Abstract
Bacteria respond to environmental stresses using a variety of signaling and gene expression pathways, with translational mechanisms being the least well understood. Here, we identified a tRNA methyltransferase in Pseudomonas aeruginosa PA14, trmJ, which confers resistance to oxidative stress. Analysis of tRNA from a trmJ mutant revealed that TrmJ catalyzes formation of Cm, Um, and, unexpectedly, Am. Defined in vitro analyses revealed that tRNAMet(CAU) and tRNATrp(CCA) are substrates for Cm formation, tRNAGln(UUG), tRNAPro(UGG), tRNAPro(CGG) and tRNAHis(GUG) for Um, and tRNAPro(GGG) for Am. tRNASer(UGA), previously observed as a TrmJ substrate in Escherichia coli, was not modified by PA14 TrmJ. Position 32 was confirmed as the TrmJ target for Am in tRNAPro(GGG) and Um in tRNAGln(UUG) by mass spectrometric analysis. Crystal structures of the free catalytic N-terminal domain of TrmJ show a 2-fold symmetrical dimer with an active site located at the interface between the monomers and a flexible basic loop positioned to bind tRNA, with conformational changes upon binding of the SAM-analog sinefungin. The loss of TrmJ rendered PA14 sensitive to H2O2 exposure, with reduced expression of oxyR-recG, katB-ankB, and katE. These results reveal that TrmJ is a tRNA:Cm32/Um32/Am32 methyltransferase involved in translational fidelity and the oxidative stress response.
INTRODUCTION
Post-transcriptional ribonucleoside modifications are found in all RNA molecules, with those in tRNA playing roles in tRNA structure and function including maturation, stability, turnover, and fidelity of decoding (1). Modifications on the anticodon stem–loop (ASL) are particularly crucial for maintaining the efficiency and fidelity of translation by stabilizing codon-anticodon pairing, promoting codon recognition, ordering ASL structure, and preventing translational frameshifting (2–5). At the systems level, the dozens of tRNA modifications in yeast have been shown to play critical roles in cellular adaptation to environmental stresses, with reprogramming of tRNA wobble modifications leading to selective translation of critical stress response transcripts (6–8). For example, up-regulation of wobble mcm5U in tRNAARG(UCU) by exposure to the DNA-damaging agent, methylmethane sulfonate (MMS), enhanced the translation of transcripts enriched with the AGA cognate codon (8), while H2O2 exposure increased the level of wobble m5C in tRNALeu(CAA), which increased translation of UUG-enriched transcripts that were critical to cellular resistance to H2O2 (7). Here, we begin to explore the role of tRNA modifications in the oxidative stress response of prokaryotes, using the PA14 strain of Pseudomonas aeruginosa.
As an opportunistic pathogen, P. aeruginosa is one of the most common nosocomial pathogens and causes acute and chronic respiratory infections in hospitalized and immunocompromised patients (9), with the US Center for Disease Control and Prevention identifying multidrug-resistant P. aeruginosa as a serious threat (10). The complex pathophysiology of P. aeruginosa depends on both host response and bacterial virulence factors, with neutrophils playing a particularly important role in P. aeruginosa infections (11). As a defense against the reactive oxygen species generated by phagocytes, P. aeruginosa possesses a variety of protective mechanisms, including the antioxidant catalases, superoxide dismutases and peroxiredoxins, along with DNA and protein damage repair systems and tight regulation of iron homeostasis (12,13). The P. aeruginosa adaptive response to stress is controlled at both transcriptional and post-transcriptional levels, with the well-defined OxyR, SoxR, OhrR and OspR systems regulating transcription of genes having a critical role in pathogenic defense response against oxidative stress (14–17). Conversely, the control of adaptive response to oxidative stress at the post-transcriptional level is poorly understood.
We have identified an Escherichia coli trmJ homolog in P. aeruginosa (PA14_14690) that confers resistance to oxidative stress. Here, we present a biochemical characterization showing that PA14 trmJ encodes a tRNA (cytidine(32)/uridine(32)/adenosine(32)-2′-O)-methyltransferase that regulates the expression of oxidative stress response genes. Complementary structural analysis of the catalytic N-terminal domain of TrmJ (TrmJ–NTD) in its free form and bound to the S-adenosyl-L-methionine (SAM) analogue in sinefungin forms a basis to design competitive inhibitors specific for this important human pathogen.
MATERIALS AND METHODS
Chemicals and reagents
Single-stranded synthetic DNAs and PCR primers were purchased from Integrated DNA Technologies. UltraPure™ 1 M Tris–HCl, pH 7.5 and molecular biology grade MgCl2 (1 M) for enzymatic assay were purchased from Thermo, Invitrogen. S-Adenosyl-l-methionine (Adomet or SAM) was purchased from Sigma.
Expression and purification of P. aeruginosa PA14 TrmJ
The gene encoding the complete TrmJ (Genbank accession: KFL12361.1) from Pseudomonas aeruginosa PA14, with codons optimized for bacterial expression, was purchased from Genscript. The DNA corresponding to the complete TrmJ protein spanning residues 1–257 (‘TrmJ’) and 1–167 (comprising the N-terminal SAM binding domain, referred to as ‘TrmJ–NTD’) were cloned into the pNIC28-Bsa4 vector that contains six histidine residues and a TEV cleavage site at the N-terminal end. E. coli BL21 (DE3) Rosetta T1R cells were grown at 37°C to an OD600 of 1. The proteins were then overexpressed by the addition of 0.5 mM IPTG at 18°C for 18 h. Cells were pelleted and stored at −80°C. All proteins were purified at 4°C. The thawed pellets were resuspended in 30 ml of lysis buffer (20 mM Na-HEPES, pH 7.5, 0.3 M NaCl, 5% glycerol, 0.5 mM TCEP), sonicated in the presence of a cocktail of protease inhibitors (product 539134, Calbiochem), and the lysates were cleared by centrifugation at 22 000 rpm for 30 min. The proteins were purified by immobilized metal affinity chromatography (IMAC) using Ni-NTA agarose (Qiagen) and by size-exclusion on a HiLoad 16/60 Superdex 75 column preparative grade (GE Healthcare) for TrmJ–NTD (20 686 Da, confirmed by mass spectrometry) and on a HiLoad 16/60 Superdex 200 column for TrmJ (31 170 Da). TrmJ was concentrated to 20 mg/ml while TrmJ–NTD was concentrated to 50 mg/ml by ultrafiltration in 20 mM Na-HEPES, pH 7.5, 0.3 M NaCl, 5% (v/v) glycerol, 0.5 mM TCEP. All proteins eluted as dimers in gel filtration.
Bacterial strains, plasmids and growth conditions
The bacterial strains and plasmids used in this study are listed in Supplementary Table S1 (18–23). Bacterial cells were cultured on lysogeny broth with antibiotics at 37°C at the following concentrations: 100 μg/ml amplicillin and 15 μg/ml gentamycin for plasmid maintenance in E. coli; 200 μg/ml carbenicillin for plasmid maintenance in PA14 strains; 200 μg/ml carbenicillin, 75 μg/ml gentamycin for maintenance of insertion mutations in trmJ mutant strain and trmJC strain.
Construction of trmJ mutant strain
A trmJ mutant strain was constructed by insertional inactivation using a suicide vector pKNOCK-Gm (18). The 200-bp fragment of trmJ (PA14_14690) was amplified from the PA14 genomic DNA with primer pairs BT3878/BT3879 (Supplementary Table S2), and cloned into pKNOCK-Gm at SmaI site, yielding pKNOCK-trmJ. The resulting clone was used to transform E. coli BW20767, and E. coli BW20767 containing pKNOCK-trmJ was used to transfer pKNOCK-trmJ into PA14 by conjugation. PA14 transconjugants harboring chromosomal insert of the suicide vectors at trmJ gene were selected on lysogeny agar plate containing 75 μg/ml gentamycin and BW20767 was counterselected using 15 μg/ml chloramphenicol. The trmJ mutant strain was subsequently screened by colony PCR, and confirmed by Southern blot.
Construction of trmJC strain
The primer pairs BT3902/BT3992 (Supplementary Table S2) were used to amplify full length trmJ gene (PA14_14690) from the PA14 genomic DNA. Purified PCR product was cloned into pBBR1-MCS4 (21) at SmaI site, yielding the complementation plasmid pBBR-trmJ. The complementation plasmid was used to transform into trmJ mutant strain by electroporation. The complementation strain is designated trmJC. The trmJC strain was selected on lysogeny agar plate containing 75 μg/ml gentamycin and 200 μg/ml carbenicillin and confirmed by PCR.
