Abstract
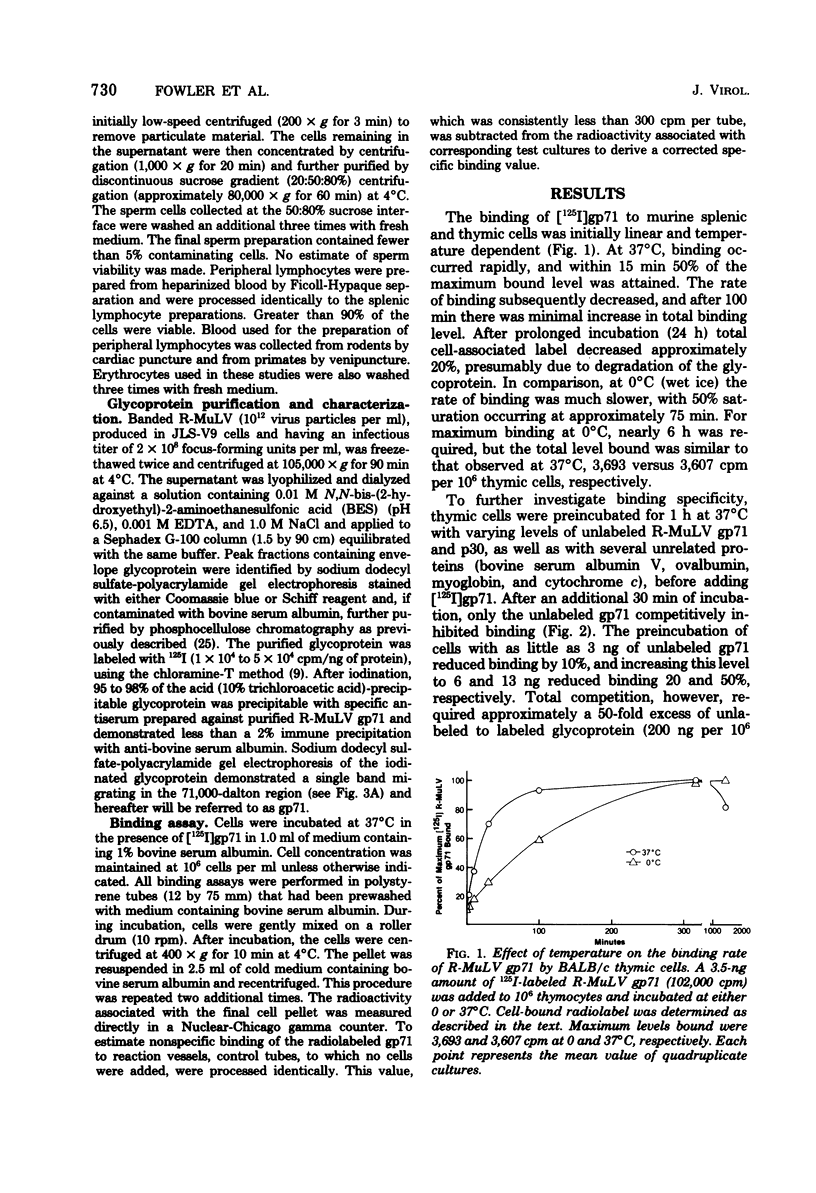
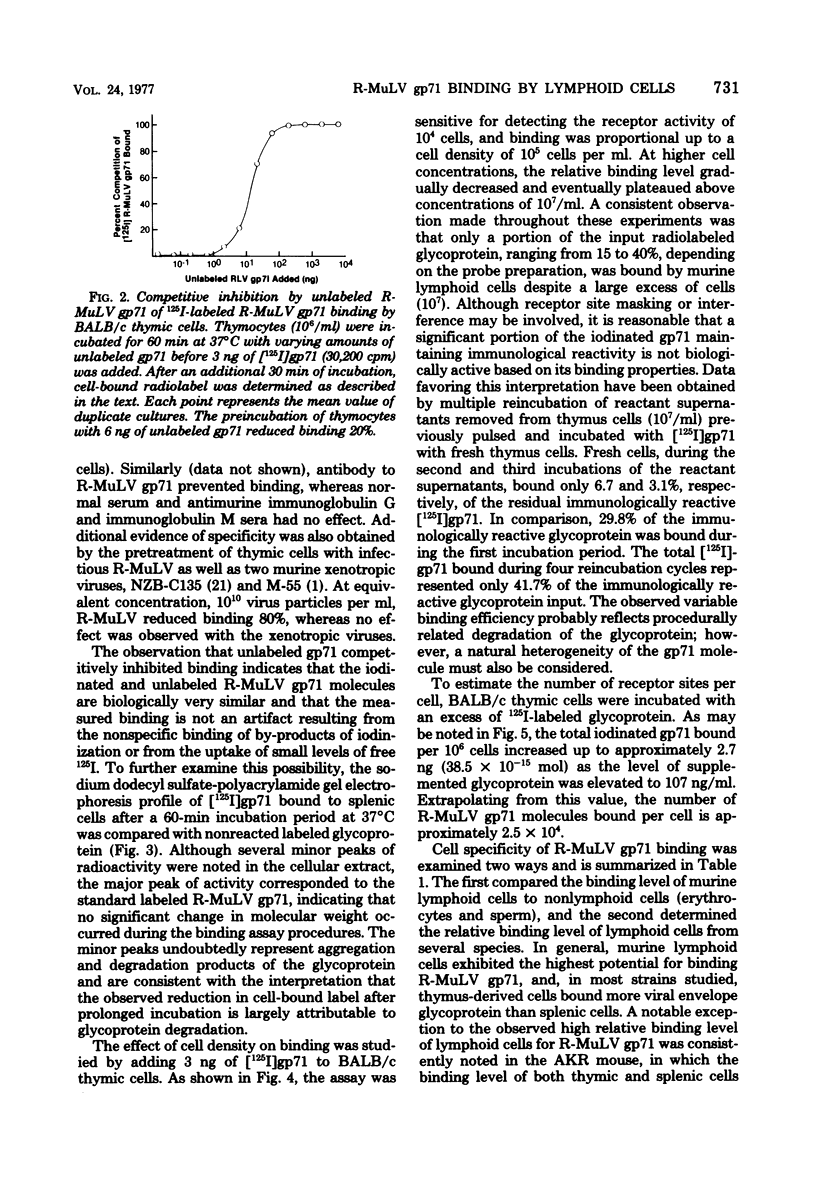
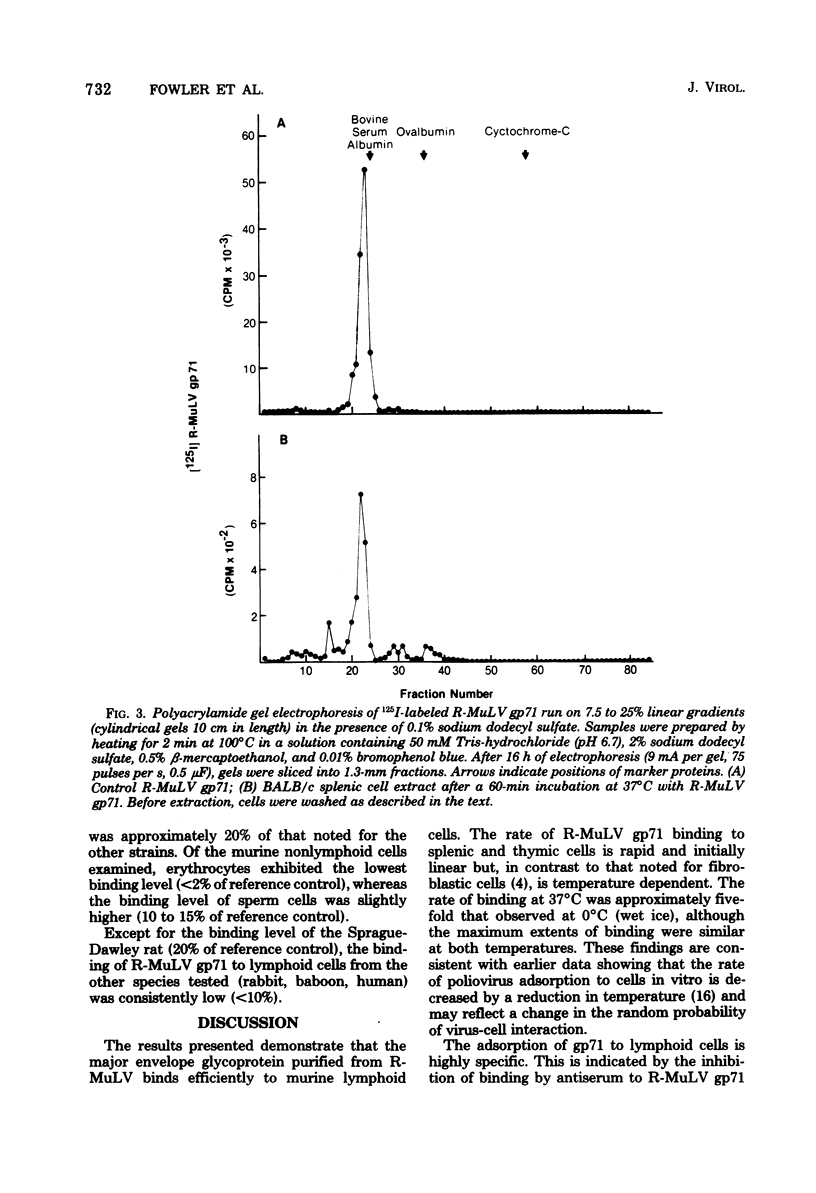
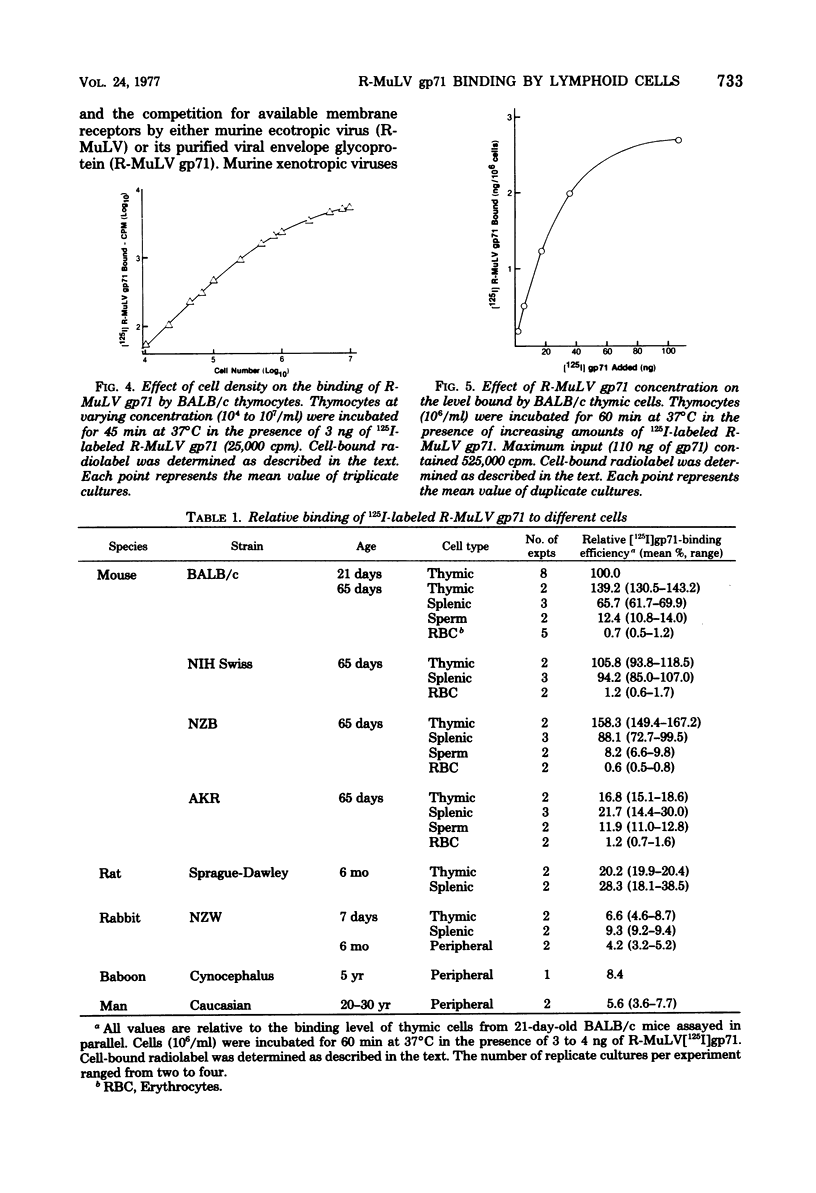
The major envelope glycoprotein (gp71) purified from Rauscher leukemia virus (R-MuLV) binds efficiently to murine lymphoid cells but not to either murine nonlymphoid cells or lymphoid cells from other species. Binding of 125I-labeled R-MuLV gp71 was competitively inhibited by unlabeled glycoprotein, as well as by whole R-MuLV, but not by murine xenotropic viruses, R-MuLV p30, and several unrelated proteins. Polyacrylamide gel electrophoresis profiles of iodinated gp71 after binding to lymphoid cells were similar to prebound profiles. Antibody to R-MuLV gp71 prevented binding, whereas normal serum had no effect. Adsorption of the glycoprotein to murine lymphoid cells occurs rapidly and is time and temperature dependent. The procedure described is sensitive for detecting the binding activity of approximately 10(4) cells. Binding was proportional up to 2.5 X 10(5) cells per ml and plateaued above 10(7) cells per ml. In the presence of excess R-MuLV gp71, BALB/c thymocytes bound approximately 2.4 X 10(4) molecules per cell.