Abstract
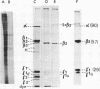
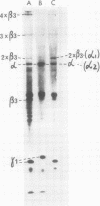
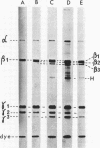
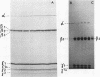
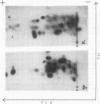
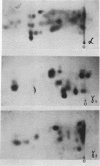
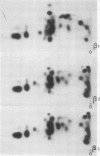
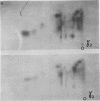
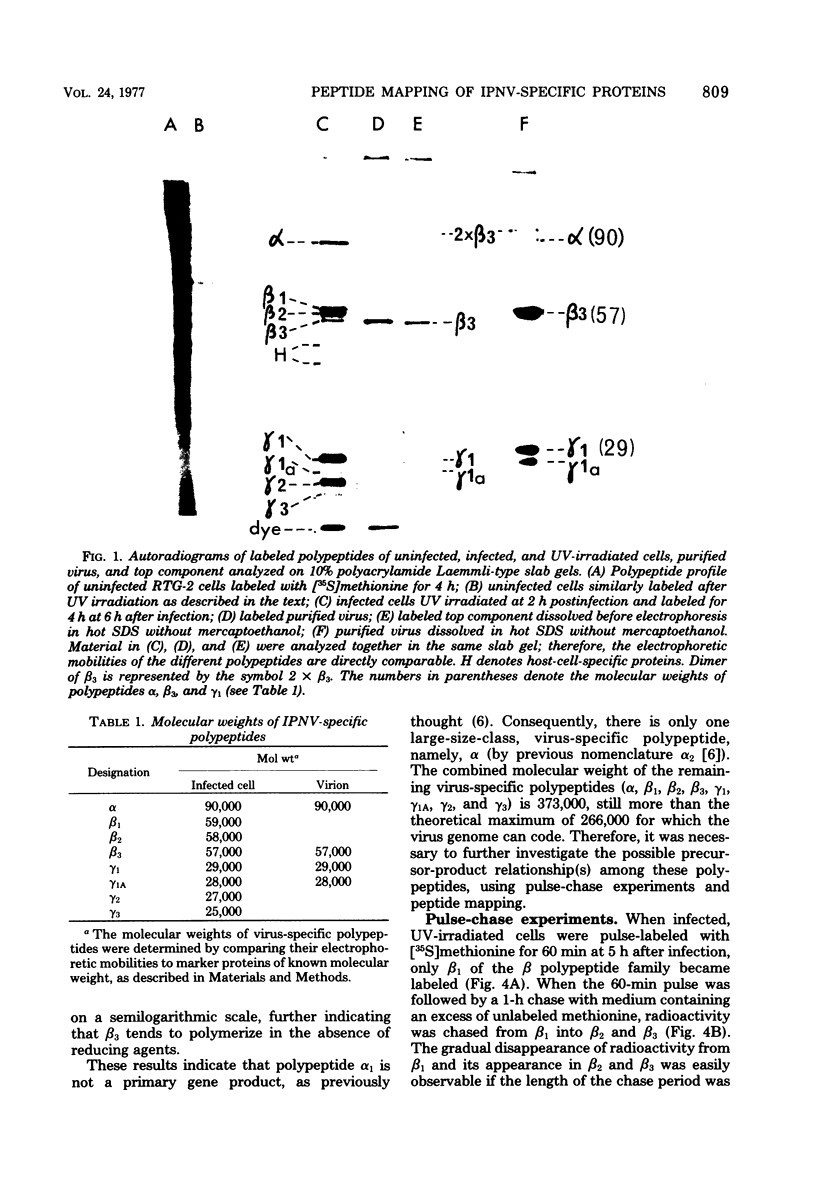
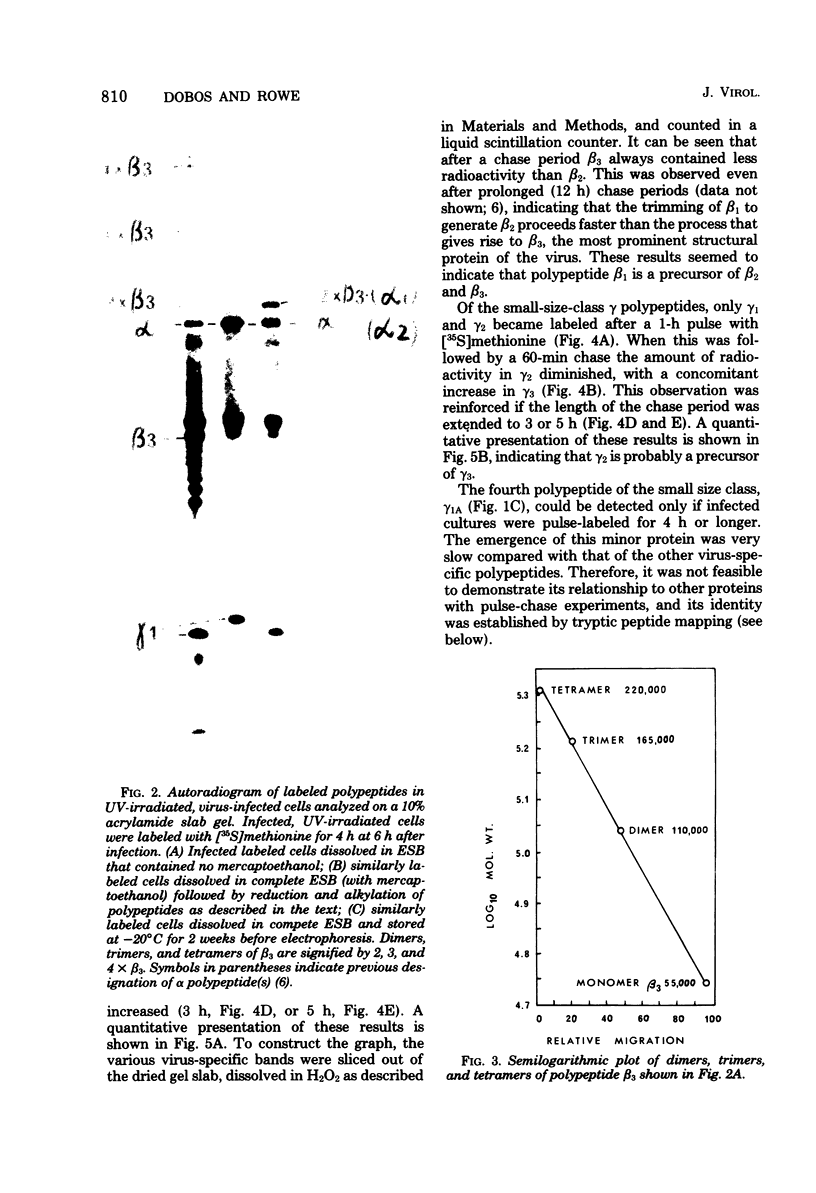