Isolation and purification of total tRNA from P. aeruginosa strains
Total tRNA was isolated from 50 ml of exponential phase cells (OD600 ≈ 0.4). The cultures were harvested and pelleted by centrifugation at 8000 × g for 10 min at 4°C. The bacterial cell pellet was processed for small RNA species isolation using the PureLink miRNA Isolation Kit following manufacturer's instructions. tRNA in the small RNA population was purified by size-exclusion HPLC using an Agilent SEC3 300 Å, 7.8 × 300 mm column operated at 60°C with a mobile phase of 100 mM ammonium acetate at a flow rate of 0.5 ml/min. tRNA-containing fractions were collected and desalted using an Ambion Millipore 10K MWCO spin-filter system. The quality and concentration of the purified total tRNAs were characterized using a Bioanalyzer (Agilent Small RNA Kit).
In vitro methylation of P. aeruginosa TrmJ using total tRNA as a substrate
Total tRNA isolated from PA14 wild-type and trmJ mutant strains was used for determining the activity of purified PA14 TrmJ. Five micrograms of total tRNA isolated from PA14 wild-type and trmJ mutant were mixed with 50 μM S-adenosyl-l-methionine (SAM) and 5 μM of purified PA14 TrmJ protein in 100 μl reaction volume in 50 mM Tris–HCl, pH 7.5 and 5 mM MgCl2. After incubation at 37°C, the reactions were analyzed as described below (see below).
Identification of tRNA species as substrate for PA14 TrmJ and determination of 2′-O-methylation position in tRNA
tRNA substrates for reaction with PA14 TrmJ were prepared by in vitro T7 transcription. A series of P. aeruginosa tRNA genes were linked to a T7 promoter sequence (Supplementary Table S2) and PCR-amplified using Phusion high fidelity DNA polymerase (Thermo Fisher Scientific) with primers shown in Supplementary Table S2: tRNASer(UGA), tRNAMet(CAU), tRNATrp(CCA), tRNAGln(UUG), tRNAPro(GGG) tRNAPro(UGG), tRNAPro(CGG) or tRNAHis(GUG). PCR product was then used as template for in vitro transcription using Megashortscript T7 transcription kit (Ambion) following manufacturer's instructions. At the end of reaction, the DNA template was removed using DNase. The in vitro transcribed tRNA was further purified and examined for its quality and concentration as described above. Ten micrograms of in vitro transcribed tRNAs were mixed with 50 μM SAM in methylation buffer (50 mM Tris–HCl, pH 7.5 and 5 mM MgCl2) in the presence or absence of PA14 TrmJ. The reactions were incubated at 37°C for 1 h. After incubation, the reactions were collected for analysis by mass spectrometry (see below).
Analysis of ribonucleosides by HPLC-coupled tandem quadrupole mass spectrometry
The ribonucleoside modifications in the various tRNA samples were identified and quantified with chromatography-coupled mass spectrometry (LC–MS) as described previously (24,25). Briefly, tRNA was digested with Benzonase, bacterial alkaline phosphatase, and phosphodiesterase in the presence of deaminase inhibitors (0.5 μg/ml coformycin, 5 μg/ml tetrahydrouridine) and antioxidants (50 μM desferrioxamine, 50 μM butylated hydroxytoluene) at 37°C for 8 h, after which proteins were removed by microfiltration and the filtrate mixtures were analyzed by LC–MS. The ribonucleosides in the enzymatic hydrolysate samples were fractionated on a Thermo Hypersil Gold aQ column (100 × 2.1 mm, 1.9 μm particle size). The mobile phases consisted of 0.1% (v/v) formic acid in water (solvent A) and 0.1% (v/v) formic acid in acetonitrile (solvent B). The samples were fractionated using a flow rate of 0.3 ml/min with a gradient of acetonitrile in 0.1% formic acid as follows: 0–15.3 min, 0%; 15.3–18.7 min, 1%; 18.7–20 min, 6%; 20–24 min, 6%; 24–27.3 min, 100%; and 27.3–41 min, 0%. The HPLC column was maintained at 25°C and directly connected to a triple quadrupole mass spectrometer (Agilent LC/QQQ 6490) operated in positive ion mode. MS parameters were as follows: gas temperature, 50°C; gas flow, 11 l/min; nebulizer, 20 psi; sheath gas flow, 12 l/min; and capillary voltage, 1800 V. The mass spectrometer was operated in MRM mode to detect and quantify ribonucleosides based on the parameters of retention time, m/z of the transmitted parent ion, m/z of the monitored product, fragmentor voltages, and collision energy as noted in Supplementary Table S3. The dwell time was 100 ms. The identities of ribonucleosides were confirmed by comparison of retention time, exact mass, and CID fragmentation to synthetic standards.
RNA fragment analysis HPLC-coupled QTOF mass spectrometry
Five micrograms of in vitro methylated tRNA transcripts were digested with either 5 U RNase T1 and dephosphorylated with 10 U bacterial alkaline phosphatase in 10 mM ammonium acetate buffer (pH 7.0) at 37°C for 4 h in the presence of deaminase inhibitors and antioxidants noted earlier, followed by purification of RNA fragments by solid-phase extraction using a ZipTipC18 (Millipore). The purified RNA fragments were fractionated by HPLC using an Amide-HILIC, TSK-gel Amide-80 column (2.0 mm ID × 150 mm, 3 μm particle sizes) maintained at 50°C and operated at a flow rate of 0.1 ml/min with a gradient of 8 mM ammonium acetate in acetonitrile as follows: 0–2 min, 10%; 2–3 min, 15%; 3–5.5 min, 30%; 5.5-20.5 min, 60%; 20.5–25 min, 70%; 25–35 min, 10%. Eluent from the HPLC column entered a quadrupole time-of-flight mass spectrometer (Agilent 6520) with an electrospray ionization source operated in negative ion mode. MS parameters were as follows: gas temperature, 325°C; drying gas, 8 l/min; nebulizer, 30 psi; fragmentor voltage, 250 V; skimmer voltage, 65 V, and capillary voltage, 3000 V. The mass range was from m/z 100 to 2000. The MS/MS analysis with collision-induced dissociation (CID) was performed to obtain RNA sequence information from the RNase T1 digestion products. Collision energies were varied from 25 to 40 V and products were scanned from 100 to 2000 m/z. The resulting CID mass spectra were analyzed with SOS (26). The fragment sequences were determined based on their a-B, w and y ions.
Hydrogen peroxide susceptibility test
PA14 wild-type, trmJ mutant and trmJC strains were grown to exponential phase at 37°C in lysogeny broth to an OD600 of 0.4. Cultures were diluted to an OD600 of 0.1 in fresh lysogeny broth and treated with either 0, 5, 10, 15 or 20 mM H2O2 for 25 min, followed by 10-fold serial dilutions in PBS buffer. The appropriate cell dilutions were subsequently plated on lysogeny agar plates and incubated overnight at 37°C. Following incubation, colonies were counted and percent surviving of treated cultures was determined relative to the viability of cells without H2O2 treatment.
Catalase activity assay
PA14 wild-type, trmJ mutant and trmJC strains were grown to exponential phase at 37°C in a shaking incubator in lysogeny broth to an OD600 of 0.4 and treated with either 0 or 10 mM H2O2 for 25 min. Cell lysates were prepared by sonication and crude protein was used for the catalase activity assay. The catalase activity was measured spectrophotometrically using 10 mM H2O2 in 50 mM phosphate buffer, pH 7.0, and reported in specific activity, U mg−1 protein. One unit (U) of catalase was defined as the activity that decomposes 1.0 μmol H2O2 min−1 at pH 7.0 at 25°C.
Semi-quantitative real-time PCR analysis of oxyR, katA, katB, katE, ankB and recG transcripts in response to H2O2
PA14 wild-type, trmJ mutant and trmJC strains were grown to exponential phase at 37°C in lysogeny broth to an OD600 of 0.4. Cultures were treated with either 0 or 10 mM H2O2 for 25 min, followed by extraction of total RNA by hot phenol-chloroform method. The RNA samples were treated with DNaseI and the contamination of genomic DNA in RNA samples with DNaseI treatment were checked by PCR analysis of 16S rRNA. The RNA integrity was examined with RNA gel electrophoresis. The concentrations of purified RNA samples were measured with Thermo Scientific NanoDrop™ 2000. Two micrograms of purified RNA samples (A260/280 of 2) were reverse transcribed with random hexamer primers using RevertAid Reverse Transcriptase (Thermo Fisher Scientific). Template cDNA (1 ng) and 200 nM primers for either oxyR, katA, katB, katE ankB, recG or 16S rRNA (listed in Supplementary Table S2) were used in 20 μL reaction of KAPA SYBR FAST qPCR kit Master Mix (ABI Prism). The reaction mixtures were incubated for 3 min at 95°C followed by 40 cycles of denaturation at 95°C for 3 s, annealing at 57°C (16S, oxyR, recG, katE and ankB) and 64°C (katA and katB) for 20 s and extension at 72°C for 45 s. Data were obtained using STEPONE software v2.1 (Applied Biosystems). The level of 16S rRNA expression was used to normalize target gene expression in the corresponding strain. The relative gene expression was determined using the 2−ΔΔCT method (27). Expression of a gene was quantified as fold-change relative to the level in the PA14 wild-type strain without H2O2 treatment.
