Abstract
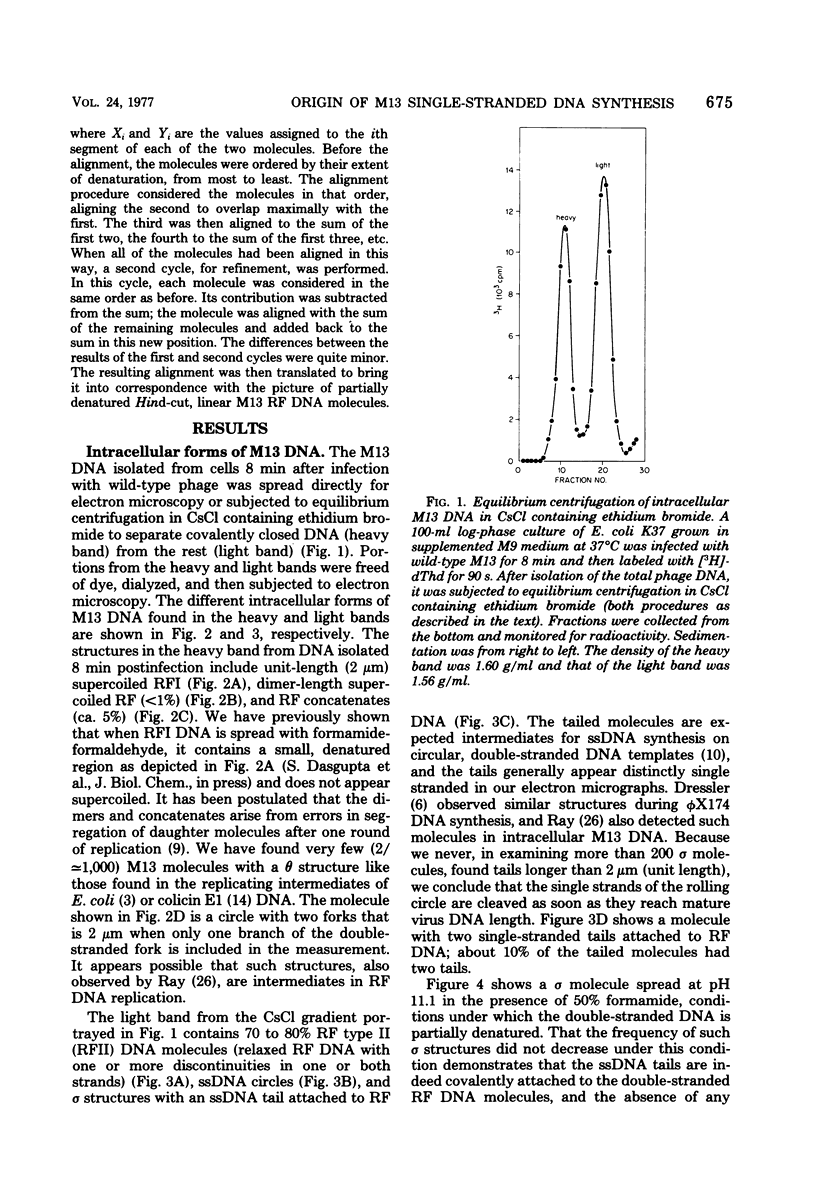
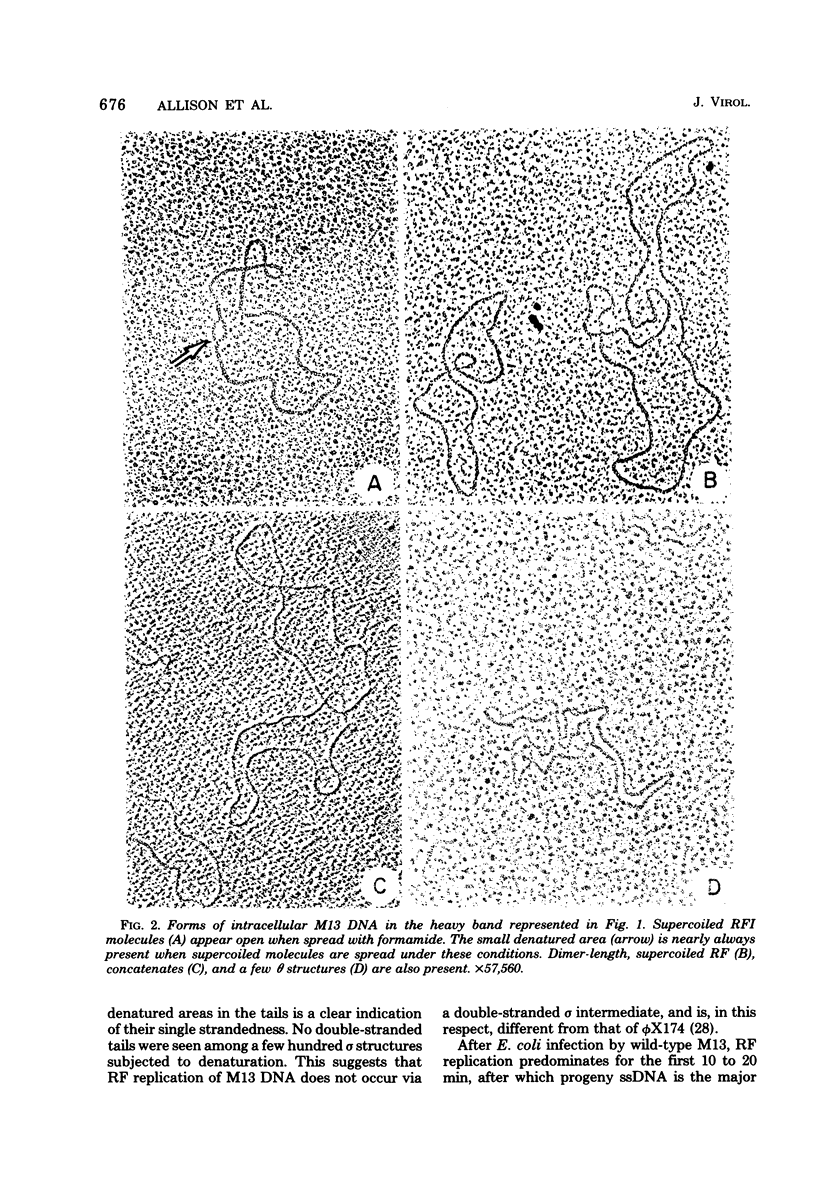
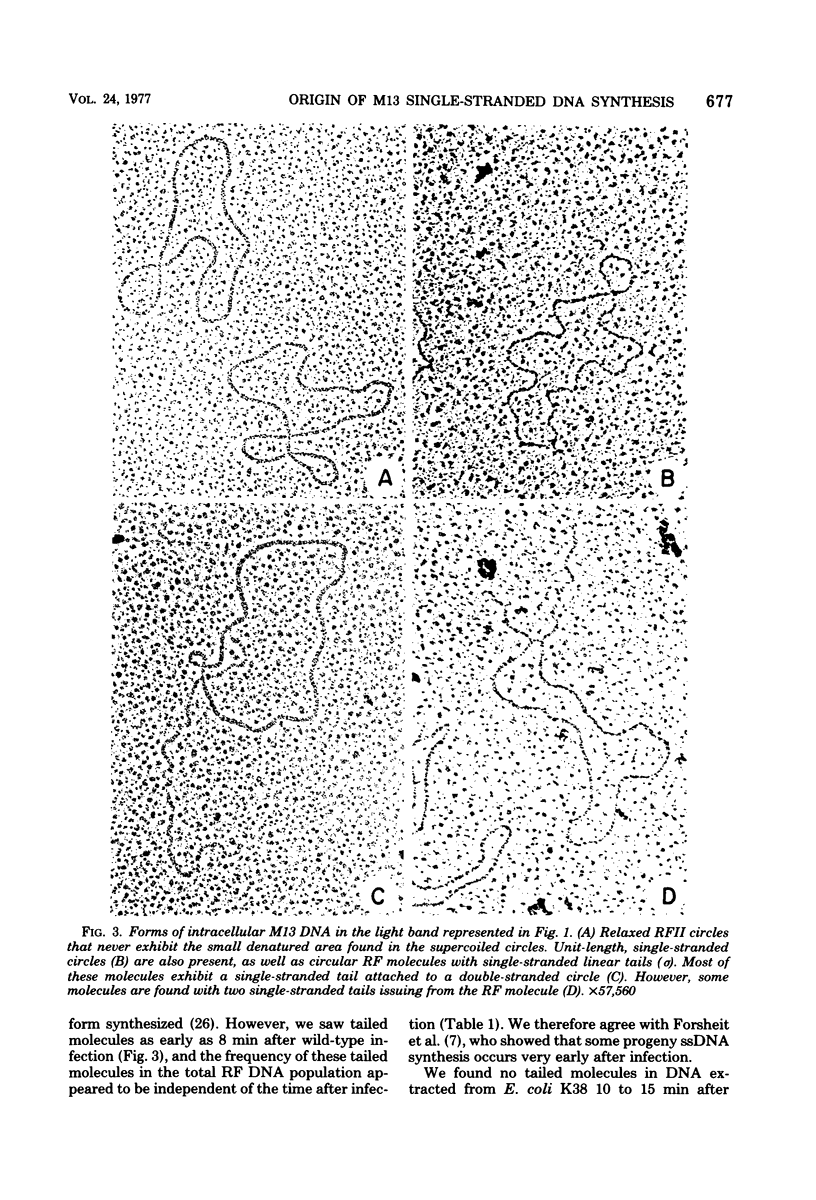
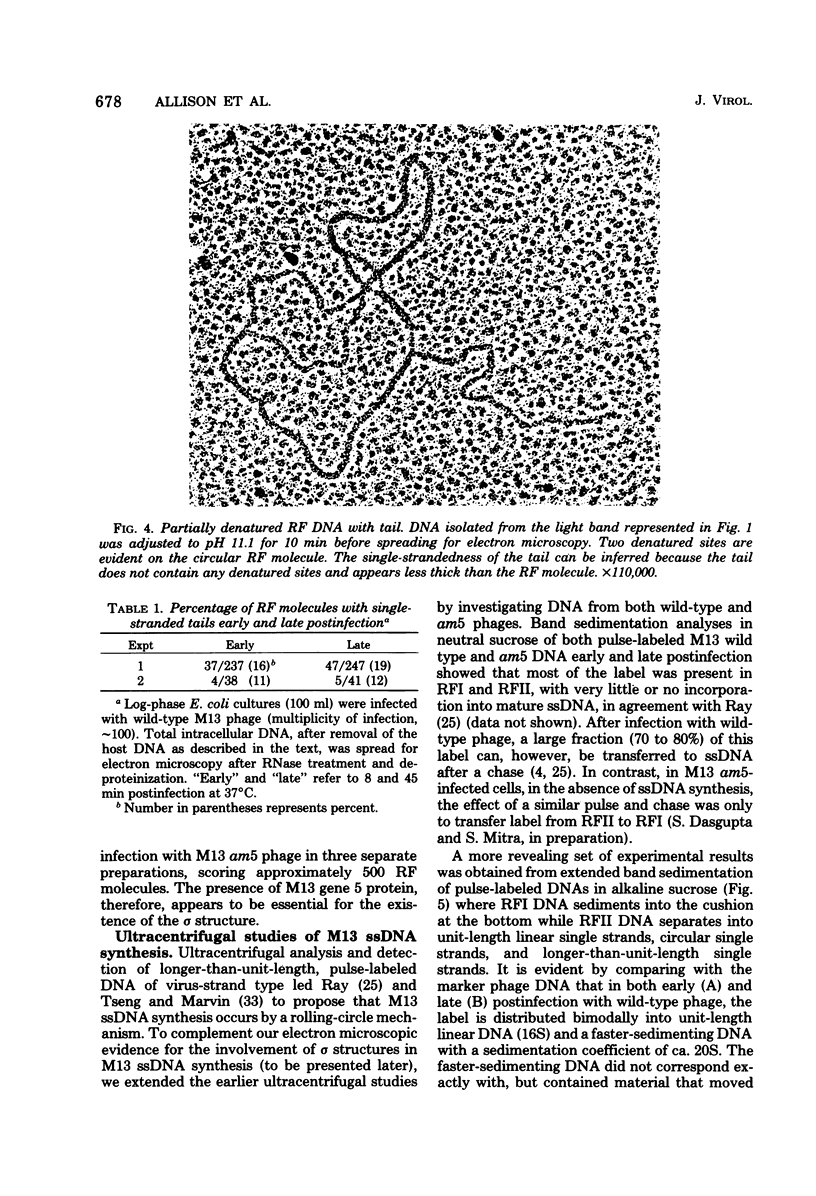
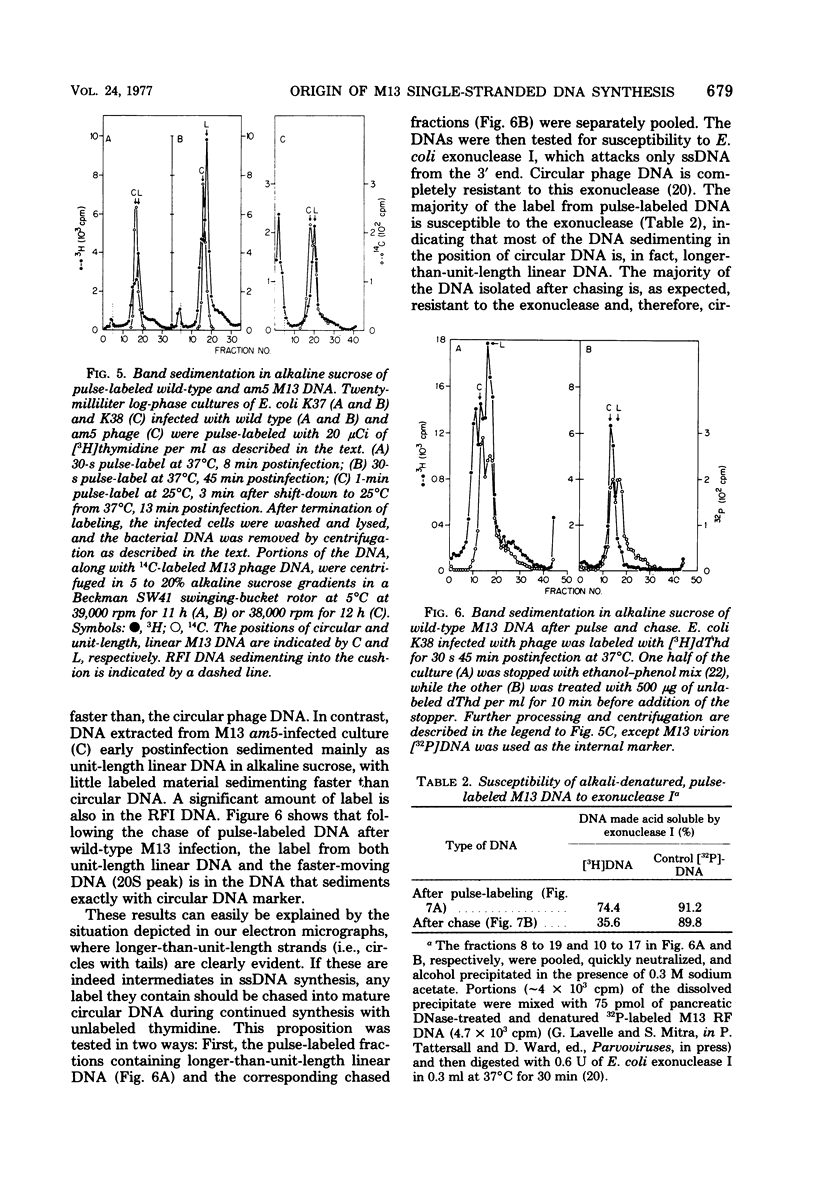
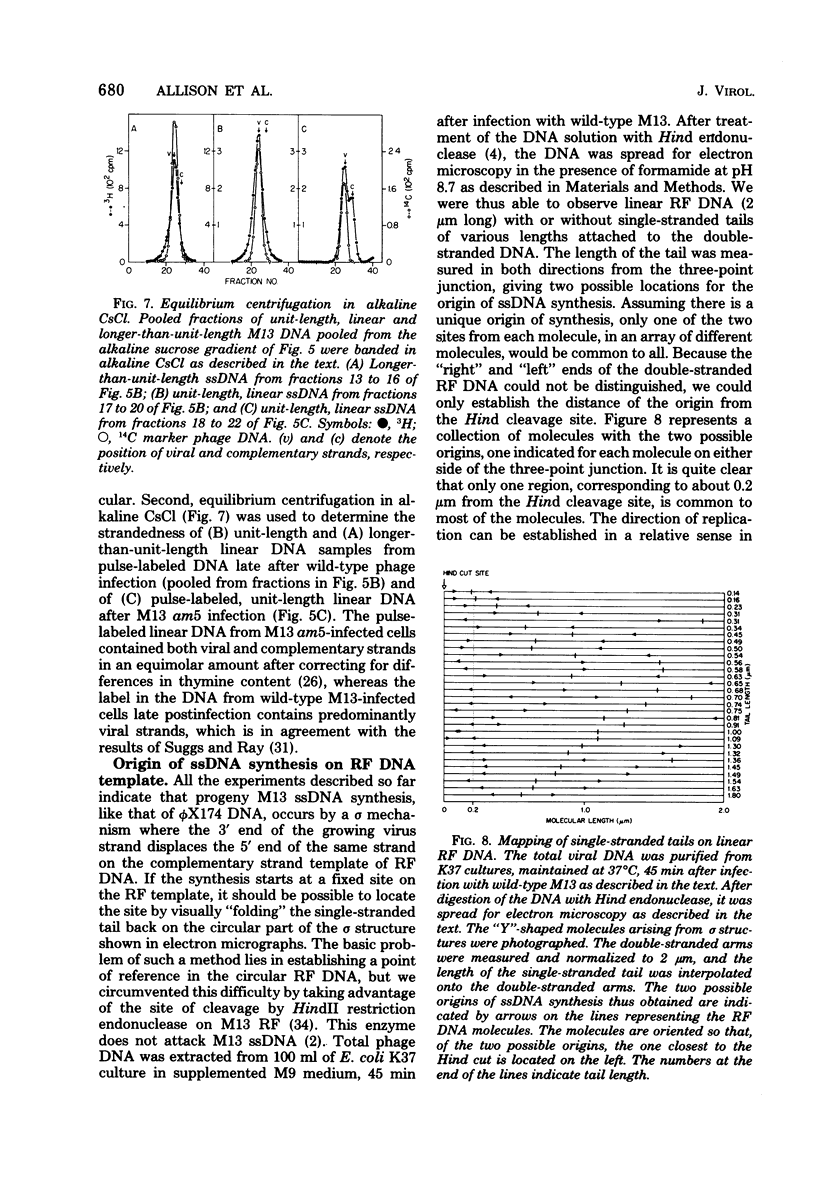
Intracellular forms of M13 phage DNA isolated after infection of Escherichia coli with wild-type phage have been studied by electron microscopy and ultracentrifugation. The data indicate the involvement of rolling-circle intermediates in single-stranded DNA synthesis. In addition to single-stranded circular DNA, we observed covalently closed and nicked replicative-form (RF) DNAs, dimer RF DNAs, concatenated RF DNAs, RF DNAs with single-stranded tails (theta, rolling circles), and, occasionally, RF DNAs with theta structures. The tails in theta molecules are always single stranded and are never longer than the DNA from mature phage; the proportion of theta to other RF molecules does not change significantly with time after infection. The origin of single-stranded DNA synthesis has been mapped by electron microscopy at a unique location on RF DNA by use of partial denaturation mapping and restriction endonuclease digestion. This location is between gene IV and gene II, and synthesis proceeds in a counterclockwise direction on the conventional genetic map.