Abstract
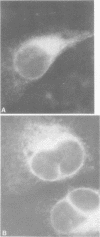
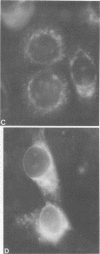
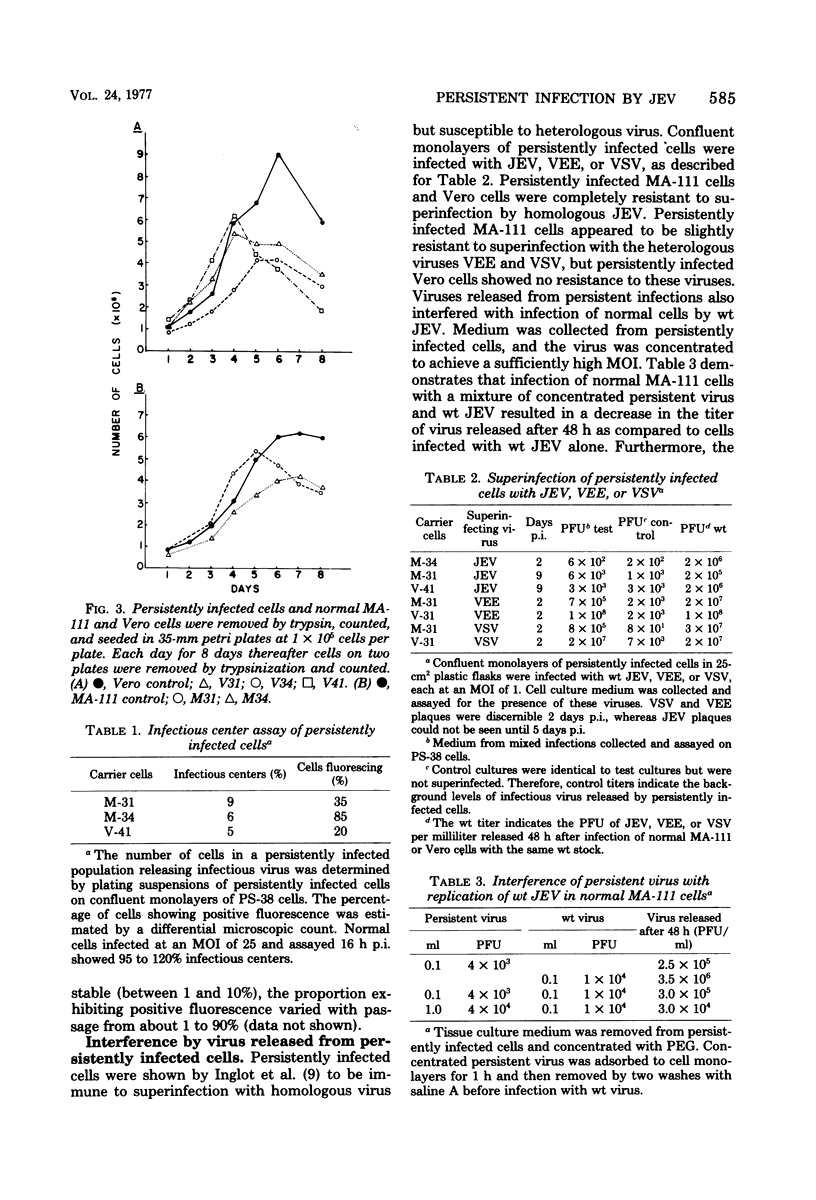
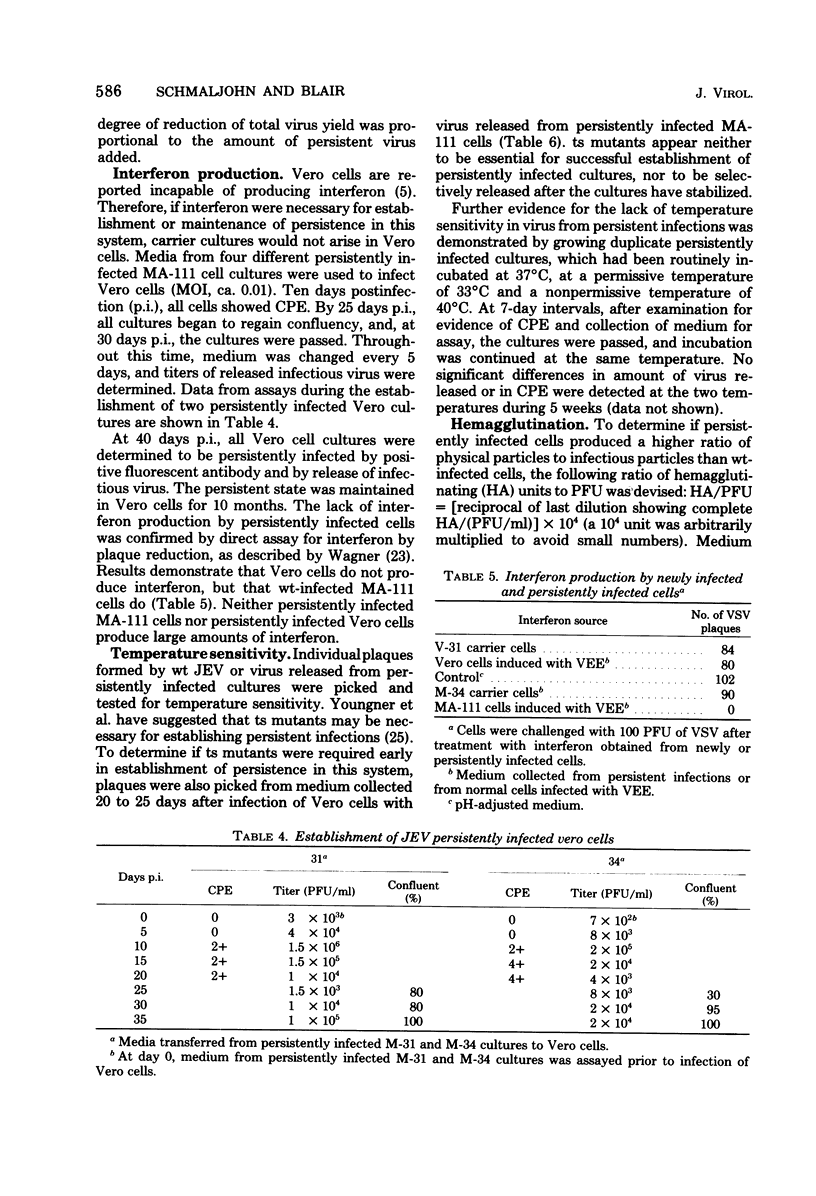
Persistent infections were established by serial undiluted passage of flavivirus Japanese encephalitis virus in a line of rabbit kidney cells (MA-111). The persistently infected cells resembled uninfected cells in most respects. Low levels of infectious virions were released from a small percentage of cells, and a larger and more variable percentage was shown to possess viral antigen by fluorescent-antibody staining. Released viruses were shown to interfere with replication of wild-type Japanese encephalitis virus. Persistently infected MA-111 cells could not be superinfected with homologous wild-type Japanese encephalitis virus but could be superinfected with two heterologous viruses. Transfer of cell culture medium from persistently infected MA-111 cells to a line of African green monkey kidney cells (Vero) resulted in similar persistent infections in the latter cells. Temperature sensitivity and host-cell interferon production were not involved in establishment or maintenance of persistence. Determination of ratios of physical particles to infectious particles revealed that many defective, noninfectious viruses were present, suggesting that defective interfering particles may be responsible for persistency.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bruton C. J., Kennedy S. I. Defective-interfering particles of Semliki Forest Virus: structural differences between standard virus and defective-interfering particles. J Gen Virol. 1976 Jun;31(3):383–395. doi: 10.1099/0022-1317-31-3-383. [DOI] [PubMed] [Google Scholar]
- CLARKE D. H., CASALS J. Techniques for hemagglutination and hemagglutination-inhibition with arthropod-borne viruses. Am J Trop Med Hyg. 1958 Sep;7(5):561–573. doi: 10.4269/ajtmh.1958.7.561. [DOI] [PubMed] [Google Scholar]
- COLE F. E., Jr, HETRICK F. M. PERSISTENT INFECTION OF HUMAN CONJUNCTIVA CELL CULTURES BY MYXOVIRUS PARAINFLUENZA 3. Can J Microbiol. 1965 Jun;11:513–521. doi: 10.1139/m65-068. [DOI] [PubMed] [Google Scholar]
- Della-Porta A. J., Westaway E. G. Rapid preparation of hemagglutinins of togaviruses from infected cell culture fluids. Appl Microbiol. 1972 Jan;23(1):158–160. doi: 10.1128/am.23.1.158-160.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmyter J., Melnick J. L., Rawls W. E. Defectiveness of interferon production and of rubella virus interference in a line of African green monkey kidney cells (Vero). J Virol. 