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen P. T., Mullins J. A., Saviolakis G. A., Strickland J. E., Fowler A. K., Hellman A. Direct isolation of xenotropic retraviruses from the NIH swiss mouse uterus. Virology. 1977 Jun 1;79(1):239–243. doi: 10.1016/0042-6822(77)90349-x. [DOI] [PubMed] [Google Scholar]
- Besmer P., Baltimore D. Mechanism of restriction of ecotropic and xenotropic murine leukemia viruses and formation of pseudotypes between the two viruses. J Virol. 1977 Mar;21(3):965–973. doi: 10.1128/jvi.21.3.965-973.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloyd M. W., Bolognesi D. P., Bigner D. D. Immunofluorescent analysis of expression of the RNA tumor virus major glycoprotein, gp71, on the surfaces of normal murine cells. Cancer Res. 1977 Mar;37(3):931–938. [PubMed] [Google Scholar]
- DeLarco J., Todaro G. J. Membrane receptors for murine leukemia viruses: characterization using the purified viral envelope glycoprotein, gp71. Cell. 1976 Jul;8(3):365–371. doi: 10.1016/0092-8674(76)90148-3. [DOI] [PubMed] [Google Scholar]
- Dent P. B. Immunodepression by oncogenic viruses. Prog Med Virol. 1972;14:1–35. [PubMed] [Google Scholar]
- Fowler A. K., Kouttab N. M., Kind P. D., Strickland J. E., Hellman A. Oncornaviral protein modulation in mouse uterine tissue by estrogen (38467). Proc Soc Exp Biol Med. 1975 Jan;148(1):14–18. doi: 10.3181/00379727-148-38467. [DOI] [PubMed] [Google Scholar]