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blakesley R. W., Wells R. D. 'Single-stranded' DNA from phiX174 and M13 is cleaved by certain restriction endonucleases. Nature. 1975 Oct 2;257(5525):421–422. doi: 10.1038/257421a0. [DOI] [PubMed] [Google Scholar]
- Dasgupta S., Mitra S. The role of Escherichia coli dnaG function in coliphage M13 DNA synthesis. Eur J Biochem. 1976 Aug 1;67(1):47–51. doi: 10.1111/j.1432-1033.1976.tb10630.x. [DOI] [PubMed] [Google Scholar]
- Davis R. W., Davidson N. Electron-microscopic visualization of deletion mutations. Proc Natl Acad Sci U S A. 1968 May;60(1):243–250. doi: 10.1073/pnas.60.1.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dressler D. The rolling circle for phiX DNA replication. II. Synthesis of single-stranded circles. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1934–1942. doi: 10.1073/pnas.67.4.1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsheit A. B., Ray D. S., Lica L. Replication of bacteriophage M13. V. Single-strand synthesis during M13 infection. J Mol Biol. 1971 Apr 14;57(1):117–127. doi: 10.1016/0022-2836(71)90122-7. [DOI] [PubMed] [Google Scholar]
- Fujimura R. K., Volkin E. Biochemical analysis of the naturally repaired sections of bacteriophage T5 deoxyribonucleic acid. I. Bromodeoxyuridine incorporation into parental deoxyribonucleic acid in the absence of deoxyribonucleic acid replication. Biochemistry. 1968 Oct;7(10):3488–3498. doi: 10.1021/bi00850a025. [DOI] [PubMed] [Google Scholar]
- Gefter M. L. DNA replication. Annu Rev Biochem. 1975;44:45–78. doi: 10.1146/annurev.bi.44.070175.000401. [DOI] [PubMed] [Google Scholar]
- Gilbert W., Dressler D. DNA replication: the rolling circle model. Cold Spring Harb Symp Quant Biol. 1968;33:473–484. doi: 10.1101/sqb.1968.033.01.055. [DOI] [PubMed] [Google Scholar]
- Godson G. N., Vapnek D. A simple method of preparing large amounts of phiX174 RF 1 supercoiled DNA. Biochim Biophys Acta. 1973 Apr 11;299(4):516–520. doi: 10.1016/0005-2787(73)90223-2. [DOI] [PubMed] [Google Scholar]
- Horiuchi K., Zinder N. D. Origin and direction of synthesis of bacteriophage fl DNA. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2341–2345. doi: 10.1073/pnas.73.7.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inman R. B., Schnös M. Partial denaturation of thymine- and 5-bromouracil-containing lambda DNA in alkali. J Mol Biol. 1970 Apr 14;49(1):93–98. doi: 10.1016/0022-2836(70)90378-5. [DOI] [PubMed] [Google Scholar]
