Abstract
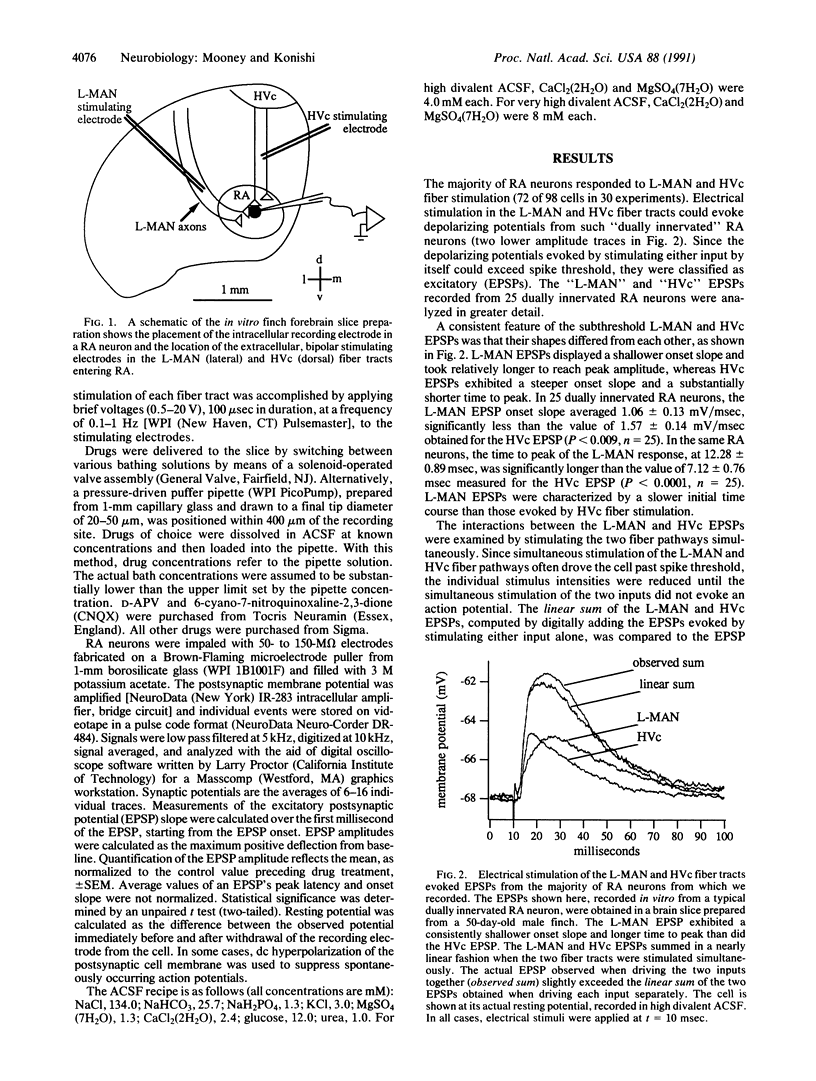
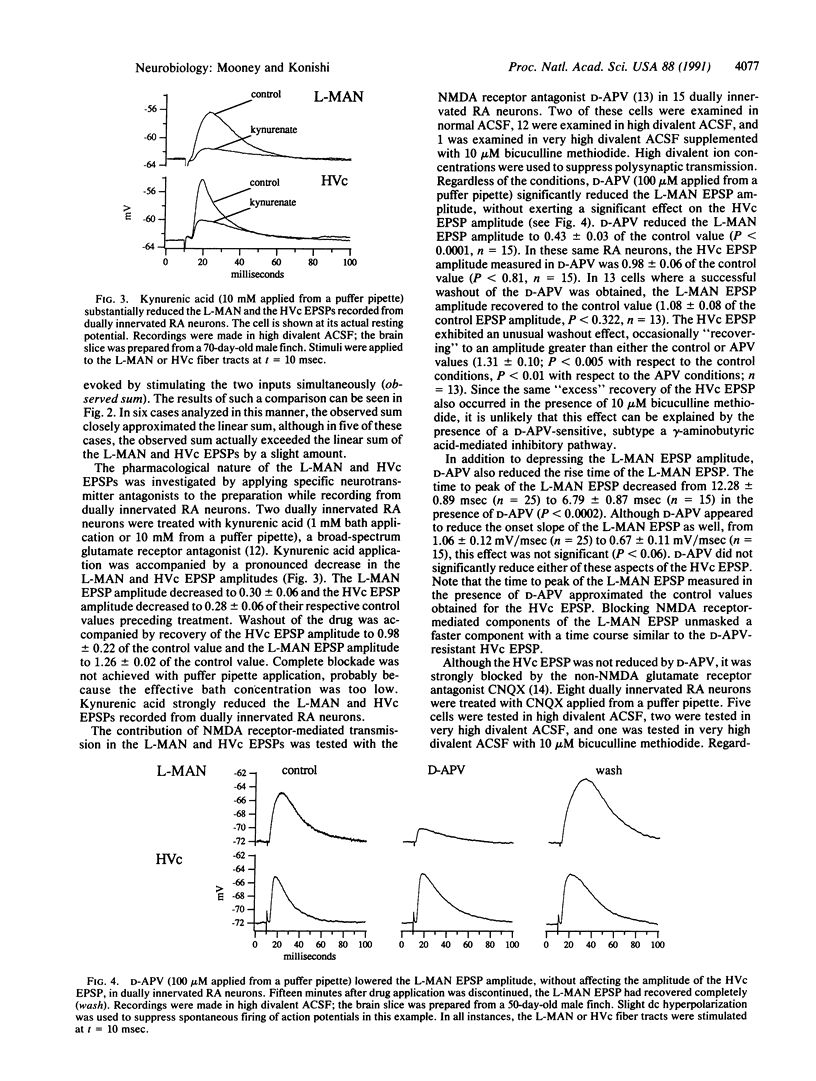
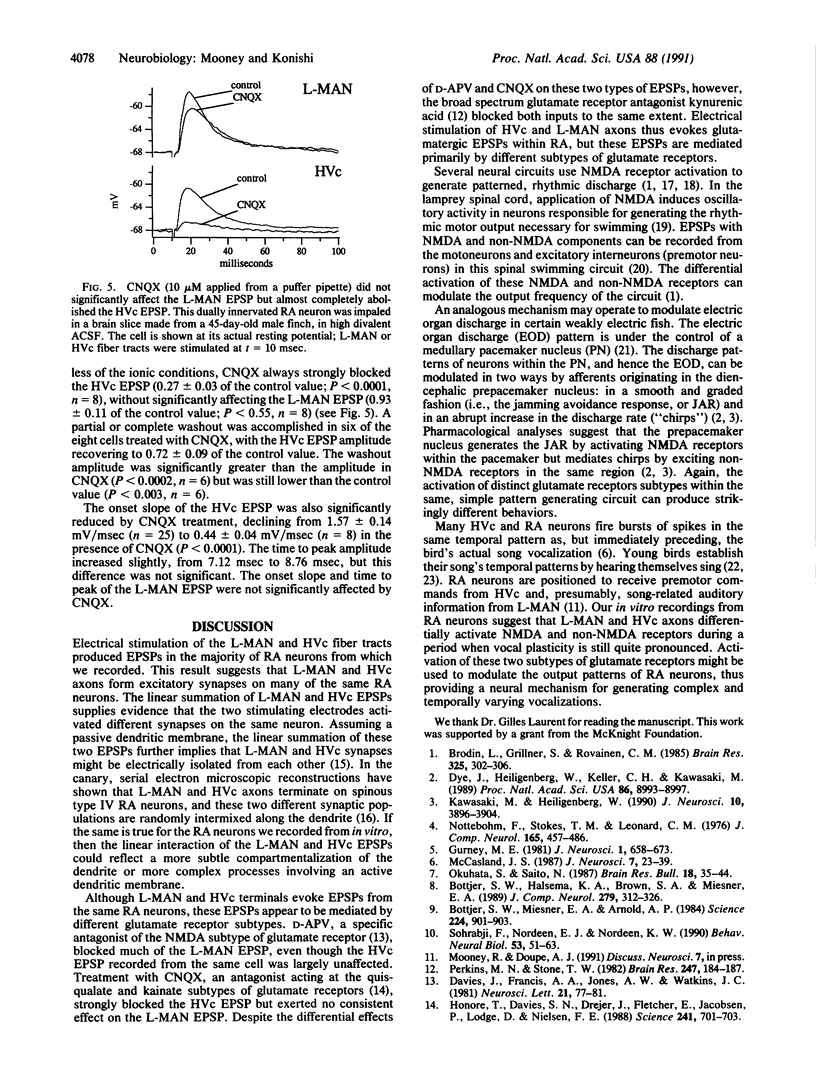
Although neural circuits mediating various simple behaviors have been delineated, those generating more complex behaviors are less well described. The discrete structure of avian song control nuclei promises that circuits controlling complex behaviors, such as birdsong, can also be understood. To this end, we developed an in vitro brain slice preparation containing the robust nucleus of the archistriatum (RA), a forebrain song control nucleus, and its inputs from two other song nuclei, the caudal nucleus of the ventral hyperstriatum (HVc) and the lateral part of the magnocellular nucleus of the anterior neostriatum (L-MAN). Using intracellular recordings, we examined the pharmacological properties of the synapses made on RA neurons by L-MAN and HVc axons. Electrical stimulation of the L-MAN and the HVc fiber tracts evoked excitatory postsynaptic potentials (EPSPs) from >70% of RA neurons when slices were prepared from male birds of 40-90 days of age, suggesting that many individual RA neurons receive excitatory input from L-MAN and HVc axons. The "L-MAN" EPSPs were blocked by the N-methyl-D-aspartate (NMDA) receptor antagonist D-(-)-2-amino-5-phosphonovaleric acid (D-APV) as well as the broad-spectrum glutamate receptor antagonist kynurenic acid but were relatively unaffected by the non-NMDA receptor blocker 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX). In contrast, "HVc" EP-SPs were relatively insensitive to D-APV but almost completely abolished by CNQX. These experiments suggest that L-MAN and HVc axons provide pharmacologically distinct types of excitatory input to many of the same RA neurons.