Abstract
We evaluated whether genital inflammation affects the selection of the transmitted virus. Among South African women, we found that preinfection genital inflammation facilitates transmission of less infectious human immunodeficiency virus, but highly infectious viruses are able to establish infection regardless of inflammation status. This suggests that viral phenotype can influence transmission risk.
Keywords: female genital tract, inflammation, HIV acquisition, cytokine, viral infectivity
A severe genetic bottleneck is observed during heterosexual human immunodeficiency virus (HIV) transmission; in approximately 80% of cases, only a single viral variant will establish infection, despite the diverse viral population in the donor [1, 2]. This transmitted founder virus is rarely the dominant variant, suggesting that transmission is not entirely stochastic but favors certain viral phenotypes [3–5]. The most likely determinants of this bottleneck are the physical and immunological properties of the healthy genital mucosa, which constitutes an efficient barrier to HIV acquisition and is able to block most unprotected sexual exposures [6]. Genital inflammation has been shown to increase the risk of HIV acquisition [7] and may result in infection with multiple viral variants [2]. However, the impact of inflammation on the characteristics of viruses that establish infection has not yet been established.
We hypothesized that a healthy genital mucosa confers a stringent barrier to HIV infection, allowing only the most infectious viruses to establish infection. The presence of inflammation, which potentially compromises the epithelial barrier and recruits activated HIV target cells, may enable less infectious variants to establish infection. We investigated this possibility by measuring the infectivity of recently transmitted viruses and relating this to cytokine concentrations in preinfection cervicovaginal lavage (CVL) samples. To our knowledge, our study is the first to show that preinfection genital inflammation can affect the selection of the transmitted viral phenotype by facilitating transmission of less infectious HIV.
METHODS
Participants
Acute HIV infection plasma and preinfection CVL samples were obtained from 27 women enrolled in the Centre for the AIDS Programme of Research in South Africa (CAPRISA) 004 tenofovir gel trial [8]. The Research Ethics Committees of the Universities of KwaZulu-Natal and Cape Town approved this study, and participants provided written informed consent.
Cytokine Measurement
Concentrations of interleukin 1α, 1β, 6, 7, 8, and 10 (IL-1α, IL-1β, IL-6, IL-7, IL-8, and IL-10), granulocyte-macrophage colony-stimulating factor, interferon γ–inducible protein 10, monocyte chemoattractant protein 1, macrophage inflammatory protein (MIP) 1α, MIP-1β, and tumor necrosis factor (TNF) α were measured in CVL samples, as described elsewhere, using Milliplex Human Cytokine kits (Millipore) [7].
Virus Isolation and Generation of Pseudoviruses
Virus was isolated from plasma using µMACS VitalVirus HIV isolation kits according to protocol (Miltenyi Biotec). Single genome env amplicons were generated from these isolates, cloned into pcDNA3.1D/V5-His TOPO (Invitrogen)according to the manufacturer's instructions, and cotransfected with the subtype B backbone vector pSG3Δenv (National Institutes of Health AIDS Reagent Program; catalog No. 11051) into HEK 293T cells to generate pseudoviruses (PSVs) (Supplementary Material).
Viral Infectivity
Viral isolates and PSVs were serially diluted and added to TZM-bl cells in the presence or absence of 20 µg/mL diethylaminoethyl (DEAE)–dextran (Sigma). Luciferase activity was quantified 48 hours later, and viral infectivity was calculated as relative light units (RLUs) generated per picogram of reverse-transcriptase (RT) activity (RLUs/pg RT) in each stock, as measured with the Roche colorimetric RT assay.
Statistical Analysis
Unsupervised hierarchical clustering was used to cluster women according to their cytokine profiles. Linear regression was used to examine the relationship between cytokines, cytokine factor scores, and viral infectivity (Supplementary Material). P values were adjusted for multiple comparisons using a false discovery rate step-down procedure.