- Inselburg J., Fuke M. Isolation of catenated and replicating DNA molecules of colicin factor E1 from minicells. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2839–2842. doi: 10.1073/pnas.68.11.2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson M. K., Lark K. G. DNA replication in Escherichia coli: evidence for two classes of small deoxyribonucleotide chains. J Mol Biol. 1973 Feb 5;73(4):371–396. doi: 10.1016/0022-2836(73)90088-0. [DOI] [PubMed] [Google Scholar]
- KLEINSCHMIDT A. K., LANG D., JACHERTS D., ZAHN R. K. [Preparation and length measurements of the total desoxyribonucleic acid content of T2 bacteriophages]. Biochim Biophys Acta. 1962 Dec 31;61:857–864. [PubMed] [Google Scholar]
- Lee C. S., Davis R. W., Davidson N. A physical study by electron microscopy of the terminally reptitious, circularly permuted DNA from the coliphage particles of Escherichia coli 15. J Mol Biol. 1970 Feb 28;48(1):1–22. doi: 10.1016/0022-2836(70)90215-9. [DOI] [PubMed] [Google Scholar]
- Lyons L. B., Zinder N. D. The genetic map of the filamentous bacteriophage f1. Virology. 1972 Jul;49(1):45–60. doi: 10.1016/s0042-6822(72)80006-0. [DOI] [PubMed] [Google Scholar]
- Mazur B. J., Model P. Regulation of coliphage f1 single-stranded DNA synthesis by a DNA-binding protein. J Mol Biol. 1973 Aug 5;78(2):285–300. doi: 10.1016/0022-2836(73)90117-4. [DOI] [PubMed] [Google Scholar]
- Mitra S., Stallions D. R. The role of Escherichia coli dna A gene and its integrative suppression in M13 coliphage DNA synthesis. Eur J Biochem. 1976 Aug 1;67(1):37–45. doi: 10.1111/j.1432-1033.1976.tb10629.x. [DOI] [PubMed] [Google Scholar]
- Model P., Zinder N. D. In vitro synthesis of bacteriophage f1 proteins. J Mol Biol. 1974 Feb 25;83(2):231–251. doi: 10.1016/0022-2836(74)90389-1. [DOI] [PubMed] [Google Scholar]
- Pratt D., Erdahl W. S. Genetic control of bacteriophage M13 DNA synthesis. J Mol Biol. 1968 Oct 14;37(1):181–200. doi: 10.1016/0022-2836(68)90082-x. [DOI] [PubMed] [Google Scholar]
- Pratt D., Laws P., Griffith J. Complex of bacteriophage M13 single-stranded DNA and gene 5 protein. J Mol Biol. 1974 Feb 5;82(4):425–439. doi: 10.1016/0022-2836(74)90239-3. [DOI] [PubMed] [Google Scholar]