- Fowler A. K., Reed C. D., Todaro G. J., Hellman A. Activation of C-type RNA virus markers in mouse uterine tissue. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2254–2257. doi: 10.1073/pnas.69.8.2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler A. K., Strickland J. E., Kouttab N. M., Hellman A. RNA tumor virus expression in mouse uterine tissue during pregnancy. Biol Reprod. 1977 Apr;16(3):344–348. doi: 10.1095/biolreprod16.3.344. [DOI] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellman A., Fowler A. K. Hormone-activated expression of the C-type RNA tumour virus genome. Nat New Biol. 1971 Sep 29;233(39):142–144. doi: 10.1038/newbio233142a0. [DOI] [PubMed] [Google Scholar]
- Hellman A., Fowler A. K., Steinman H. G., Buzzerd P. M. Studies of the blastogenic response of murine lymphocyte. 3. Specific viral transformation. Proc Soc Exp Biol Med. 1972 Oct;141(1):106–109. doi: 10.3181/00379727-141-36726. [DOI] [PubMed] [Google Scholar]
- Hellman A., Fowler A. K., Strickland J. E., Kouttab N. M. A possible physiological function for type C RNA viruses. Bibl Haematol. 1975 Oct;(43):161–165. doi: 10.1159/000399119. [DOI] [PubMed] [Google Scholar]