Protein crystallization
Crystallization conditions were screened at 20°C with a concentration of TrmJ–NTD protein of 20 mg/ml using the vapor diffusion method and commercial crystallization screens in Intelli 96-3 wells sitting drop plates. Three precipitant: protein ratios (1:1, 1:2 and 2:1) were tested using a Phoenix crystallization robot (Art Robbins Instruments) and drop volumes of 0.3 μl. Optimized crystals of the free TrmJ–NTD dimer (form I) were obtained using the vapor diffusion method in hanging drops and 24-well trays, by mixing a volume of 1 μl of protein with 2 μl of a precipitant solution containing Bis–Tris propane at pH 6.5, 0.2 M ammonium chloride, 25% (w/v) PEG 3,350. These crystals diffracted to 1.70 Å at SLS. A second crystal form of the TrmJ–NTD dimer was obtained by mixing 1 μl of protein with 2 μl of Morpheus screen conditions A5 (Molecular Dimensions) containing 0.03 M MgCl2, 0.03 M CaCl2, 20% (w/v) PEG MME550, 10% (w/v) PEG 20000, 0.1 M MOPS/Na-HEPES at pH 7.5. These crystals (form II) only diffracted to about 7 Å at SLS. Following three hours soaking with 1 mM sinefungin, however, diffraction to 1.8–2.2 Å was obtained at the Taiwan Light Source.
Data collection and structure determination
Prior to data collection, crystals were briefly soaked in their respective precipitating solution supplemented with 20% (v/v) glycerol and rapidly frozen in liquid nitrogen. X-ray diffraction data were collected at the PXIII beamline at SLS (Villigen, Switzerland) for the free TrmJ–NTD crystal (form I). Free TrmJ–NTD crystal (form II) and the complex with sinefungin were collected at Taiwan Light source NSRRC, beamline 13B1.
Diffraction intensities were integrated with XDS (28), scaled, merged and truncated with SCALA/TRUNCATE (29). The structures of TrmJ–NTD was determined by molecular replacement using the CCP4 software (29) with the structure from E. coli (PDB code: 4CND) as search probe. The TrmJ protein from P. aeruginosa and E. coli share 53% overall amino acid sequence identity. The structure of TrmJ–NTD from P. aeruginosa was built iteratively at the computer graphics using COOT (30) and refined using Autobuster (31). The geometrical parameters for sinefungin were generated using coordinates 4R8S from the PDB (www.rcsb.org). Buried solvent accessible surface areas upon dimer formation were calculated using the PDBePISA server (www.ebi.ac.uk/pdbe/pisa/) and structure comparison was performed with the DALI server (http://ekhidna.biocenter.helsinki.fi/dali_server/start). The quality of the structures was assessed using the MOLPROBITY server (molprobity.biochem.duke.edu/) and figures were generated using the Pymol software (Schrodinger). Data collection and structure refinement parameters are summarized in Table 1. The atomic coordinates and structure factors are deposited with the Protein Data Bank under accession codes 5GMC and 5GMB for the free TrmJ–NTD protein, and 5GM8 for TrmJ–NTD bound to sinefungin.
Table 1. Crystallographic data and refinement statistics for TrmJ–NTD.
| TrmJ–NTD-free I | TrmJ–NTD-free II | TrmJ–NTD-sinefungin | |
|---|---|---|---|
| Data collection | |||
| X-ray source | SLS X06DA | NSRRC, 13B1 | NSRRC, 13B1 |
| Wavelength (Å) | 1.000 | 1.000 | 1.000 |
| Crystallographic parameters | |||
| Space group | P21 | P43212 | P21 |
| Unit cell dimensions (Å) | 65.44, 65.04, 93.19 | 59.80, 59.50, 124.18 | 56.52, 66.61, 98.58 |
| a, b, c (Å); α, β, γ (°) | 90.0, 110.4, 90.0 | 90.0, 90.0, 90.0 | 90.0, 101.96, 90.0 |
| Dimers per asymmetric unit | 2 | 0.5 | 2 |
| Data collection statistic | |||
| Resolution limit (Å) | 24.89–1.70 (1.79–1.70) | 29.58–1.62 (1.68–1.62) | 25.94–2.24 (2.26–2.24) |
| No. of observed reflections | 212 396 (13 543) | 288 407 (6001) | 123 095 (9971) |
| No. of unique reflections | 75 444 (5209) | 29 132 (1200) | 34 918 (2849) |
| Completeness (%) | 93.4 (93.4) | 95.4 (71.5) | 95.5 |
| Redundancy | 2.8 (2.6) | 9.9 (5.0) | 3.5 (3.5) |
| I/σ(I) | 12.1 (2.5) | 19.0 (3.9) | 8.0 (1.9) |
| Rmerge (%) | 0.042 (0.416) | 0.062 (0.73) | 0.099 (0.635) |
| Refinement | |||
| Reflections used for refinement | 75 444 | 27 532 | 34 917 |
| Correlation coefficient | 0.954 | 0.953 | 0.942 |
| R factor (Rwork/Rfree) (%) | 17.7/19.9 | 17.9/20.4 | 0.19/0.22 |
| Model contents | |||
| Protein atoms | 5,155 | 1,295 | 5,172 |
| Water molecules | 652 | 166 | 286 |
| Ligand atoms | – | – | 5172 |
| Root mean square deviation | |||
| Distances (Å) | 0.010 | 0.010 | 0.010 |
| Bond angles (°) | 1.01 | 1.02 | 1.10 |
| Ramachandran plot | |||
| Favored | 92.3 | 91.2 | 91.9 |
| Allowed | 7.7 | 9.8 | 8.1 |
| Outlier | 0 | 0 | 0 |
| PDB Code | 5GMC | 5GMB | 5GM8 |
aRmerge = Σ|Iobs−Iav|/ΣIav, over all symmetry-related observations.
bRwork = Σ|Fobs−<|Fcalc|>|/Σ|Fobs|, over all reflections included in the refinement.
cRfree is calculated with 5% of reflections excluded from the refinement. In this formula, <|Fcalc|> denotes the expectation value of |Fcalc| under the probability distribution used to define the likelihood function that is maximized in the refinement. Values in parentheses are for the outermost resolution shell of data.
Statistical analysis
Statistical analysis was performed using Graphpad Prism (GraphPad Software). Student's t-test was used to determine the statistical significance.
RESULTS
Identification of tRNA-related genes that confer resistance to oxidative stress
Using bioinformatics analysis, we identified 26 genes potentially involved in tRNA modifications in P. aeruginosa (Supplementary Table S4). A study in yeast showed that Cm, among other modifications, is increased following exposure to H2O2 (6). We then selected PA14_14690 (trmJ) whose product is sharing 53% amino-acid sequence identity and 68% similarity to E. coli TrmJ and predicted to be involved in the synthesis of Cm for further study. In the H2O2 susceptibility assay, we observed that trmJ mutant has increased sensitivity to H2O2 (Figure 1). That the H2O2 sensitivity was due to the loss of trmJ, the PA14 mutant lacking this gene was transformed with an expression vector containing full-length trmJ, which restored H2O2 resistance to wild-type levels (Figure 1).
Figure 1.
Loss of trmJ causes an increased susceptibility to H2O2. Phenotypic analysis of cytotoxicity induced by H2O2 in PA14 wild-type strain (grey bar), trmJ mutant strain (black bar), and the trmJ complementation strain or trmJC (white bar). The data represent mean ± SD for three biological replicates. Asterisks denote the values with significant difference by Student's t-test (P ≤ 0.05).
PA14_14690 encodes a tRNA (cytidine(32)/uridine(32)-2′-O)-methyltransferase-like protein of P. aeruginosa PA14: PA14 TrmJ
A protein sequence alignment of PA14_14690 with TrmJ homologs with known structure from E. coli was constructed using Endscript-http://endscript.ibcp.fr (Figure 2) (32), which revealed that PA14_14690 possesses the conserved region of TrmJ-specific motif, a consensus ‘TXARXR’ sequence (residues 79–84) (33). This motif has previously been shown to be critical for binding of SAM/SAH and tRNA (33,34). SAM becomes SAH (S−Adenosyl Homocysteine) after losing its methyl group in the methylation reaction. PA14_14690 also contains a conserved arginine at position 23, which is proposed to be involved in its catalytic activity. However, another catalytic residue, the tyrosine at position 141, is replaced by phenylalanine. In addition to arginine positions 82 and 84 in the TrmJ-specific motif, PA14_14690 also contains more key conserved tRNA binding residues including arginine at positions 100, 101 and 105. Therefore, we hypothesized that PA14_14690 encodes a tRNA (cytidine(32)/uridine(32)-2′-O)-methyltransferase-like protein. Subsequent biochemical analysis validated this assignment.