- Ray D. S. Replication of bacteriophage M13. II. The role of replicative forms in single-strand synthesis. J Mol Biol. 1969 Aug 14;43(3):631–643. doi: 10.1016/0022-2836(69)90364-7. [DOI] [PubMed] [Google Scholar]
- Schekman R., Weiner A., Kornberg A. Multienzyme systems of DNA replication. Science. 1974 Dec 13;186(4168):987–993. doi: 10.1126/science.186.4168.987. [DOI] [PubMed] [Google Scholar]
- Schröder C. H., Kaerner H. C. Replication of bacteriophage phichi174 replicative form DNA in vivo. J Mol Biol. 1972 Nov 14;71(2):351–362. doi: 10.1016/0022-2836(72)90356-7. [DOI] [PubMed] [Google Scholar]
- Staudenbauer W. L., Hofschneider P. H. Membrane attachment of replicating parental DNA molecules of bacteriophage M13. Biochem Biophys Res Commun. 1971 Mar 19;42(6):1035–1041. doi: 10.1016/0006-291x(71)90008-8. [DOI] [PubMed] [Google Scholar]
- Staudenbauer W. L., Hofschneider P. H. Replication of bacteriophage M-13. Positive role of gene-5 protein in single-strand-DNA synthesis. Eur J Biochem. 1973 May 2;34(3):569–576. doi: 10.1111/j.1432-1033.1973.tb02797.x. [DOI] [PubMed] [Google Scholar]
- Suggs S. V., Ray D. S. Replication of bacteriophage M13. XI. Localization of the origin for M13 single-strand synthesis. J Mol Biol. 1977 Feb 15;110(1):147–163. doi: 10.1016/s0022-2836(77)80103-4. [DOI] [PubMed] [Google Scholar]
