Abstract
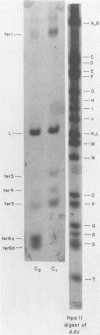
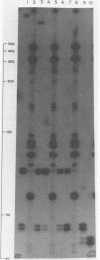
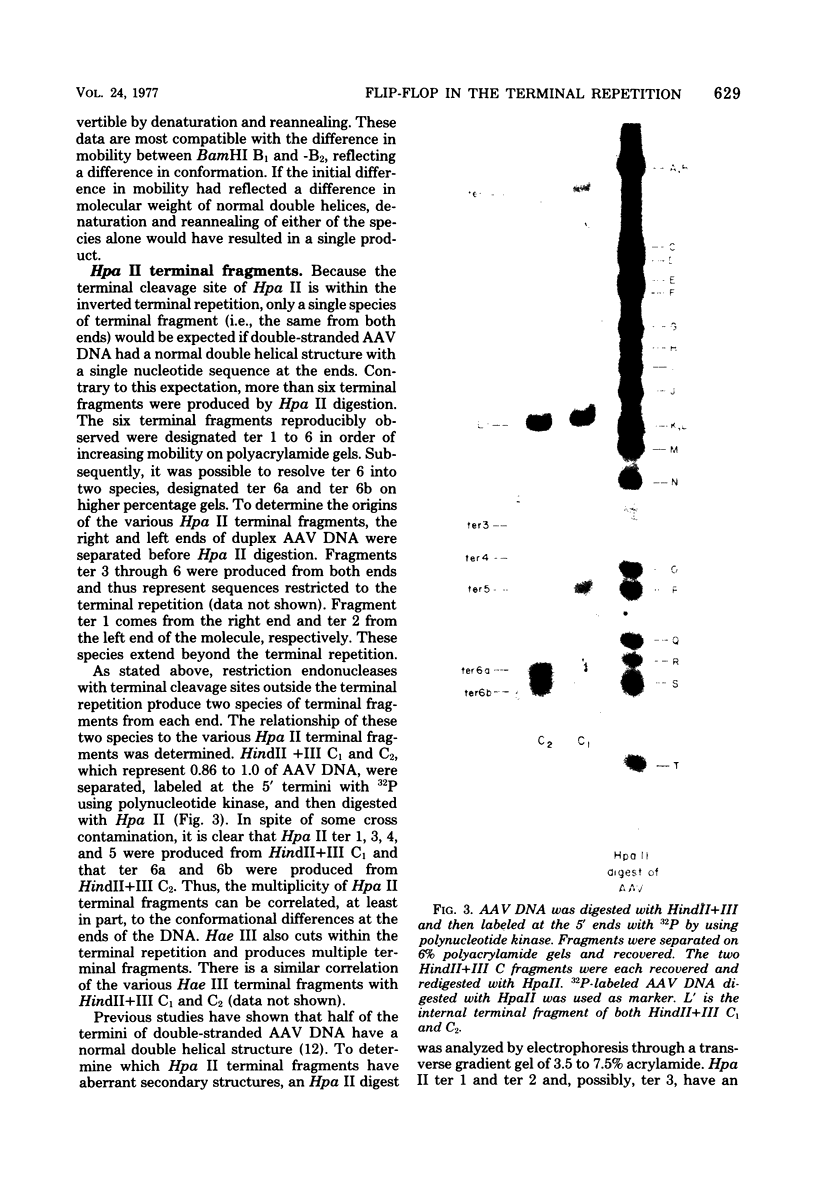
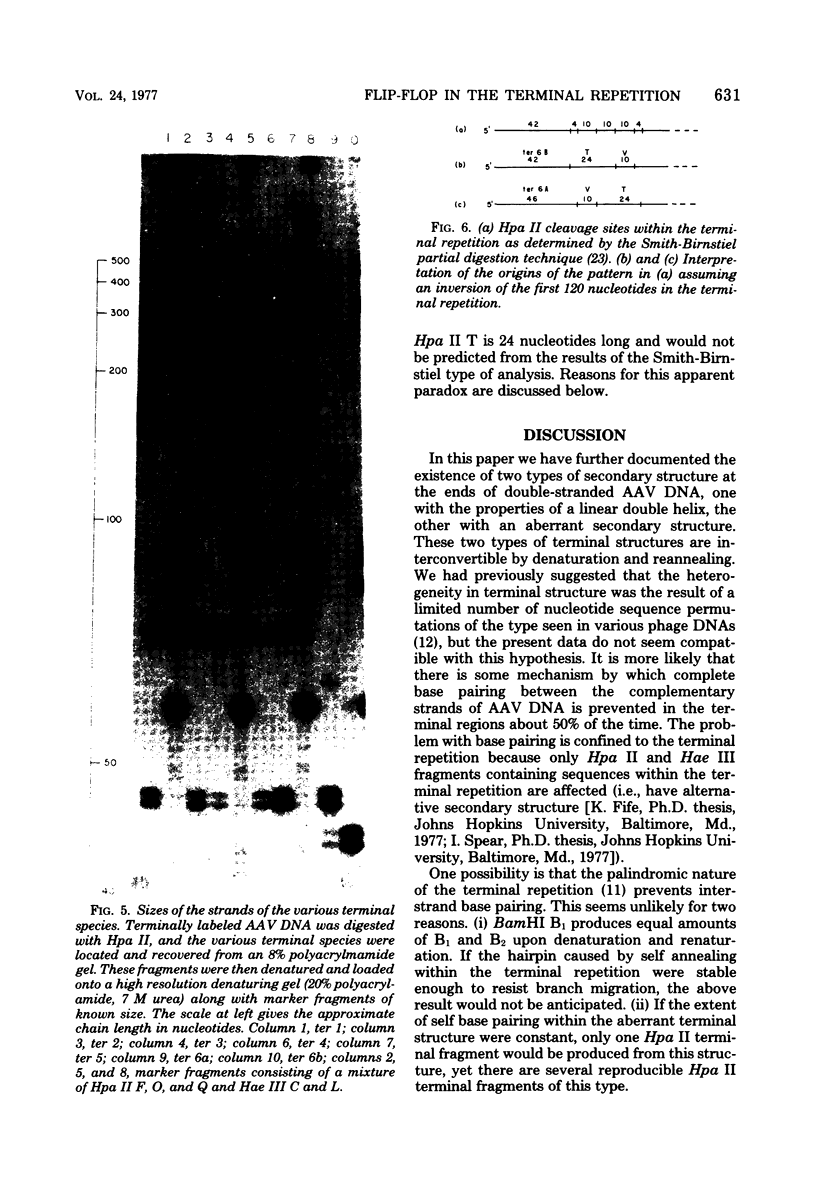
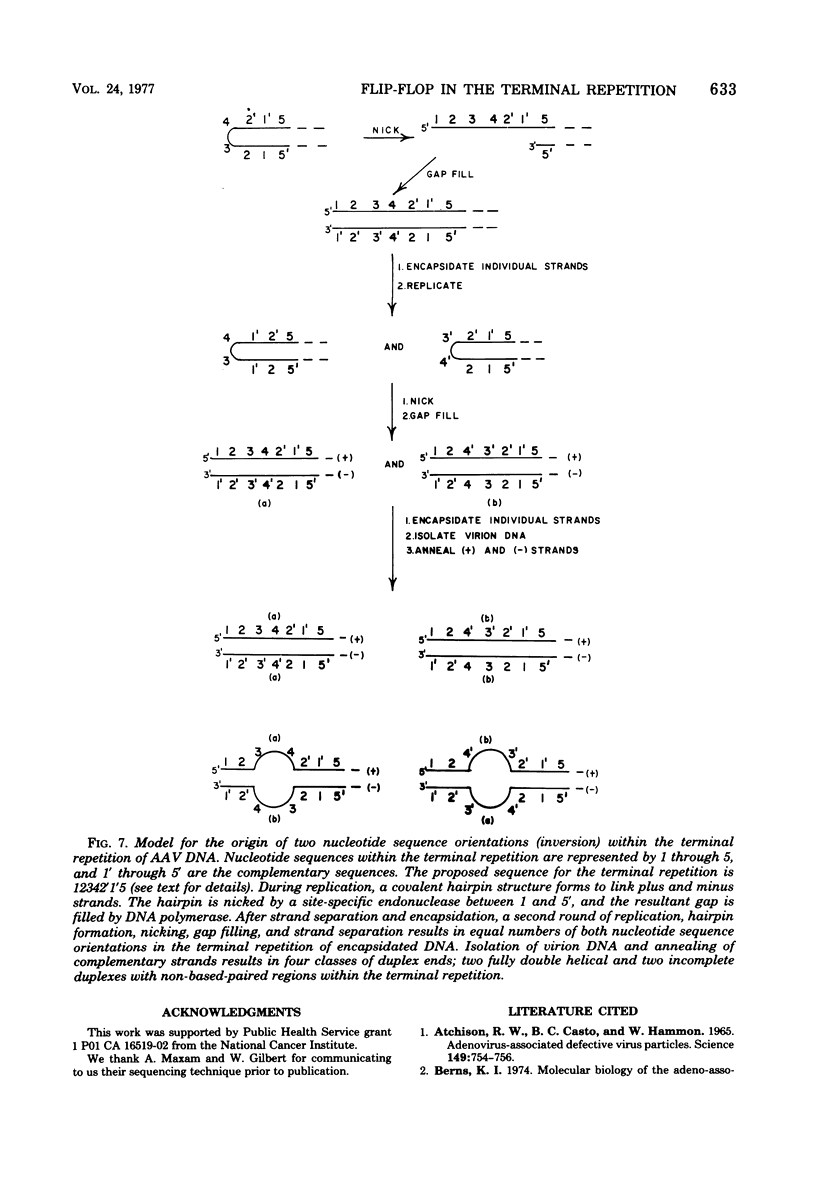
Duplex adeno-associated virus (AAV) DNA, produced by annealing plus and minus virion single strands, has been digested with several bacterial restriction endonucleases. These studies reveal the existence of alternate secondary structures at the termini of duplex AAV DNA. Analysis of the sites of endo R-Hpa II cleavage, the products of complete endo R-Hpa II digestion, and the multiple terminal secondary structures leads to the conclusion that there are two possible nucleotide sequences at each end of AAV DNA. A model that attributes the terminal nucleotide sequence heterogeneity to two possible orientations of the first 120 nucleotides at each end of the DNA is proposed; in one case the sequence is 1 to 120; in the other case the sequence is inverted. An origin of the inversion is suggested based on previously described intermediates in AAV DNA replication.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ATCHISON R. W., CASTO B. C., HAMMON W. M. ADENOVIRUS-ASSOCIATED DEFECTIVE VIRUS PARTICLES. Science. 1965 Aug 13;149(3685):754–756. doi: 10.1126/science.149.3685.754. [DOI] [PubMed] [Google Scholar]