RESULTS
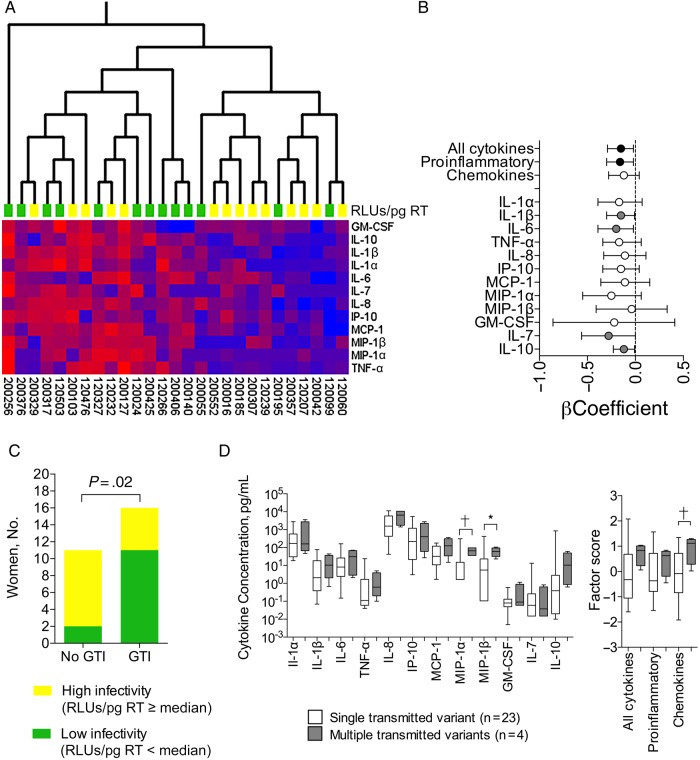
HIV isolates were obtained a median of 34 days after infection from plasma of 27 women who seroconverted during the CAPRISA 004 trial [8] (Supplementary Table 1). Viral infectivity was quantified using the TZM-bl, assay and hierarchical clustering was used to group women according to their preinfection cervicovaginal cytokine profiles, measured a median of 4.6 months before infection (range, 0.5–13.5 months). Women with higher genital cytokine levels were more likely to be infected by viruses with lower infectivity (Figure 1A). A cytokine profile including TNF-α, MIP-1α, IL-7, and IL-10 significantly separated women according to the infectivity of their viral isolates (Supplementary Figure 1).
Figure 1.
Preinfection genital inflammation was associated with acquisition of less infectious human immunodeficiency virus (HIV). A, Unsupervised hierarchical clustering was used to visualize the variation in cytokine concentrations in individual women and to cluster women according to the similarities of their cytokine expression profiles (using Qlucore Omics Explorer software, version 3.0). Women from whom less infectious viruses were isolated (value below median for relative light units per picogram of reverse-transcriptase [RLUs/pg RT]; n = 13; green blocks) had up-regulated cytokine concentrations in preinfection cervicovaginal lavage (CVL) samples and tended to cluster together. Women from whom highly infectious viruses were isolated (RLUs/pg RT at or above the median) had lower cytokine concentrations and also clustered together (n = 14; yellow blocks). Cytokine concentrations are indicated using a color scale, ranging from blue (low) to red (high). The dendrogram above the heat map illustrates degrees of relatedness between genital cytokine profiles evident among the various women. Patient identity numbers are indicated below the heat map. B, RLUs/pg RT and cytokine concentrations were either log10-transformed or converted to categorical variables (MIP-1α, MIP-1β, GM-CSF, and IL-7), and β coefficients were calculated using linear regression. Factor scores for each functional group of cytokines were generated using confirmatory factor analysis. Circles indicate β coefficients from regression analyses; error bars, 95% confidence intervals. Black circles indicate associations between cytokine factors and RLUs/pg RT that were statistically significant (unadjusted P < .05); gray circles, associations between individual cytokines and infectivity that were statistically significant before adjustment for multiple comparisons. Regression analyses were adjusted for potential confounders, including tenofovir or placebo assignment, virus subtype, tropism, time between infection and virus isolation, age, hormonal contraceptive use, number of sex acts per month, and herpes simplex virus 2 status. C, To estimate baseline levels of inflammation, cytokine factor scores including all 12 cytokines were calculated for 27 HIV-infected women and 54 uninfected controls. Women with cytokine scores at or above the median were considered to have genital inflammation, and those with scores less than the median did not. High- and low-infectivity viruses were defined as those with RLUs/pg RT at or above the median or below the median, respectively. P values were calculated using the Fisher exact test. GTI, genital tract inflammation. D, Relationship between cytokines and multiple variant transmission. Box-and-whisker plots show medians with ranges of cytokine concentrations and factor scores. Mann–Whitney U test was used to compare cytokine concentrations and factor scores between women infected by single (n = 23; white boxes) or multiple (n = 4; gray boxes) HIV variants. *Statistically significant before but not after adjustment for multiple comparisons. †Statistically significant (cytokine factors) or significant after adjustment for multiple comparisons (cytokines). P values for cytokine comparisons were adjusted for multiple comparisons using a false discovery rate step-down procedure. Abbreviations: GM-CSF, granulocyte-macrophage colony-stimulating factor; IL-1α, IL-1β, IL-6, IL-7, IL-8, and IL-10, interleukin 1α, 1β, 6, 7, 8, and 10; IP, interferon γ–inducible protein; MCP, monocyte chemoattractant protein; MIP, macrophage inflammatory protein; TNF, tumor necrosis factor.