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bottjer S. W., Halsema K. A., Brown S. A., Miesner E. A. Axonal connections of a forebrain nucleus involved with vocal learning in zebra finches. J Comp Neurol. 1989 Jan 8;279(2):312–326. doi: 10.1002/cne.902790211. [DOI] [PubMed] [Google Scholar]
- Bottjer S. W., Miesner E. A., Arnold A. P. Forebrain lesions disrupt development but not maintenance of song in passerine birds. Science. 1984 May 25;224(4651):901–903. doi: 10.1126/science.6719123. [DOI] [PubMed] [Google Scholar]
- Brodin L., Grillner S., Rovainen C. M. N-Methyl-D-aspartate (NMDA), kainate and quisqualate receptors and the generation of fictive locomotion in the lamprey spinal cord. Brain Res. 1985 Jan 28;325(1-2):302–306. doi: 10.1016/0006-8993(85)90328-2. [DOI] [PubMed] [Google Scholar]
- Brodin L., Grillner S. The role of putative excitatory amino acid neurotransmitters in the initiation of locomotion in the lamprey spinal cord. I. The effects of excitatory amino acid antagonists. Brain Res. 1985 Dec 23;360(1-2):139–148. doi: 10.1016/0006-8993(85)91229-6. [DOI] [PubMed] [Google Scholar]
- Canady R. A., Burd G. D., DeVoogd T. J., Nottebohm F. Effect of testosterone on input received by an identified neuron type of the canary song system: a Golgi/electron microscopy/degeneration study. J Neurosci. 1988 Oct;8(10):3770–3784. doi: 10.1523/JNEUROSCI.08-10-03770.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale N., Roberts A. Dual-component amino-acid-mediated synaptic potentials: excitatory drive for swimming in Xenopus embryos. J Physiol. 