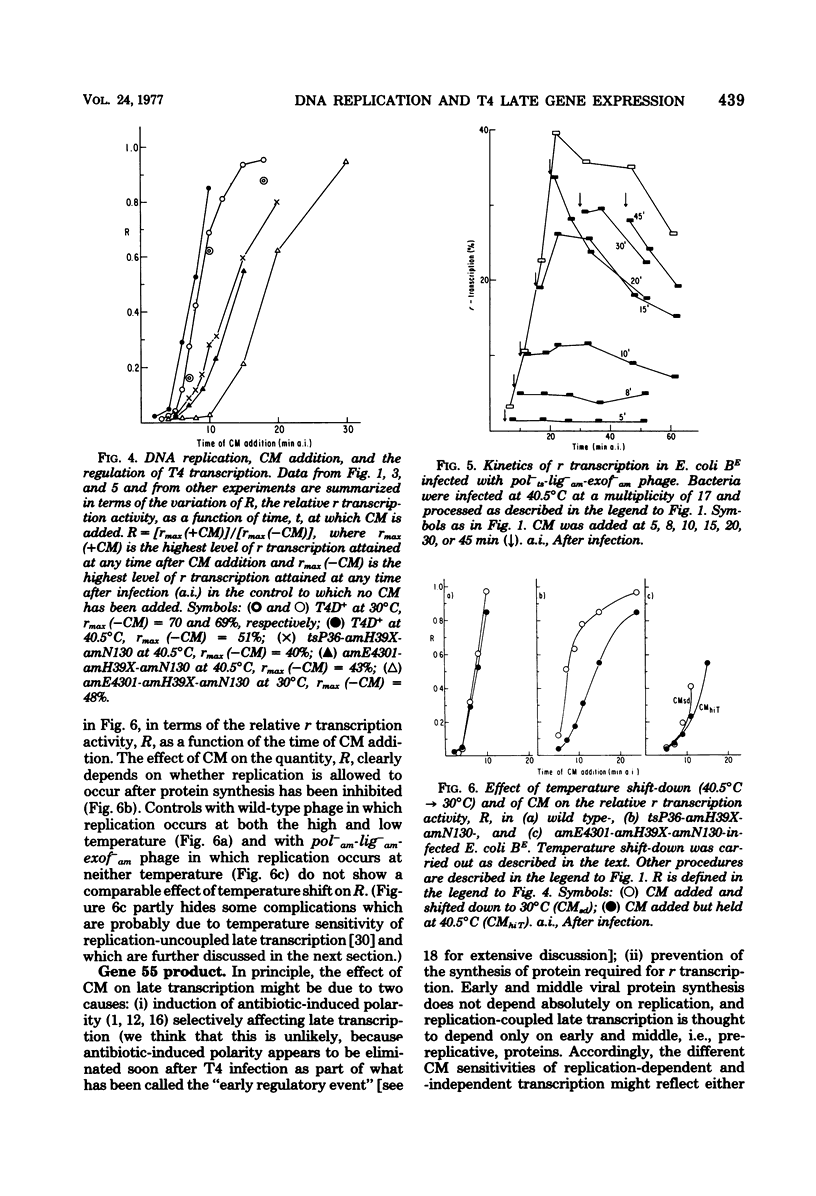
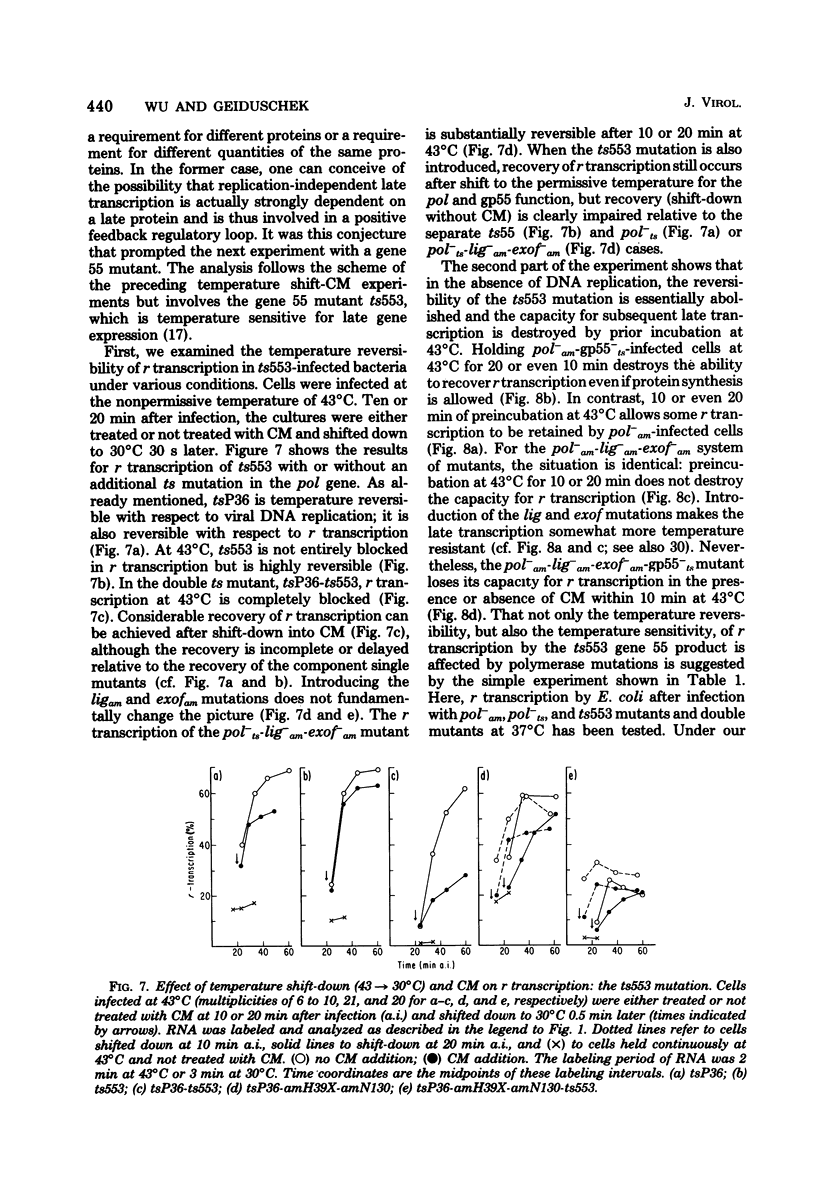
This paper further explores the relationship of viral DNA replication to bacteriophage T4 late gene expression. It is shown that replication coupled and -independent late transcription make different qualitative or quantitative demands on phage protein synthesis. In further analysis of these different protein synthesis requirements, experiments were performed with a temperature-sensitive mutant in T4 gene 55 (ts553). It is known that the gene 55 product regulates T4 late gene expression and binds to RNA polymerase. In the experiments presented here, it is shown that the temperature sensitivity of the ts553 gene 55 protein depends on whether it is involved in replication-coupled or -independent T4 late transcription. This is evidence that the proteins constituting the transcription apparatus interact differently with late transcription units in T4 DNA, depending on whether late transcription is replication coupled or independent.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alpers D. H., Tomkins G. M. Sequential transcription of the genes of the lactose operon and its regulation by protein synthesis. J Biol Chem. 1966 Oct 10;241(19):4434–4443. [PubMed] [Google Scholar]
- Bolle A., Epstein R. H., Salser W., Geiduschek E. P. Transcription during bacteriophage T4 development: requirements for late messenger synthesis. J Mol Biol. 1968 Apr 28;33(2):339–362. doi: 10.1016/0022-2836(68)90193-9. [DOI] [PubMed] [Google Scholar]
- Bolle A., Epstein R. H., Salser W., Geiduschek E. P. Transcription during bacteriophage T4 development: synthesis and relative stability of early and late RNA. J Mol Biol. 1968 Feb 14;31(3):325–348. doi: 10.1016/0022-2836(68)90413-0. [DOI] [PubMed] [Google Scholar]
- Coppo A., Manzi A., Pulitzer J. F. Host mutant (tabD)-induced inhibition of bacteriophage T4 late transcription. II. Genetic characterization of mutants. J Mol Biol. 1975 Aug 25;96(4):601–624. doi: 10.1016/0022-2836(75)90141-2. [DOI] [PubMed] [Google Scholar]
- Coppo A., Manzi A., Pulitzer J. F., Takahashi H. Host mutant (tabD)-induced inhibition of bacteriophage T4 late transcription. I. Isolation and phenotypic characterization of the mutants. J Mol Biol. 1975 Aug 25;96(4):579–600. doi: 10.1016/0022-2836(75)90140-0. [DOI] [PubMed] [Google Scholar]