The relationships between individual cytokines and viral infectivity were then investigated using linear regression to analyze RLUs/pg RT as a continuous variable and adjust for potential confounders, including study arm, time between infection and virus isolation, age, hormonal contraceptive use, viral tropism, subtype, sex acts per month, and herpes simplex virus 2 status (Supplementary Tables 1 and 2). To reduce the complexity of the data set, we also used confirmatory factor analysis to group cytokines into factors, including all 12 cytokines, proinflammatory cytokines only (IL-1α, IL-1β, IL-6, and TNF-α), and chemokines (IL-8, interferon γ–inducible protein 10, monocyte chemoattractant protein 1, MIP-1α, MIP-1β). After adjustment for confounders, the factor including all cytokines, the proinflammatory factor, and IL-1β, IL-6, IL-7, and IL-10 were inversely associated with viral infectivity (Figure 1B; Supplementary Figure 2).
We then classified women according to the level of inflammation in their genital tracts. To estimate baseline levels, we included cytokine data for 54 HIV-uninfected controls, because these women were shown elsewhere to have lower levels of inflammation than women who later became infected [7]. We found that only 2 of 11 women (18%) who did not have preinfection genital inflammation were infected by viruses with low infectivity (RLUs/pg RT below the median), compared with 11 of 16 (69%) with inflammation (Figure 1C). Interestingly, fewer women with genital inflammation (5 of 16; 31%) than without inflammation (9 of 11; 82%) were infected by highly infectious viruses (RLUs/pg RT at or above the median). Similar results were obtained when comparing women infected by the most highly infectious viruses (RLUs/pg RT at ≥75th percentile) with those infected by viruses with very low infectivity (RLUs/pg RT at <25th percentile) (Supplementary Figure 3).
Because viral infectivity is the sum of multiple steps in the viral life cycle, we evaluated whether the first step, viral entry, was driving the relationship with genital inflammation. We found an association between genital inflammation and the DEAE-dextran dependency of the isolates (Supplementary Figure 4), as well as the PSV infectivity (Supplementary Figure 5), suggesting that viral entry efficiency may be the main driver.
Next we examined whether genital inflammation affects the genetic bottleneck by allowing for the transmission of multiple viral variants as opposed to a single virus. Although this finding was not significant (Fisher exact test; P = .10), of the 27 women studied, all 4 multivariant transmissions were detected in women grouped as having inflammation. Furthermore, the chemokine factor score and MIP-1α concentrations were higher in women with multiple transmitted viral variants (Figure 1D).
Finally, we found that viral infectivity was not associated with markers of HIV disease progression, including plasma viral load set point (β coefficient, −0.55 (95% confidence interval, −1.37 to .27; P = .18)] and time taken for CD4+ T cell counts to fall to <350/µL or antiretroviral therapy initiation (hazard ratio, 0.40; 95% confidence interval, .12–1.31; P = .13), even after adjustment for genital inflammation.
DISCUSSION
HIV prevalence in young South African women is extremely high, yet the biological factors driving this prevalence are not well understood. One important factor could be the high prevalence of inflammatory genital conditions that increase HIV acquisition risk [7]. In the current study, we showed that in the absence of preinfection genital inflammation most women became infected by highly infectious viruses. However, in women with inflammation, an increase in HIV infections was observed, which primarily involved viruses of low infectivity. This suggests that a healthy genital mucosa provides an effective barrier against less infectious viruses, which can be compromised by genital inflammation. The transmission probability of a virus would thus depend not only on the efficiency of the mucosal barrier and viral concentration in semen [6] but also on viral phenotype.
Interestingly, we also found that fewer women with preinfection genital inflammation were infected by highly infectious viruses, compared with women without inflammation. This could suggest that within the viral quasispecies in semen or within the infected population, less infectious viruses might outnumber those with higher infectivity.
Our findings are in line with those of former studies showing that transmitted founder viruses exhibit higher infectivity than chronic viruses [9] and that genital ulceration or inflammation can reduce the selection bias for consensuslike variants during transmission, allowing for less fit viruses to establish infection [3]. In contrast, Deymier et al [4] did not observe selection for higher infectivity in a study of 6 transmission pairs, although this could be due to the use of DEAE-dextran in their infectivity assay and/or the presence of subclinical inflammation. The genital cytokines that predicted transmission of less infectious viruses, including proinflammatory IL-1β, IL-6, TNF-α, and hematopoietic IL-7, have been shown to directly induce HIV expression [10], whereas at lower concentrations, IL-10 synergizes with inflammatory cytokines to enhance HIV replication [11]. The chemokine MIP-1α recruits CCR5+ cells needed to establish infection [12].