1985 Jun;363:35–59. doi: 10.1113/jphysiol.1985.sp015694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale N., Roberts A. Excitatory amino acid receptors in Xenopus embryo spinal cord and their role in the activation of swimming. J Physiol. 1984 Mar;348:527–543. doi: 10.1113/jphysiol.1984.sp015123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J., Francis A. A., Jones A. W., Watkins J. C. 2-Amino-5-phosphonovalerate (2APV), a potent and selective antagonist of amino acid-induced and synaptic excitation. Neurosci Lett. 1981 Jan 1;21(1):77–81. doi: 10.1016/0304-3940(81)90061-6. [DOI] [PubMed] [Google Scholar]
- Dye J., Heiligenberg W., Keller C. H., Kawasaki M. Different classes of glutamate receptors mediate distinct behaviors in a single brainstem nucleus. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8993–8997. doi: 10.1073/pnas.86.22.8993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurney M. E. Hormonal control of cell form and number in the zebra finch song system. J Neurosci. 1981 Jun;1(6):658–673. doi: 10.1523/JNEUROSCI.01-06-00658.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honoré T., Davies S. N., Drejer J., Fletcher E. J., Jacobsen P., Lodge D., Nielsen F. E. Quinoxalinediones: potent competitive non-NMDA glutamate receptor antagonists. Science. 1988 Aug 5;241(4866):701–703. doi: 10.1126/science.2899909. [DOI] [PubMed] [Google Scholar]