- Goff C. G. Chemical structure of a modification of the Escherichia coli ribonucleic acid polymerase alpha polypeptides induced by bacteriophage T4 infection. J Biol Chem. 1974 Oct 10;249(19):6181–6190. [PubMed] [Google Scholar]
- Guha A., Szybalski W. Fractionation of the complementary strands of coliphage T4 DNA based on the asymmetric distribution of the poly U and poly U,G binding sites. Virology. 1968 Apr;34(4):608–616. doi: 10.1016/0042-6822(68)90082-2. [DOI] [PubMed] [Google Scholar]
- Guha A., Szybalski W., Salser W., Geiduschek E. P., Pulitzer J. F., Bolle A. Controls and polarity of transcription during bacteriophage T4 development. J Mol Biol. 1971 Jul 28;59(2):329–349. doi: 10.1016/0022-2836(71)90054-4. [DOI] [PubMed] [Google Scholar]
- Horvitz H. R. Polypeptide bound to the host RNA polymerase is specified by T4 control gene 33. Nat New Biol. 1973 Aug 1;244(135):137–140. doi: 10.1038/newbio244137a0. [DOI] [PubMed] [Google Scholar]
- Imamoto F. Evidence for premature termination of transcription of the tryptophan operon in polarity mutants of Escherichia coli. Nature. 1970 Oct 17;228(5268):232–235. doi: 10.1038/228232a0. [DOI] [PubMed] [Google Scholar]
- Lembach K. J., Kuninaka A., Buchanan J. M. The relationship of DNA replication to the control of protein synthesis in protoplasts of T4-infected Escherichia coli B. Proc Natl Acad Sci U S A. 1969 Feb;62(2):446–453. doi: 10.1073/pnas.62.2.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse D. E., Yanofsky C. Polarity and the degradation of mRNA. Nature. 1969 Oct 25;224(5217):329–331. doi: 10.1038/224329a0. [DOI] [PubMed] [Google Scholar]
- Pulitzer J. F. Function of T4 gene 55. I. Characterization of temperature-sensitive mutations in the "maturation" gene 55. J Mol Biol. 1970 Apr 28;49(2):473–488. doi: 10.1016/0022-2836(70)90258-5. [DOI] [PubMed] [Google Scholar]
- Ratner D. The interaction bacterial and phage proteins with immobilized Escherichia coli RNA polymerase. J Mol Biol. 1974 Sep 15;88(2):373–383. doi: 10.1016/0022-2836(74)90488-4. [DOI] [PubMed] [Google Scholar]
- Riva S., Cascino A., Geiduschek E. P. Coupling of late transcription to viral replication in bacteriophage T4 development. J Mol Biol. 1970 Nov 28;54(1):85–102. doi: 10.1016/0022-2836(70)90447-x. [DOI] [PubMed] [Google Scholar]