- Hirsch M. S., Black P. H., Tracy G. S., Leibowitz S., Schwartz R. S. Leukemia virus activation in chronic allogeneic disease. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1914–1917. doi: 10.1073/pnas.67.4.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häyry P., Rago D., Defendi V. Inhibition of phytohemagglutinin- and alloantigen-induced lymphocyte stimulation by Rauscher leukemia virus. J Natl Cancer Inst. 1970 Jun;44(6):1311–1319. [PubMed] [Google Scholar]
- JOKLIK W. K., DARNELL J. E., Jr The adsorption and early fate of purified poliovirus in HeLa cells. Virology. 1961 Apr;13:439–447. doi: 10.1016/0042-6822(61)90275-6. [DOI] [PubMed] [Google Scholar]
- Lee J. C., Ihle J. N. Characterization of the blastogenic and cytotoxic responses of normal mice to ecotropic C-type viral gp71. J Immunol. 1977 Mar;118(3):928–934. [PubMed] [Google Scholar]
- Lerner R. A., Wilson C. B., Villano B. C., McConahey P. J., Dixon F. J. Endogenous oncornaviral gene expression in adult and fetal mice: quantitative, histologic, and physiologic studies of the major viral glycorprotein, gp70. J Exp Med. 1976 Jan 1;143(1):151–166. doi: 10.1084/jem.143.