A limitation of the current study is the relatively small number of isolates that had matching preinfection CVL samples available for cytokine analysis. Although few of the women were infected by multiple viral variants, limiting the statistical power, multivariant transmission was correlated with higher genital MIP-1α levels and higher chemokine factor scores, suggesting a possible role for target cell recruitment in multivariant transmission. We did not find an association between viral infectivity and disease progression, suggesting that other host factors, such as immune activation, also contribute to disease progression or that infectivity does not confer a long-term advantage. In conclusion, our results show that genital inflammation before infection can affect the transmitted viral phenotype by facilitating transmission of less infectious HIV variants.
Supplementary Data
Supplementary materials are available at http://academic.oup.com/cid. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Acknowledgments. We acknowledge the CAPRISA 004 and the Tenofovir Gel Research for AIDS Prevention Science (TRAPS) study team for coordinating the collection, processing, storage, and shipping of the samples. We also thank the Western Province Blood Transfusion Service in Cape Town for providing HIV-negative donor blood.
Disclaimer. The views expressed by the authors do not necessarily reflect the views of the US Agency for International Development (USAID), Gilead Sciences, Eastern Virginia Medical School, or Contraception Research and Development. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Financial support. The parent trial (CAPRISA 004) was supported by the USAID, FHI 360 (USAID cooperative agreement GPO-A-00-05-00022-00, contract 132119), and the Technology Innovation Agency (LIFElab) of the South African government's Department of Science & Technology. The research infrastructure was supported by the Comprehensive International Program of Research on AIDS, National Institutes of Health (grant AI51794). This work was also supported by the Clinical Infectious Diseases Research Initiative (CIDRI), South Africa (grant to P. S.), the Poliomyelitis Research Foundation of South Africa (J.-A. S. P.), the University of Cape Town Clinical Infectious Diseases Research Initiative (Wellcome Trust; grant to L. M. and P. S.) the National Research Foundation of South Africa, the Fogarty Training program (J.-A. S. P. and L. M.), and the Claude Leon Foundation and CIDRI (postdoctoral fellowship to P. S.).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Abrahams MR, Anderson JA, Giorgi EE et al. . Quantitating the multiplicity of infection with human immunodeficiency virus type 1 subtype C reveals a non-Poisson distribution of transmitted variants. J Virol 2009; 83:3556–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haaland RE, Hawkins PA, Salazar-Gonzalez J et al. . Inflammatory genital infections mitigate a severe genetic bottleneck in heterosexual transmission of subtype A and C HIV-1. PLoS Pathog 2009; 5:e1000274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carlson JM, Schaefer M, Monaco DC et al. . HIV transmission: selection bias at the heterosexual HIV-1 transmission bottleneck. Science 2014; 345:1254031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deymier MJ, Ende Z, Fenton-May AE et al. . Heterosexual transmission of subtype C HIV-1 selects consensus-like variants without increased replicative capacity or interferon-α resistance. PLoS Pathog 2015; 11:1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boeras DI, Hraber PT, Hurlston M et al. . Role of donor genital tract HIV-1 diversity in the transmission bottleneck. Proc Natl Acad Sci U S A 2011; 108:E1156–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hughes JP, Baeten JM, Lingappa JR et al. . Determinants of per-coital-act HIV-1 infectivity among African HIV-1-serodiscordant couples. J Infect Dis 2012; 205:358–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Masson L, Passmore JAS, Liebenberg LJ et al. . Genital inflammation and the risk of HIV acquisition in women. Clin Infect Dis 2015; 61:260–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abdool Karim Q, Abdool Karim SS, Frohlich JA et al. . Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science 2010; 329:1168–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parrish NF, Gao F, Li H et al. . Phenotypic properties of transmitted founder HIV-1. Proc Natl Acad Sci U S A 2013; 110:6626–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chêne L, Nugeyre MT, Barré-Sinoussi F, Israël N. High-level replication of human immunodeficiency virus in thymocytes requires NF-kappaB activation through interaction with thymic epithelial cells. J Virol 1999; 73:2064–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weissman D, Poli G, Fauci AS. IL-10 synergizes with multiple cytokines in enhancing HIV production in cells of monocytic lineage. J Acquir Immune Defic Syndr Hum Retrovirol 1995; 9:442–9. [PubMed] [Google Scholar]
- 12.Stanford MM, Issekutz TB. The relative activity of CXCR3 and CCR5 ligands in T lymphocyte migration: concordant and disparate activities in vitro and in vivo. J Leukoc Biol 2003; 74:791–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.