- Berns K. I., Adler S. Separation of two types of adeno-associated virus particles containing complementary polynucleotide chains. J Virol. 1972 Feb;9(2):394–396. doi: 10.1128/jvi.9.2.394-396.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berns K. I., Kelly T. J., Jr Letter: Visualization of the inverted terminal repetition in adeno-associated virus DNA. J Mol Biol. 1974 Jan 15;82(2):267–271. doi: 10.1016/0022-2836(74)90344-1. [DOI] [PubMed] [Google Scholar]
- Berns K. I., Kort J., Fife K. H., Grogan E. W., Spear I. Study of the fine structure of adeno-associated virus DNA with bacterial restriction endonucleases. J Virol. 1975 Sep;16(3):712–719. doi: 10.1128/jvi.16.3.712-719.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berns K. I., Rose J. A. Evidence for a single-stranded adenovirus-associated virus genome: isolation and separation of complementary single strands. J Virol. 1970 Jun;5(6):693–699. doi: 10.1128/jvi.5.6.693-699.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter B. J., Fife K. H., de la Maza L. M., Berns K. I. Genome localization of adeno-associated virus RNA. J Virol. 1976 Sep;19(3):1044–1053. doi: 10.1128/jvi.19.3.1044-1053.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter B. J., Khoury G., Denhardt D. T. Physical map and strand polarity of specific fragments of adenovirus-associated virus DNA produced by endonuclease R-EcoRI. J Virol. 1975 Sep;16(3):559–568. doi: 10.1128/jvi.16.3.559-568.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denhardt D. T., Eisenberg S., Bartok K., Carter B. J. Multiple structures of adeno-associated virus DNA: analysis of terminally labeled molecules with endonuclease R-Hae III. J Virol. 1976 May;18(2):672–684. doi: 10.1128/jvi.18.2.672-684.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fife K. H., Berns K. I., Murray K. Structure and nucleotide sequence of the terminal regions of adeno-associated virus DNA. Virology. 1977 May 15;78(2):475–477. doi: 10.1016/0042-6822(77)90124-6. [DOI] [PubMed] [Google Scholar]