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy J. A., Kazan P., Varnier O., Kleiman H. Murine xenotropic type C viruses I. Distribution and further characterization of the virus in NZB mice. J Virol. 1975 Oct;16(4):844–853. doi: 10.1128/jvi.16.4.844-853.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy J. A. Xenotropic viruses: murine leukemia viruses associated with NIH Swiss, NZB, and other mouse strains. Science. 1973 Dec 14;182(4117):1151–1153. doi: 10.1126/science.182.4117.1151. [DOI] [PubMed] [Google Scholar]
- Notkins A. L., Mergenhagen S. E., Howard R. J. Effect of virus infections on the function of the immune system. Annu Rev Microbiol. 1970;24:525–538. doi: 10.1146/annurev.mi.24.100170.002521. [DOI] [PubMed] [Google Scholar]
- RAUSCHER F. J. A virus-induced disease of mice characterized by erythrocytopoiesis and lymphoid leukemia. J Natl Cancer Inst. 1962 Sep;29:515–543. [PubMed] [Google Scholar]
- Sarma P. S., Cheong M. P., Hartley J. W., Huebner R. J. A viral interference test for mouse leukemia viruses. Virology. 1967 Sep;33(1):180–184. doi: 10.1016/0042-6822(67)90111-0. [DOI] [PubMed] [Google Scholar]
- Strand M., August J. T. Structural proteins of ribonucleic acid tumor viruses. Purification of envelope, core, and internal components. J Biol Chem. 1976 Jan 25;251(2):559–564. [PubMed] [Google Scholar]