Figure 2.
Amino acid sequence alignment of TrmJ proteins. The TrmJ-specific motif is underlined (dashed line) and identical residues are highlighted in red. Residues known to be involved in tRNA binding and catalytic activity of TrmJ (33) are marked by ★ and #, respectively. Abbreviations: Ec, Escherichia coli; Pa, Pseudomonas aeruginosa.
Inactivation of trmJ abolishes Cm, Um, or Am formation in P. aeruginosa tRNA
TrmJ has been reported to methylate the 2′-O ribose moiety in tRNA (33,34). Here, we tested this hypothesis for PA14 TrmJ in vivo and in vitro. Analysis of ribonucleosides in total tRNA from wild-type PA14 by HPLC-coupled triple quadrupole mass spectrometry (LC–MS/MS) relative to standards revealed at least 25 species including Cm, Um and Am (Supplementary Table S3). When we analyzed ribonucleosides in tRNA isolated from the trmJ mutant strain, we observed reduced levels of Cm, Um and Am compared to wild-type (Figure 3A). This result suggests that deletion of trmJ led to hypo-modification of 2′-O-ribose position of C, U and A in tRNA. With respect to the PA14 wild-type strain, the level of Cm, Um and Am was significantly reduced in trmJ mutant strain (P < 0.05). However, the level of Cm does not drop as much as Um and Am, which suggests functional redundancy for Cm formation.
Figure 3.
Loss of TrmJ reduces levels of Cm, Um and Am in total tRNA. The levels of Cm, Um and Am in tRNA isolated from wild-type, trmJ mutant and trmJC strains of PA14 were quantified by LC–MS/MS. Relative to wild-type cells, loss of TrmJ in trmJ mutant strain lowered levels of Cm, Um and Am (A) while complementation of trmJ mutant strain (trmJC) restored the ribonucleoside levels (B). Data represent mean ± SD for three biological replicates, with asterisks denoting significant differences by Student's t-test (P ≤ 0.05).
To further validate formation of Cm, Um, and Am in tRNA by PA14 TrmJ, the sequence encoding the full-length of PA14 TrmJ protein (PA14_14690) was cloned into a plasmid that was used to transform the trmJ mutant strain as described in Materials and Methods. LC–MS/MS analysis revealed that restoration of PA14 TrmJ expression in trmJ mutant increased the levels of Cm, Um, and Am (Figure 3B) without changing other modifications (Supplementary Table S3). The observation of a higher level of Am in the complemented strain compared to wild-type (Figure 3B) could be due to nonspecific TrmJ activity caused by higher protein levels in the complemented strain, given that complementation was achieved with a medium copy number vector with strong promoter. That TrmJ was responsible for altered levels of Cm, Um and Am was evident in in vitro reactions of purified PA14 TrmJ with total tRNA isolated from wild-type and trmJ mutant. Significant changes in Cm, Um, and Am levels were only apparent with tRNA isolated from the trmJ mutant strain (Figure 4). These results lead to the conclusion that PA14 TrmJ is a 2′-O-tRNA methyltransferase catalyzing the formation of Cm, Um and Am.
Figure 4.
Purified PA14 TrmJ catalyzes Cm, Um and Am formation in total tRNA isolated from P. aeruginosa trmJ mutant. (A) Total tRNA extracted from the trmJ mutant strain was reacted with PA14 TrmJ and SAM (white bar) or left untreated (black bar), followed by LC–MS/MS quantification of Cm, Um and Am. (B) Total tRNA extracted from wild-type P. aeruginosa was reacted with PA14 TrmJ and SAM (white bar) or left untreated (black bar), followed by LC–MS/MS quantification of Cm, Um and Am. Data represent mean ± SD for three biological replicates for (A) and mean ± SD for two biological replicates for (B). Asterisks denote a significant difference by Student's t-test (P ≤ 0.05).
PA14 TrmJ catalyzes 2′-O-methylation of C, U and A at position 32 in the tRNA anticodon loop
The preceding results establish that PA14 TrmJ is a 2′-O-tRNA methyltransferase but without regard to location. Since E. coli TrmJ methylates position 32 in the tRNA anticodon loop, we sought to map the location of PA14 TrmJ products in tRNA. We first identified substrate tRNAs for PA14 TrmJ using the in vitro methylation assay with tRNAs synthesized by in vitro transcription. As candidate tRNAs, we chose four P. aeruginosa tRNA isoacceptors previously reported as substrates for E. coli TrmJ: tRNASer(UGA) (Cm32) tRNAMet(CAU) (Cm32), tRNATrp(CCA) (Cm32) and tRNAGln(UUG) (Um32), as well as four tRNA species possessing A or U at position 32: tRNAPro(GGG) (Am32), tRNAPro(UGG) (Um32), tRNAPro(CGG) (Um32) and tRNAHis(GUG) (Um32). The results from LC–MS/MS quantification of Cm, Um and Am in these reactions are shown in Figure 5. All tRNA species except tRNASer(UGA) proved to be substrates for PA14 TrmJ, though tRNAHis(GUG) was minimally modified. tRNAPro(GGG) was the most efficient substrate, with Am formation an order of magnitude higher than Um or Cm in the other substrates.
Figure 5.
In vitro methylation of synthetic tRNAs by purified PA14 TrmJ. (A) The indicated tRNAs were prepared by in vitro transcription and used as substrates for in vitro methylation reactions with PA14 TrmJ and SAM. (B) Following LC–MS/MS analysis, signals for Cm, Um and Am were normalized to total signal for canonical A, C, G and U. Data represent mean ± SD for three biological replicates.
To confirm the location of PA14 TrmJ methylation, tRNAPro(GGG) and tRNAGln(UUG) were subjected to in vitro methylation by PA14 TrmJ and the modification at position 32 assessed by LC-QTOF mass spectrometric analysis of RNase T1 digests of the tRNAs. As shown in Figure 6A, a doubly-charged negative ion with m/z 617.6017 was detected at 18.9 min in the RNase T1 digestion of tRNAPro(GGG), which is consistent with CAmUG. This sequence was corroborated in CID analysis (Figure 6B and C). Similarly, a doubly-charged negative ion with m/z 759.5922 was detected at 19.9 min, with subsequent CID analysis consistent with UmUUUG released from tRNAGln(UUG) (Figure 6D–F). Based on these results, we conclude that PA14 TrmJ catalyzes 2′-O-ribose methylation at position 32 in the tRNA anticodon loop.
Figure 6.
Mapping PA14 TrmJ methylation in tRNA by LC-QTOF analysis of RNase T1 digestion products. (A) Extracted ion chromatogram for m/z 617.6017 released from tRNAPro(GGG) by RNase T1. (B) The mass spectrum for the tRNAPro(GGG) oligo eluting at 18.9 min shows an (M-2H)−2 ion with m/z 617.6017 and a sodium adduct at m/z 628.5938. (C) CID of m/z 617.6017 yields products consistent with CAmUG. (D) Extracted ion chromatogram for m/z 759.5922 released from tRNAGln(UUG) by RNase T1. (E) The mass spectrum for the tRNAGln(UUG) oligo eluting at 19.9 min shows an (M-2H)−2 ion with m/z 759.5922 and sodium adducts at m/z 770.5839 and 781.5740. (F) CID spectrum of m/z 759.5922 yields products consistent with UmUUUG.