- Gerry H. W., Kelly T. J., Jr, Berns K. I. Arrangement of nucleotide sequences in adeno-associated virus DNA. J Mol Biol. 1973 Sep 15;79(2):207–225. doi: 10.1016/0022-2836(73)90001-6. [DOI] [PubMed] [Google Scholar]
- Glynn I. M., Chappell J. B. A simple method for the preparation of 32-P-labelled adenosine triphosphate of high specific activity. Biochem J. 1964 Jan;90(1):147–149. doi: 10.1042/bj0900147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauswirth W. W., Berns K. I. Origin and termination of adeno-associated virus DNA replication. Virology. 1977 May 15;78(2):488–499. doi: 10.1016/0042-6822(77)90125-8. [DOI] [PubMed] [Google Scholar]
- Hoggan M. D., Blacklow N. R., Rowe W. P. Studies of small DNA viruses found in various adenovirus preparations: physical, biological, and immunological characteristics. Proc Natl Acad Sci U S A. 1966 Jun;55(6):1467–1474. doi: 10.1073/pnas.55.6.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeppesen P. G. A method for separating DNA fragments by electrophoresis in polyacrylamide concentration gradient slab gels. Anal Biochem. 1974 Mar;58(1):195–207. doi: 10.1016/0003-2697(74)90458-8. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayor H. D., Torikai K., Melnick J. L., Mandel M. Plus and minus single-stranded DNA separately encapsidated in adeno-associated satellite virions. Science. 1969 Dec 5;166(3910):1280–1282. doi: 10.1126/science.166.3910.1280. [DOI] [PubMed] [Google Scholar]