- Kawasaki M., Heiligenberg W. Different classes of glutamate receptors and GABA mediate distinct modulations of a neuronal oscillator, the medullary pacemaker of a gymnotiform electric fish. J Neurosci. 1990 Dec;10(12):3896–3904. doi: 10.1523/JNEUROSCI.10-12-03896.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi M. The role of auditory feedback in the control of vocalization in the white-crowned sparrow. Z Tierpsychol. 1965 Dec;22(7):770–783. [PubMed] [Google Scholar]
- McCasland J. S. Neuronal control of bird song production. J Neurosci. 1987 Jan;7(1):23–39. doi: 10.1523/JNEUROSCI.07-01-00023.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nottebohm F., Stokes T. M., Leonard C. M. Central control of song in the canary, Serinus canarius. J Comp Neurol. 1976 Feb 15;165(4):457–486. doi: 10.1002/cne.901650405. [DOI] [PubMed] [Google Scholar]
- Okuhata S., Saito N. Synaptic connections of thalamo-cerebral vocal nuclei of the canary. Brain Res Bull. 1987 Jan;18(1):35–44. doi: 10.1016/0361-9230(87)90031-1. [DOI] [PubMed] [Google Scholar]
- Perkins M. N., Stone T. W. An iontophoretic investigation of the actions of convulsant kynurenines and their interaction with the endogenous excitant quinolinic acid. Brain Res. 1982 Sep 9;247(1):184–187. doi: 10.1016/0006-8993(82)91048-4. [DOI] [PubMed] [Google Scholar]
- Sohrabji F., Nordeen E. J., Nordeen K. W. Selective impairment of song learning following lesions of a forebrain nucleus in the juvenile zebra finch. Behav Neural Biol. 1990 Jan;53(1):51–63. doi: 10.1016/0163-1047(90)90797-a. [DOI] [PubMed] [Google Scholar]
- Wallén P., Grillner S. N-methyl-D-aspartate receptor-induced, inherent oscillatory activity in neurons active during fictive locomotion in the lamprey. J Neurosci. 1987 Sep;7(9):2745–2755. doi: 10.1523/JNEUROSCI.07-09-02745.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]