- Riva S., Cascino A., Geiduschek E. P. Uncoupling of late transcription from DNA replication in bacteriophage T4 development. J Mol Biol. 1970 Nov 28;54(1):103–119. doi: 10.1016/0022-2836(70)90448-1. [DOI] [PubMed] [Google Scholar]
- Stevens A. An isotopic study of DNA-dependent RNA polymerase of E. coli following T4 phage infection. Biochem Biophys Res Commun. 1970 Oct 23;41(2):367–373. doi: 10.1016/0006-291x(70)90513-9. [DOI] [PubMed] [Google Scholar]
- Stevens A. Deoxyribonucleic acid dependent ribonucleic acid polymerases from two T4 phage-infected systems. Biochemistry. 1974 Jan 29;13(3):493–503. doi: 10.1021/bi00700a015. [DOI] [PubMed] [Google Scholar]
- Stevens A. New small polypeptides associated with DNA-dependent RNA polymerase of Escherichia coli after infection with bacteriophage T4. Proc Natl Acad Sci U S A. 1972 Mar;69(3):603–607. doi: 10.1073/pnas.69.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WIBERG J. S., DIRKSEN M. L., EPSTEIN R. H., LURIA S. E., BUCHANAN J. M. Early enzyme synthesis and its control in E. coli infected with some amber mutants of bacteriophage T4. Proc Natl Acad Sci U S A. 1962 Feb;48:293–302. doi: 10.1073/pnas.48.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter G., Seifert W., Zillig W. Modified DNA-dependent RNA polymerase from E. coli infected with bacteriophage T4. Biochem Biophys Res Commun. 1968 Feb 15;30(3):240–247. doi: 10.1016/0006-291x(68)90441-5. [DOI] [PubMed] [Google Scholar]
- Wood W. B., Revel H. R. The genome of bacteriophage T4. Bacteriol Rev. 1976 Dec;40(4):847–868. doi: 10.1128/br.40.4.847-868.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu R., Geiduschek E. P. The role of replication proteins in the regulation of bacteriophage T4 transcription. I. Gene 45 and hydroxymethyl-C-containing DNA. J Mol Biol. 1975 Aug 25;96(4):513–538. doi: 10.1016/0022-2836(75)90137-0. [DOI] [PubMed] [Google Scholar]
- Wu R., Geiduschek E. P. The role of replication proteins in the regulation of bacteriophage T4 transcription. II. Gene 45 and late transcription uncoupled from replication. J Mol Biol. 1975 Aug 25;96(4):539–562. doi: 10.1016/0022-2836(75)90138-2. [DOI] [PubMed] [Google Scholar]
- Wu R., Yeh Y. C. DNA arrested mutants of gene 59 of bacteriophage T4. II. Replicative intermediates. Virology. 1974 May;59(1):108–122. doi: 10.1016/0042-6822(74)90209-8. [DOI] [PubMed] [Google Scholar]