Structure of the N-terminal domain of TrmJ from P. aeruginosa
Initial attempts to crystallize the full-length TrmJ protein from P. aeruginosa yielded only poorly diffracting crystals. Sequence comparison with the TrmJ protein from E. coli (34) suggested that residues 166-GKPTKMEK-173 connect the N- to the C-terminal domain of TrmJ in a flexible way in the absence of a tRNA ligand. We set out to first determine the structure of the N-terminal catalytic domain of TrmJ (hereafter named TrmJ–NTD) spanning residues 1 to 167 of the protein by eliminating this source of conformational heterogeneity hindering formation of well-ordered crystals. The TrmJ–NTD protein was obtained with a good yield and crystallized readily in two forms using two distinct sets of precipitants (labeled as ‘free I’ and ‘free II’ Table 1). A summary of diffraction data collection and structure refinement statistics for TrmJ–NTD is given in Table 1. The TrmJ–NTD monomer adopts the ‘SPOUT’ fold (35) – a variant of the classical Rossmann nucleotide-binding fold with the N- and C-termini in close proximity and a central β-sheet composed of seven parallel β-strands surrounded by eight α-helices (Figure 7A and B). The additional four residues derived from cloning at the N-terminal end of the polypeptide participate in the formation of helix α1. A peculiar topological feature also found in other SPOUT methyltransferases is the presence of a knot in the C-terminal region of the protein (Figure 7B). An automatic superimposition of TrmJ–NTD from P. aeruginosa with structures deposited in the PDB returned TrmJ from E. coli (residues 1–164, PDB code: 4CND) as the closest homologue (59% amino-acid sequence identity) with r.m.s.d. of 1.3 Å (34). Both proteins are tRNA cytidine/uridine 2′-O-methyltransferase. The TrmJ–NTD structure is also closely related to various putative ribosomal RNA methyltransferases including HI038 from Haemophilus influenzae (PDB code: 3ILK), SpoU from Rhodobacter sphaeroides (PDB code: 3ONP) and the RlmB 23S rRNA methylransferase from E. coli (PDB code: 1GZ0, (36)) that founded this family of methyltransferases (36). A structure-based sequence alignment is displayed in Figure 7. Both the free and sinefungin bound TrmJ–NTD crystals contain two dimers per asymmetric unit consistent with the observation that the protein eluted as a dimer in gel filtration. The buried surface area at the interface of the TrmJ–NTD dimer is 1210 Å2, which represents approximately 14% of the total accessible surface area per monomer (8430 Å2) and is comparable to the interface between the N-Terminal Domain (NTD) of TrmJ from E. coli of 1400 Å2 (34), reflecting the conserved mode of TrmJ dimerization between the two species. Dimerization of the NTD is mediated mainly by the centrally located helix α8 that crisscrosses with its counterpart from the adjacent monomer as well as by one face of β-turn 136–142 that interacts with α2 from the other monomer, while it also forms part of the cofactor binding pocket, on its opposite face (Figures 7 and 8).
Figure 7.
Overall structure of TrmJ–NTD from P. aeruginosa. (A) Topology of the SAM binding domain of TrmJ–NTD. The residue numbers and N- and C-termini of the polypeptide chain are indicated. (B) Structure of the TrmJ–NTD monomer using the same color code. (C) Left panel: Structure of the TrmJ–NTD homodimer. One monomer is depicted as ‘ribbons’ while a surface view is given for the other monomer. The SAM binding loop and the putative RNA binding residues are colored in purple and blue, respectively. The right panel shows a view rotated by 180° along a vertical axis emphasizing the tight dimer interface contributed by two long helices and projecting loops.
Figure 8.
Sinefungin (SFG) binding by TrmJ–NTD from P. aeruginosa. (A) Two sinefungin molecules (sticks) are bound to the TrmJ–NTD homodimer displayed as an orange ribbons. An electron density map with Fourier coefficients mFo-DFc and phases where the SFG molecules were omitted from the calculation is displayed in green at a level of 3 Å. (B and C) Magnified views of the cofactor-binding site highlighting residues interacting with SFG. Hydrogen bonds are displayed as dashed lines. (C) TrmJ–NTD is shown as a molecular surface and SFG is represented as sticks with the base deeply buried in the pocket whilst the amino-acid tail projects towards the solvent. (D) Structure based sequence alignment of the NTD domains of TrmJ from Pseudomonas aeruginosa (PA14 TrmJ, this work), Haemophilus influenzae TrmJ (HiTrmJ), Sulfolobus acidocaldarius (SaTrmJ) and E. coli (EcTrmJ) (34). Strictly conserved residues are highlighted in red and conserved residues in cyan. Deletions are indicated by dashes. The secondary structure of PA14 TrmJ is shown above the sequence.
Structure of TrmJ–NTD bound to sinefungin and tRNA binding
A crystal form with two TrmJ–NTD dimers per asymmetric unit, was obtained in the presence of PEG MME550 and PEG 20000, but these crystals diffracted very poorly in the absence of bound ligand. Nevertheless, these crystals could be used to obtain a complex between TrmJ–NTD and the isosteric SAM analogue sinefungin by soaking for 3 h leading to good diffraction (Table 1), while soaking of free I native crystals led to severe degradation of their diffraction quality. Each TrmJ–NTD dimer binds two sinefungin molecules such that two tRNA molecules can in principle bind simultaneously at opposite faces of the TrmJ–NTD dimer (Figure 7). A view of the TrmJ–NTD dimer electrostatic surface reveals basic patches at the dimer surface. In this respect, the basic loop spanning residues 82-RDRRIPWL-90 is well positioned to bind the target tRNA substrate. This segment appears to be flexible both in the free I TrmJ–NTD and in the sinefungin-bound structures and probably requires tRNA to become ordered, as was indeed recently observed for a complex between the TrmD methyltransferase and tRNA (37).
Upon sinefungin binding, an outward opening movement towards the adjacent monomer occurs in loop 115-GREYAG-120 that acts as a lid to grant access to the inhibitor. The sinefungin molecule adopts a bent conformation within a pocket formed at the TrmJ–NTD dimer interface. Sinefungin appears to be primarily held via its adenine ring and sugar moieties, while its amino-acid tail is more mobile and extends towards the solvent. The catalytic residues Arg23 (from monomer A) and Ser 143 (monomer B) are both within hydrogen bonding distances of the sinefungin amino-group (which substitutes the methyl group in SAM) and make contact with two bound water molecules (Figure 8).
Molecular basis for TrmJ-dependent H2O2 sensitivity in P. aeruginosa
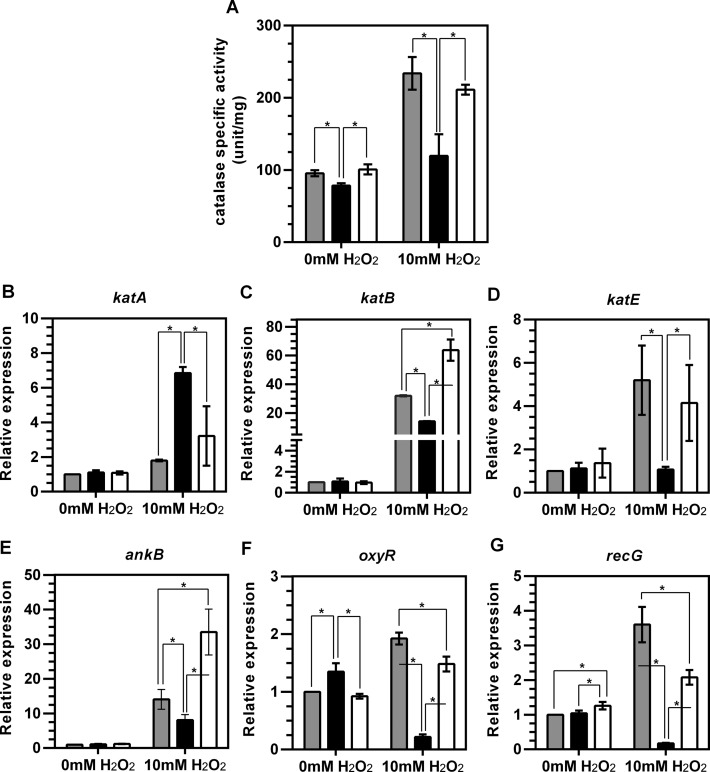
Having established that PA14_14690 codes for a tRNA:Cm32/Um32/Am32 methyltransferase that confers resistance to H2O2 in P. aeruginosa, we initiated studies to define the molecular basis for this behavior. We first assessed the role of catalase activity in the H2O2 sensitivity of the trmJ mutant. Catalase is one of the major enzymes involved in detoxification of H2O2, with PA14 possessing three genes (katA, katB, and katE) coding for catalases. Under unstressed conditions, catalase activity in the trmJ mutant strain (78 ± 3.2 unit/mg) was significantly lower than the wild-type and trmJC strains (95 ± 4.2 and 101 ± 6.9 unit/mg, respectively) (Figure 9A). This difference more than doubled when the cells were exposed to a roughly LD50 dose of H2O2, with catalase activity increasing 2.5-fold in wild-type cells compared to only 1.5-fold in the trmJ mutant strain (Figure 9A). TrmJ complementation restored the catalase activity to nearly wild-type levels (2.1-fold, P < 0.05) (Figure 9A).
Figure 9.
Loss of trmJ causes a decreased catalase activity and alters expression of oxidative stress responsive genes. (A) Catalase activity assay in PA14 wild-type strain (grey bar), trmJ mutant strain (black bar), and the trmJ complementation strain or trmJC (white bar). (B-G) Quantitative real time PCR analysis of oxidative stress responsive genes. Bacterial cultures were grown to exponential phase (OD600 ≈ 0.4) and were then either treated or untreated with 10 mM H2O2 for 25 min. Expression of katA (B), katB (C), katE (D), ankB (E), oxyR (F), and recG (G) in trmJ mutant strain (black bar) and trmJC (white bar) was reported as fold-change in expression relative to the level of that gene in the PA14 wild-type strain (grey bar) without H2O2 treatment. The data represent mean ± SD for three biological replicates. Asterisks denote the values with significant difference by Student's t-test (P ≤ 0.05).