- Maza L. M., Carter B. J. Cleavage of adeno-associated virus DNA with Sali,Psti and Haeii restriction endonucleases. Nucleic Acids Res. 1976 Oct;3(10):2605–2616. doi: 10.1093/nar/3.10.2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson C. C. Phosphorylation of nucleic acid by an enzyme from T4 bacteriophage-infected Escherichia coli. Proc Natl Acad Sci U S A. 1965 Jul;54(1):158–165. doi: 10.1073/pnas.54.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J. A., Berns K. I., Hoggan M. D., Koczot F. J. Evidence for a single-stranded adenovirus-associated virus genome: formation of a DNA density hybrid on release of viral DNA. Proc Natl Acad Sci U S A. 1969 Nov;64(3):863–869. doi: 10.1073/pnas.64.3.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. O., Birnstiel M. L. A simple method for DNA restriction site mapping. Nucleic Acids Res. 1976 Sep;3(9):2387–2398. doi: 10.1093/nar/3.9.2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straus S. E., Sebring E. D., Rose J. A. Concatemers of alternating plus and minus strands are intermediates in adenovirus-associated virus DNA synthesis. Proc Natl Acad Sci U S A. 1976 Mar;73(3):742–746. doi: 10.1073/pnas.73.3.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tattersall P., Ward D. C. Rolling hairpin model for replication of parvovirus and linear chromosomal DNA. Nature. 1976 Sep 9;263(5573):106–109. doi: 10.1038/263106a0. [DOI] [PubMed] [Google Scholar]