1968 Oct;2(10):955–961. doi: 10.1128/jvi.2.10.955-961.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland J. J., Villarreal L. P. Persistent noncytocidal vesicular stomatitis virus infections mediated by defective T particles that suppress virion transcriptase. Proc Natl Acad Sci U S A. 1974 Aug;71(8):2956–2960. doi: 10.1073/pnas.71.8.2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland J. J., Villarreal L. P., Welsh R. M., Oldstone M. B., Kohne D., Lazzarini R., Scolnick E. Long-term persistent vesicular stomatitis virus and rabies virus infection of cells in vitro. J Gen Virol. 1976 Nov;33(2):193–211. doi: 10.1099/0022-1317-33-2-193. [DOI] [PubMed] [Google Scholar]
- Huang A. S., Baltimore D. Defective viral particles and viral disease processes. Nature. 1970 Apr 25;226(5243):325–327. doi: 10.1038/226325a0. [DOI] [PubMed] [Google Scholar]
- Inglot A. D., Albin M., Chudzio T. Persistent infection of mouse cells with Sindbis virus: role of virulence of strains, auto-interfering particles and interferon. J Gen Virol. 1973 Jul;20(1):105–110. doi: 10.1099/0022-1317-20-1-105. [DOI] [PubMed] [Google Scholar]
- Inoue Y. K., Ogura R. Studies on Japanese B encephalitis virus. III. Propagation and assay of Japanese B encephalitis virus in a stable line of porcine kidney cells. Virology. 1962 Feb;16:205–207. doi: 10.1016/0042-6822(62)90299-4. [DOI] [PubMed] [Google Scholar]
- Inoue Y. K., Yamada M. A. Clonal line of porcine kidney stable cells for assay of Japanese encephalitis virus. J Bacteriol. 1964 May;87(5):1239–1240. doi: 10.1128/jb.87.5.1239-1240.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai A., Matsumoto S., Tanabe K. Characterization of rabies viruses recovered from persistently infected BHK cells. Virology. 1975 Oct;67(2):520–533. doi: 10.1016/0042-6822(75)90452-3. [DOI] [PubMed] [Google Scholar]
- Preble O. T., Youngner J. S. Selection of temperature-sensitive mutants during persistent infection: role in maintenance of persistent Newcastle disease virus infections of L cells. J Virol. 1973 Sep;12(3):481–491. doi: 10.1128/jvi.12.3.481-491.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preble O. T., Youngner J. S. Temperature-sensitive defect of mutants isolated from L cells persistently infected with Newcastle disease virus. J Virol. 1973 Sep;12(3):472–480. doi: 10.1128/jvi.12.3.472-480.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preble O. T., Youngner J. S. Temperature-sensitive viruses and the etiology of chronic and inapparent infections. J Infect Dis. 1975 Apr;131(4):467–473. doi: 10.1093/infdis/131.4.467. [DOI] [PubMed] [Google Scholar]