These results suggest that TrmJ confers H2O2 resistance in part by upregulating the expression of catalase genes. To test this hypothesis at the transcriptional level, we quantified expression of katA, katB, and katE by semi-quantitative real-time PCR in total RNA isolated from wild-type, trmJ mutant, and trmJC strains exposed to 10 mM H2O2. In unexposed cells, the katA, katB and katE transcript levels were similar in all of the strains (Figure 9B–G). Upon exposure to 10 mM H2O2, however, katB and katE transcript levels increased more in the wild-type and trmJC strains than in the trmJ mutant (Figure 9C and D). We observed a similar pattern for ankB transcript levels (Figure 9E) as expected since katB and ankB are co-expressed on the same operon. Surprisingly, H2O2 caused higher levels of katA transcripts in trmJ mutant than in wild-type and trmJC strains (Figure 9B).
It is well recognized that OxyR is a key transcription regulator that modulates expression of antioxidant genes including katB-ankB and katA upon H2O2 stress in P. aeruginosa (16,38). We then investigated the expression level of oxyR-recG operon. The expression level of oxyR-recG was decreased in trmJ mutant strain after H2O2 exposure (Figure 9F, G). This is in good agreement with the expression pattern of katB. Considering these findings together, it suggests that trmJ is essential for a proper expression of katB during H2O2 stress through a modulation of oxyR expression.
DISCUSSION
Emerging evidence points to a variety of functions for tRNA other than as a simple adaptor molecule in protein synthesis (4,6–8). The potential for the functional diversity of tRNA is enhanced by dozens of post-transcriptional modifications of the canonical ribonucleotides, ranging from simple methylations as in 5-methylcytidine (m5C) to hyper-modified bases as in 5-methoxycarbonylmethyluridine (mcm5U). Here, we report that the gene for the tRNA methyltransferase trmJ in P. aeruginosa plays a role in the oxidative stress response, with its repertoire of position 32 anti-codon loop 2′-O-methylation products expanded from Um and Cm to include Am. This new function for TrmJ in oxidative stress response is consistent with similar roles for wobble m5C in yeast tRNA (7,8) and the m6A37 tRNA methyltransferase encoded by the yfic gene in E. coli (39).
PA14 TrmJ shares most of the important characteristics found in other bacterial homologs, including a TrmJ-specific motif (a consensus ‘TXARXR’ sequence) important for binding to SAM/SAH and tRNA, a conserved amino acid important for catalytic activity, and conserved amino acids at the C-terminus (Figure 2) (33). This conservation is reinforced in the structural analysis shown in Figures 7 and 8. Arg23 and Ser43 are evolutionary conserved and correspond to Arg23 and Ser142 in TrmJ from E. coli. However, the TrmJ–NTD catalytic site from P. aeruginosa displays an interesting difference with E. coli TrmJ: the residue structurally equivalent to Tyr140 of E. coli TrmJ, that was proposed to play a catalytic role, is substituted by Phe141 in the P. aeruginosa enzyme. The only other possible residue in the catalytic site with a hydroxyl group is Tyr118, which does not establish an H-bond with Arg21.
The substrate specificity of PA14 TrmJ also shows similarities to and notable differences from other TrmJ homologs. We found that PA14 TrmJ catalyzes the formation not only of Cm and Um at position 32 in tRNA, but also Am in vivo and in vitro (Figures 3–6). While the data in Figure 5B represent population levels of Cm, Um and Am in tRNA hydrolysates, the fact that these TrmJ products were only observed in the tRNAs that possessed the specific respective C32, U32, and A32 substrates is consistent with the established specificity of TrmJ for position 32. Though possible, it is unlikely that the Am observed in tRNAPro(GGG) occurred at positions other than 32. In the archaean Sulfolobus acidocaldarius, it has been shown that TrmJ performs ribose methylation at only C32 both in vivo and in vitro. E. coli possesses two activities catalyzing both Cm and Um in vitro and in vivo, TrmJ and TrmL, with TrmJ methylating position 32 (34,40) and TrmL methylating the wobble position 34 in leucine tRNA isoacceptors (41). Such an enzymatic redundancy may explain the observation of high background levels of Cm in the PA14 trmJ mutant strain (Figure 3). The observation of Am32 and Gm32 in tRNA with E. coli TrmJ was made with a single tRNA species that varied the identity of the ribonucleoside at position 32 and thus does not represent a biologically-relevant activity (34). In terms of substrates, 7 tRNA substrates were identified for PA14 TrmJ in vitro: tRNAMet(CAU) and tRNATrp(CCA) for C32; tRNAGln(UUG), tRNAPro(UGG), tRNAPro(CGG) and tRNAHis(GUG) for U32; and A32 in tRNAPro(GGG) (Figure 5). Similar in vitro studies with E. coli TrmJ revealed 4 substrates overlapping with PA14 (tRNAMet(CAU), tRNATrp(CCA) and tRNAGln(UUG)) and two different substrates: tRNAGln(CUG) and tRNASer(UGA) (33).
One new function of TrmJ activity arises from our observation that hypomethylation of 2′-O-ribose moiety of C32, U32, and A32 of tRNA in the PA14 trmJ mutant strain rendered the cells hypersensitive to the oxidative stress of H2O2 exposure. This parallels the resistance to oxidative stress conferred by m6A37 in tRNA in E. coli (39). One mechanism underlying the H2O2 sensitivity of the PA14 trmJ mutant strain involves reduced catalase activity (Figure 9A). It is not clear which of the 3 catalase genes in PA14 have reduced activity, since 2 genes (katB and katE) showed reduced mRNA levels in the trmJ mutant strain exposed to H2O2, while the major catalase, KatA, had higher mRNA levels (Figure 9C and D). Although KatE is generally regarded as stationary phase catalase, it has also been shown to protect bacteria against oxidative stress during logarithmic phase of growth (42–44). The expression of katB is known to be under the regulation of OxyR, an oxidation-sensing transcription regulator (16,45). The lower level of katB in the P. aeruginosa trmJ mutant during H2O2 stress may thus result from lower expression of OxyR.
So what are the mechanistic links between a defect in 2′-O-methylation of C, U and A at position 32 of the tRNA anticodon loop and H2O2 resistance in PA14? Modifications of tRNA anticodon loop have been demonstrated to play a role in stabilizing local structure and enhancing accurate codon recognition (46). For example, lack of 2-thiocytidine (s2C) at position 32 of tRNAArg can destabilize the anticodon loop (3), reduce the A-site selection rate for tRNAArg(mnm5UCU) at the AGG codon, and increase the frequency of frameshifting (47). Comparing 2-thiolation and 2′-O-methylation of ribonucleotides, both stabilize the C3′-endoform of pyrimidine nucleotides (48,49) and enhance base stacking (50). So, one possible role of 2′-O-methylation at position 32 of tRNA ribonucleotides could be to improve anticodon loop stability and anticodon base-paring properties of tRNA. Thus, it is possible that the lack of Cm32, Um32, and Am32 in tRNA could reduce the paring properties of tRNA and thus cause inefficient translation or misreading of mRNAs for proteins crucial oxidative stress response in P. aeruginosa. Evidence in yeast has shown that a defect in position 32 2′-O-methylation caused by loss of Trm7 impairs growth and translation efficiency and fidelity (51,52). Interestingly, loss of TrmJ activity did not affect PA14 susceptibility to the oxidative stressors paraquat and t-butyl hydroperoxide, which indicates a specificity of TrmJ-dependent tRNA modifications for affecting an H2O2 survival phenotype in PA14. Similarly, Chan et al. observed that Trm4 protected yeast against H2O2 toxicity, while Trm9 and Trm140 protected against exposure to alkylating agents (53). There is a strong parallel here in terms of fine-tuning the stress response to specific agents. Yeast Trm140 inserts m3C at position 32 in three tRNAs (tRNAThr(IGU), tRNASer(UGA), tRNASer(CGA)) and selectively protects against SN1-type alkylating agents such as methyl- and ethyl-methanesulfonate (MMS and EMS, respectively) but not SN2 alkylators (53). It awaits further study to determine if the oxidative stress sensitivity in PA14 is the result of reduced translation of transcripts with codon biases that match TrmJ-dependent tRNAs, as occurs with Trm4, Trm9 and Trm140 in yeast (7,53). Such a mechanism could explain how loss of TrmJ leads to reduced transcription of oxyR and its regulon, if the transcriptional regulators of oxyR or other up-stream stress response genes are enriched in TrmJ-dependent codons.