- Tabak H. F., Griffith J., Geider K., Schaller H., Kornberg A. Initiation of deoxyribonucleic acid synthesis. VII. A unique location of the gap in the M13 replicative duplex synthesized in vitro. J Biol Chem. 1974 May 25;249(10):3049–3054. [PubMed] [Google Scholar]
- Tseng B. Y., Marvin D. A. Filamentous bacterial viruses. V. Asymmetric replication of fd duplex deoxyribonucleic acid. J Virol. 1972 Sep;10(3):371–383. doi: 10.1128/jvi.10.3.371-383.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Den Hondel C. A., Schoenmakers J. G. Cleavage maps of the filamentous bacteriophages M13, fd, fl, and ZJ/2. J Virol. 1976 Jun;18(3):1024–1039. doi: 10.1128/jvi.18.3.1024-1039.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Den Hondel C. A., Schoenmakers J. G. Studies on bacteriophage M13 DNA. 1. A cleavage map of the M13 genome. Eur J Biochem. 1975 May 6;53(2):547–558. doi: 10.1111/j.1432-1033.1975.tb04098.x. [DOI] [PubMed] [Google Scholar]
- Young I. T., Levinstone D., Eden M., Tye B. K., Botstein D. Alignment of partial denaturation maps of circularly permuted DNA by computer. J Mol Biol. 1974 Jan 5;85(4):528–532. doi: 10.1016/0022-2836(74)90313-1. [DOI] [PubMed] [Google Scholar]