- Schwöbel W., Ahl R. Peristence of sindbis virus in BHK-21 cell cultures. Arch Gesamte Virusforsch. 1972;38(1):1–10. doi: 10.1007/BF01241350. [DOI] [PubMed] [Google Scholar]
- Shenk T. E., Stollar V. Defective-interfering particles of Sindbis virus. I. Isolation and some chemical and biological properties. Virology. 1973 May;53(1):162–173. doi: 10.1016/0042-6822(73)90475-3. [DOI] [PubMed] [Google Scholar]
- Sung J. S., Diwan A. R., Falkler W. A., Jr, Yang H. Y., Halstead S. B. Dengue carrier culture and antigen production in human lymphoblastoid lines. Intervirology. 1975;5(3-4):137–149. doi: 10.1159/000149891. [DOI] [PubMed] [Google Scholar]
- Thacore H., Youngner J. S. Cells persistently infected with Newcastle disease virus. II. Ribonucleic acid and protein synthesis in cells infected with mutants isolated from persistently infected L cells. J Virol. 1970 Jul;6(1):42–48. doi: 10.1128/jvi.6.1.42-48.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thacore H., Youngner J. S. Cells persistently infected with newcastle disease virus: I. Properties of mutants isolated from persistently infected L cells. J Virol. 1969 Sep;4(3):244–251. doi: 10.1128/jvi.4.3.244-251.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WAGNER R. R. Biological studies of interferon. I. Suppression of cellular infection with eastern equine encephalomyelitis virus. Virology. 1961 Mar;13:323–337. doi: 10.1016/0042-6822(61)90152-0. [DOI] [PubMed] [Google Scholar]
- Weiss B., Schlesinger S. Defective interfering passages of Sindbis virus: chemical composition, biological activity, and mode of interference. J Virol. 1973 Oct;12(4):862–871. doi: 10.1128/jvi.12.4.862-871.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngner J. S., Dubovi E. J., Quagliana D. O., Kelly M., Preble O. T. Role of temperature-sensitive mutants in persistent infections initiated with vesicular stomatitis virus. J Virol. 1976 Jul;19(1):90–101. doi: 10.1128/jvi.19.1.90-101.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngner J. S., Quagliana D. O. Temperature-sensitive mutants isolated from hamster and canine cell lines persistently infected with Newcastle disease virus. J Virol. 1975 Nov;16(5):1332–1336. doi: 10.1128/jvi.16.5.1332-1336.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ter Meulen V., Martin S. J. Genesis and maintenance of a persistent infection by canine distemper virus. J Gen Virol. 1976 Sep;32(3):431–440. doi: 10.1099/0022-1317-32-3-431. [DOI] [PubMed] [Google Scholar]