In summary, we present results demonstrating that PA14 TrmJ is a tRNA methyltransferase that catalyzes formation of Cm, Um and Am at position 32 and that TrmJ protects PA14 against H2O2-induced toxicity. The results point to a mechanism linking tRNA modifications to stress response phenotypes in bacteria.
Supplementary Material
Acknowledgments
The authors thank Mr. Ee Pin Koon for technical assistance with synthesis and purification of tRNA, Ms. Maggie Cai for technical assistance with QQQ operation, Ms. Thanyaporn Srimahaeak for handling of bacterial strains, Dr. Amnart Khongmanee and Mr. Bhawat Wongkhamprai for technical assistance with QTOF analysis, and NISB for sharing the Swiss Light Source and Taiwan Light Source synchrotron beamtime.
Footnotes
Present address: Yok Hian Chionh and Megan E. McBee, Tychan Ltd, Singapore.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
FUNDING
National Research Foundation of Singapore through its Singapore-MIT Alliance for Research and Technology; US National Science Foundation [CHE-1308839]; Chulabhorn Graduate Institute; Chulabhorn Research Institute; Mahidol University; Agilent Technologies. Funding for open access charge: Chulabhorn Research Institute and Chulabhorn Graduate Institute.
Conflict of interest statement. None declared.
REFERENCES
- 1.El Yacoubi B., Bailly M., de Crecy-Lagard V. Biosynthesis and function of posttranscriptional modifications of transfer RNAs. Annu. Rev. Genet. 2012;46:69–95. doi: 10.1146/annurev-genet-110711-155641. [DOI] [PubMed] [Google Scholar]
- 2.Bjork G.R., Wikstrom P.M., Bystrom A.S. Prevention of translational frameshifting by the modified nucleoside 1-methylguanosine. Science. 1989;244:986–989. doi: 10.1126/science.2471265. [DOI] [PubMed] [Google Scholar]
- 3.Cantara W.A., Bilbille Y., Kim J., Kaiser R., Leszczynska G., Malkiewicz A., Agris P.F. Modifications modulate anticodon loop dynamics and codon recognition of E. coli tRNAArg1,2. J. Mol. Biol. 2012;416:579–597. doi: 10.1016/j.jmb.2011.12.054. [DOI] [PubMed] [Google Scholar]
- 4.Patil A., Chan C.T., Dyavaiah M., Rooney J.P., Dedon P.C., Begley T.J. Translational infidelity-induced protein stress results from a deficiency in Trm9-catalyzed tRNA modifications. RNA Biol. 2012;9:990–1001. doi: 10.4161/rna.20531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Urbonavicius J., Qian Q., Durand J.M., Hagervall T.G., Bjork G.R. Improvement of reading frame maintenance is a common function for several tRNA modifications. EMBO J. 2001;20:4863–4873. doi: 10.1093/emboj/20.17.4863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan C.T., Dyavaiah M., DeMott M.S., Taghizadeh K., Dedon P.C., Begley T.J. A quantitative systems approach reveals dynamic control of tRNA modifications during cellular stress. PLoS Genet. 2010;6:e1001247. doi: 10.1371/journal.pgen.1001247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan C.T., Pang Y.L., Deng W., Babu I.R., Dyavaiah M., Begley T.J., Dedon P.C. Reprogramming of tRNA modifications controls the oxidative stress response by codon-biased translation of proteins. Nat. Commun. 2012;3:937. doi: 10.1038/ncomms1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Begley U., Dyavaiah M., Patil A., Rooney J.P., DiRenzo D., Young C.M., Conklin D.S., Zitomer R.S., Begley T.J. Trm9-catalyzed tRNA modifications link translation to the DNA damage response. Mol. Cell. 2007;28:860–870. doi: 10.1016/j.molcel.2007.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arancibia F., Bauer T.T., Ewig S., Mensa J., Gonzalez J., Niederman M.S., Torres A. Community-acquired pneumonia due to gram-negative bacteria and Pseudomonas aeruginosa: incidence, risk, and prognosis. Arch. Intern. Med. 2002;162:1849–1858. doi: 10.1001/archinte.162.16.1849. [DOI] [PubMed] [Google Scholar]
- 10.Solomon S.L., Oliver K.B. Antibiotic resistance threats in the United States: stepping back from the brink. Am. Family Phys. 2014;89:938. [PubMed] [Google Scholar]
- 11.Gellatly S.L., Hancock R.E. Pseudomonas aeruginosa: new insights into pathogenesis and host defenses. Pathog. Dis. 2013;67:159–173. doi: 10.1111/2049-632X.12033. [DOI] [PubMed] [Google Scholar]
- 12.Reinhart A.A., Powell D.A., Nguyen A.T., O'Neill M., Djapgne L., Wilks A., Ernst R.K., Oglesby-Sherrouse A.G. The prrF-encoded small regulatory RNAs are required for iron homeostasis and virulence of Pseudomonas aeruginosa. Infect. Immun. 2015;83:863–875. doi: 10.1128/IAI.02707-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wei Q., Minh P.N., Dotsch A., Hildebrand F., Panmanee W., Elfarash A., Schulz S., Plaisance S., Charlier D., Hassett D., et al. Global regulation of gene expression by OxyR in an important human opportunistic pathogen. Nucleic Acids Res. 2012;40:4320–4333. doi: 10.1093/nar/gks017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Atichartpongkul S., Fuangthong M., Vattanaviboon P., Mongkolsuk S. Analyses of the regulatory mechanism and physiological roles of Pseudomonas aeruginosa OhrR, a transcription regulator and a sensor of organic hydroperoxides. J. Bacteriol. 2010;192:2093–2101. doi: 10.1128/JB.01510-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lan L., Murray T.S., Kazmierczak B.I., He C. Pseudomonas aeruginosa OspR is an oxidative stress sensing regulator that affects pigment production, antibiotic resistance and dissemination during infection. Mol. Microbiol. 2010;75:76–91. doi: 10.1111/j.1365-2958.2009.06955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ochsner U.A., Vasil M.L., Alsabbagh E., Parvatiyar K., Hassett D.J. Role of the Pseudomonas aeruginosa oxyR-recG operon in oxidative stress defense and DNA repair: OxyR-dependent regulation of katB-ankB, ahpB, and ahpC-ahpF. J. Bacteriol. 2000;182:4533–4544. doi: 10.1128/jb.182.16.4533-4544.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Palma M., DeLuca D., Worgall S., Quadri L.E.N. Transcriptome analysis of the response of Pseudomonas aeruginosa to hydrogen peroxide. J. Bacteriol. 2004;186:248–252. doi: 10.1128/JB.186.1.248-252.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alexeyev M.F. The pKNOCK series of broad-host-range mobilizable suicide vectors for gene knockout and targeted DNA insertion into the chromosome of gram-negative bacteria. Biotechniques. 1999;26:824–826. doi: 10.2144/99265bm05. [DOI] [PubMed] [Google Scholar]
- 19.Geng X., Oliver G. Elucidating the molecular characteristics of organogenesis in human embryos. Genome Biol. 2010;11:130. doi: 10.1186/gb-2010-11-8-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grant S.G., Jessee J., Bloom F.R., Hanahan D. Differential plasmid rescue from transgenic mouse DNAs into Escherichia coli methylation-restriction mutants. Proc. Natl. Acad. Sci. U.S.A. 1990;87:4645–4649. doi: 10.1073/pnas.87.12.4645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kovach M.E., Elzer P.H., Hill D.S., Robertson G.T., Farris M.A., Roop R.M. 2nd, Peterson K.M. Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene. 1995;166:175–176. doi: 10.1016/0378-1119(95)00584-1. [DOI] [PubMed] [Google Scholar]
- 22.Lee D.G., Urbach J.M., Wu G., Liberati N.T., Feinbaum R.L., Miyata S., Diggins L.T., He J., Saucier M., Deziel E., et al. Genomic analysis reveals that Pseudomonas aeruginosa virulence is combinatorial. Genome Biol. 2006;7:R90. doi: 10.1186/gb-2006-7-10-r90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Metcalf W.W., Jiang W., Daniels L.L., Kim S.K., Haldimann A., Wanner B.L. Conditionally replicative and conjugative plasmids carrying lacZ alpha for cloning, mutagenesis, and allele replacement in bacteria. Plasmid. 1996;35:1–13. doi: 10.1006/plas.1996.0001. [DOI] [PubMed] [Google Scholar]
- 24.Chan C.T., Chionh Y.H., Ho C.H., Lim K.S., Babu I.R., Ang E., Wenwei L., Alonso S., Dedon P.C. Identification of N6,N6-dimethyladenosine in transfer RNA from Mycobacterium bovis Bacille Calmette-Guerin. Molecules. 2011;16:5168–5181. doi: 10.3390/molecules16065168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Su D., Chan C.T., Gu C., Lim K.S., Chionh Y.H., McBee M.E., Russell B.S., Babu I.R., Begley T.J., Dedon P.C. Quantitative analysis of ribonucleoside modifications in tRNA by HPLC-coupled mass spectrometry. Nat. Protoc. 2014;9:828–841. doi: 10.1038/nprot.2014.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rozenski J., McCloskey J.A. SOS: a simple interactive program for ab initio oligonucleotide sequencing by mass spectrometry. J. Am. Soc. Mass Spectrom. 2002;13:200–203. doi: 10.1016/S1044-0305(01)00354-3. [DOI] [PubMed] [Google Scholar]
- 27.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 28.Kabsch W. Integration, scaling, space-group assignment and post-refinement. Acta Crystallogr. D Biol. Crystallogr. 2010;66:133–144. doi: 10.1107/S0907444909047374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Collaborative Computational Project, N. The CCP4 suite: programs for protein crystallography. Acta Crystallogr. D Biol. Crystallogr. 1994;50:760–763. doi: 10.1107/S0907444994003112. [DOI] [PubMed] [Google Scholar]
- 30.Emsley P., Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 31.Smart O.S., Womack T.O., Flensburg C., Keller P., Paciorek W., Sharff A., Vonrhein C., Bricogne G. Exploiting structure similarity in refinement: automated NCS and target-structure restraints in BUSTER. Acta Crystallogr. D Biol. Crystallogr. 2012;68:368–380. doi: 10.1107/S0907444911056058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Robert X., Gouet P. Deciphering key features in protein structures with the new ENDscript server. Nucleic Acids Res. 2014;42:W320–W324. doi: 10.1093/nar/gku316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu R.J., Long T., Zhou M., Zhou X.L., Wang E.D. tRNA recognition by a bacterial tRNA Xm32 modification enzyme from the SPOUT methyltransferase superfamily. Nucleic Acids Res. 2015;43:7489–7503. doi: 10.1093/nar/gkv745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Somme J., Van Laer B., Roovers M., Steyaert J., Versees W., Droogmans L. Characterization of two homologous 2′-O-methyltransferases showing different specificities for their tRNA substrates. RNA. 2014;20:1257–1271. doi: 10.1261/rna.044503.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kurowski M.A., Sasin J.M., Feder M., Debski J., Bujnicki J.M. Characterization of the cofactor-binding site in the SPOUT-fold methyltransferases by computational docking of S-adenosylmethionine to three crystal structures. BMC Bioinformatics. 2003;4:9. doi: 10.1186/1471-2105-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Michel G., Sauve V., Larocque R., Li Y., Matte A., Cygler M. The structure of the RlmB 23S rRNA methyltransferase reveals a new methyltransferase fold with a unique knot. Structure. 2002;10:1303–1315. doi: 10.1016/s0969-2126(02)00852-3. [DOI] [PubMed] [Google Scholar]
- 37.Ito T., Masuda I., Yoshida K., Goto-Ito S., Sekine S., Suh S.W., Hou Y.M., Yokoyama S. Structural basis for methyl-donor-dependent and sequence-specific binding to tRNA substrates by knotted methyltransferase TrmD. Proc. Natl. Acad. Sci. U.S.A. 2015;112:E4197–E4205. doi: 10.1073/pnas.1422981112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heo Y.J., Chung I.Y., Cho W.J., Lee B.Y., Kim J.H., Choi K.H., Lee J.W., Hassett D.J., Cho Y.H. The major catalase gene (katA) of Pseudomonas aeruginosa PA14 is under both positive and negative control of the global transactivator OxyR in response to hydrogen peroxide. J. Bacteriol. 2010;192:381–390. doi: 10.1128/JB.00980-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Golovina A.Y., Sergiev P.V., Golovin A.V., Serebryakova M.V., Demina I., Govorun V.M., Dontsova O.A. The yfiC gene of E. coli encodes an adenine-N6 methyltransferase that specifically modifies A37 of tRNA1Val(cmo5UAC) RNA. 2009;15:1134–1141. doi: 10.1261/rna.1494409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Purta E., van Vliet F., Tkaczuk K.L., Dunin-Horkawicz S., Mori H., Droogmans L., Bujnicki J.M. The yfhQ gene of Escherichia coli encodes a tRNA:Cm32/Um32 methyltransferase. BMC Mol. Biol. 2006;7:23. doi: 10.1186/1471-2199-7-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Benitez-Paez A., Villarroya M., Douthwaite S., Gabaldon T., Armengod M.E. YibK is the 2′-O-methyltransferase TrmL that modifies the wobble nucleotide in Escherichia coli tRNALeu isoacceptors. RNA. 2010;16:2131–2143. doi: 10.1261/rna.2245910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ivanova A.B., Glinsky G.V., Eisenstark A. Role of rpoS regulon in resistance to oxidative stress and near-UV radiation in delta oxyR suppressor mutants of Escherichia coli. Free Radic. Biol. Med. 1997;23:627–636. doi: 10.1016/s0891-5849(97)00013-0. [DOI] [PubMed] [Google Scholar]
- 43.Miller C.D., Kim Y.C., Anderson A.J. Cloning and mutational analysis of the gene for the stationary-phase inducible catalase (catC) from Pseudomonas putida. J. Bacteriol. 1997;179:5241–5245. doi: 10.1128/jb.179.16.5241-5245.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vattanaviboon P., Mongkolsuk S. Expression analysis and characterization of the mutant of a growth-phase- and starvation-regulated monofunctional catalase gene from Xanthomonas campestris pv phaseoli. Gene. 2000;241:259–265. doi: 10.1016/s0378-1119(99)00483-7. [DOI] [PubMed] [Google Scholar]
- 45.Panmanee W., Hassett D.J. Differential roles of OxyR-controlled antioxidant enzymes alkyl hydroperoxide reductase (AhpCF) and catalase (KatB) in the protection of Pseudomonas aeruginosa against hydrogen peroxide in biofilm vs. planktonic culture. FEMS Microbiol. Lett. 2009;295:238–244. doi: 10.1111/j.1574-6968.2009.01605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Agris P.F. Bringing order to translation: the contributions of transfer RNA anticodon-domain modifications. EMBO Rep. 2008;9:629–635. doi: 10.1038/embor.2008.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jager G., Leipuviene R., Pollard M.G., Qian Q., Bjork G.R. The conserved Cys-X1-X2-Cys motif present in the TtcA protein is required for the thiolation of cytidine in position 32 of tRNA from Salmonella enterica serovar Typhimurium. J. Bacteriol. 2004;186:750–757. doi: 10.1128/JB.186.3.750-757.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kawai G., Yamamoto Y., Kamimura T., Masegi T., Sekine M., Hata T., Iimori T., Watanabe T., Miyazawa T., Yokoyama S. Conformational rigidity of specific pyrimidine residues in tRNA arises from posttranscriptional modifications that enhance steric interaction between the base and the 2′-hydroxyl group. Biochemistry. 1992;31:1040–1046. doi: 10.1021/bi00119a012. [DOI] [PubMed] [Google Scholar]
- 49.Yamamoto Y., Yokoyama S., Miyazawa T., Watanabe K., Higuchi S. NMR analyses on the molecular mechanism of the conformational rigidity of 2-thioribothymidine, a modified nucleoside in extreme thermophile tRNAs. FEBS Lett. 1983;157:95–99. doi: 10.1016/0014-5793(83)81123-5. [DOI] [PubMed] [Google Scholar]
- 50.Drake A.F., Mason S.F., Trim A.R. Optical studies of the base-stacking properties of 2′-O-methylated dinucleoside monophosphates. J. Mol. Biol. 1974;86:727–739. doi: 10.1016/0022-2836(74)90349-0. [DOI] [PubMed] [Google Scholar]
- 51.Guy M.P., Podyma B.M., Preston M.A., Shaheen H.H., Krivos K.L., Limbach P.A., Hopper A.K., Phizicky E.M. Yeast Trm7 interacts with distinct proteins for critical modifications of the tRNAPhe anticodon loop. RNA. 2012;18:1921–1933. doi: 10.1261/rna.035287.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pintard L., Lecointe F., Bujnicki J.M., Bonnerot C., Grosjean H., Lapeyre B. Trm7p catalyses the formation of two 2′-O-methylriboses in yeast tRNA anticodon loop. EMBO J. 2002;21:1811–1820. doi: 10.1093/emboj/21.7.1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chan C.T., Deng W., Li F., DeMott M.S., Babu I.R., Begley T.J., Dedon P.C. Highly predictive reprogramming of tRNA modifications is linked to selective expression of codon-biased genes. Chem. Res. Toxicol. 2015;28:978–988. doi: 10.1021/acs.chemrestox.5b00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.