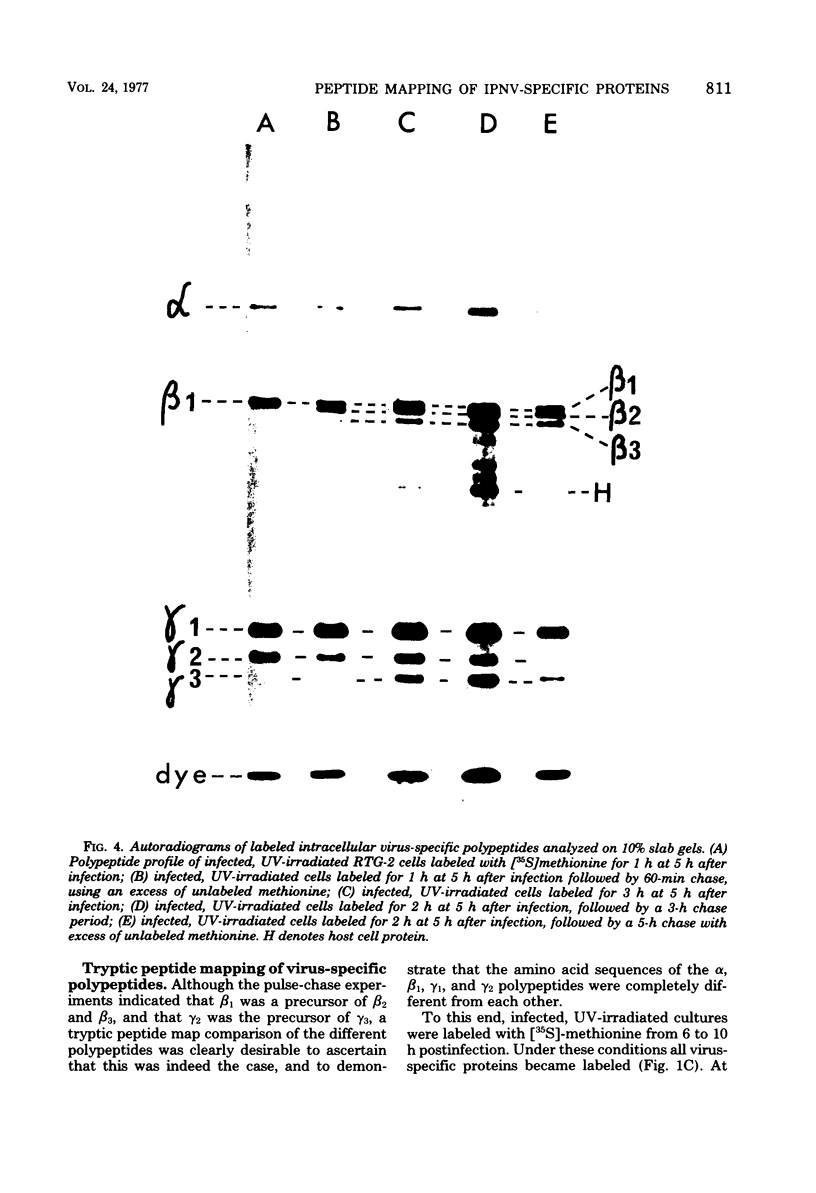
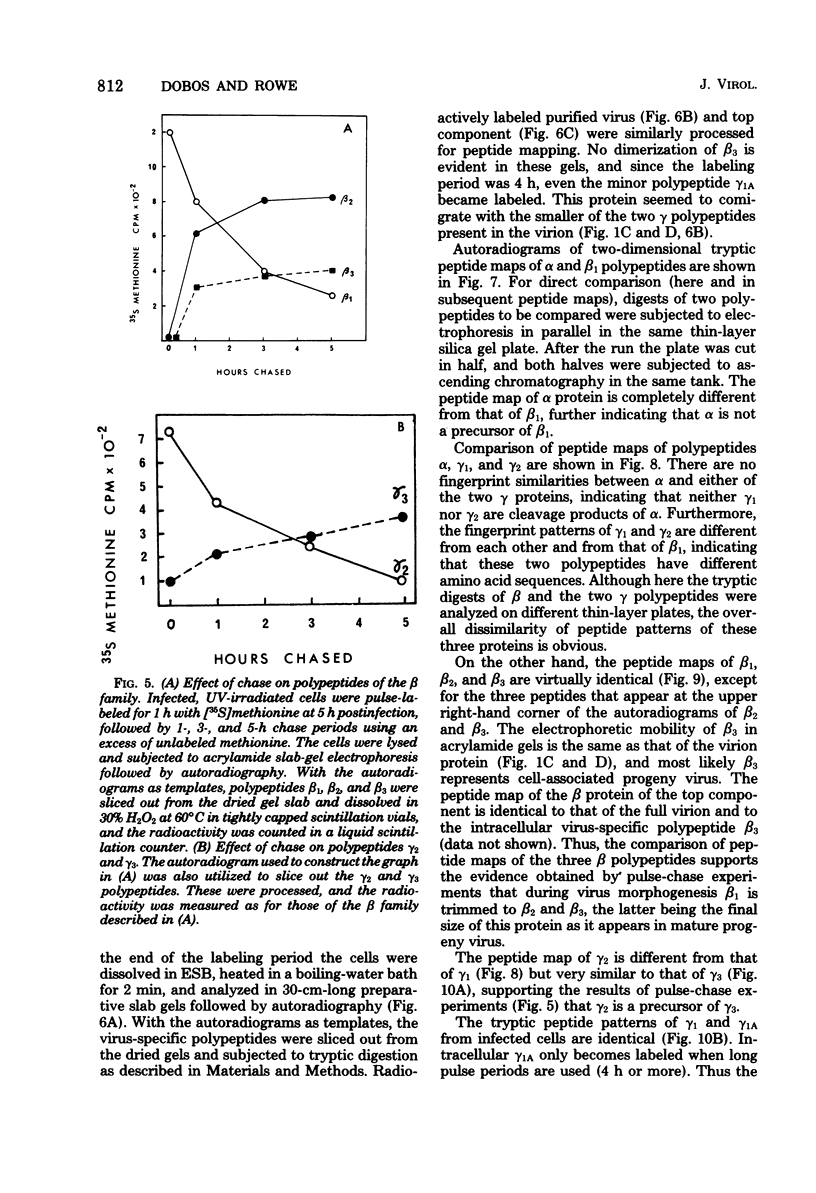
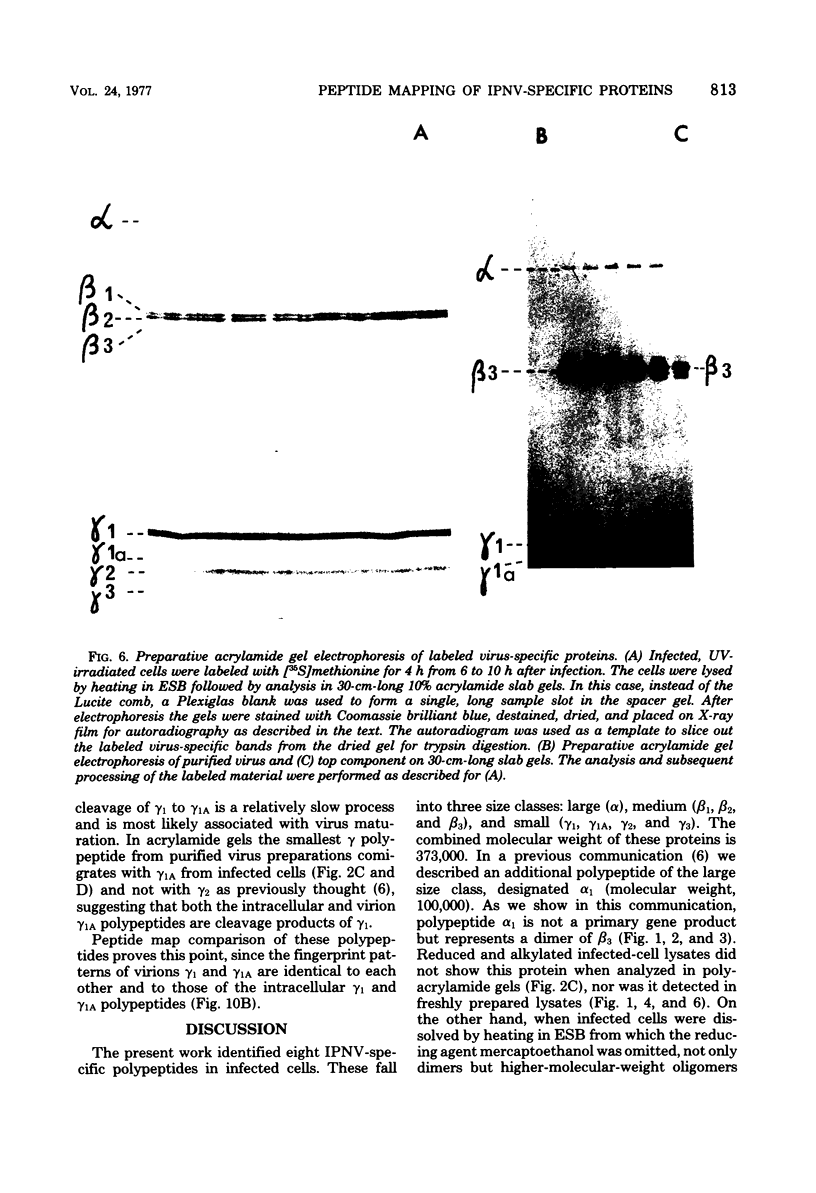
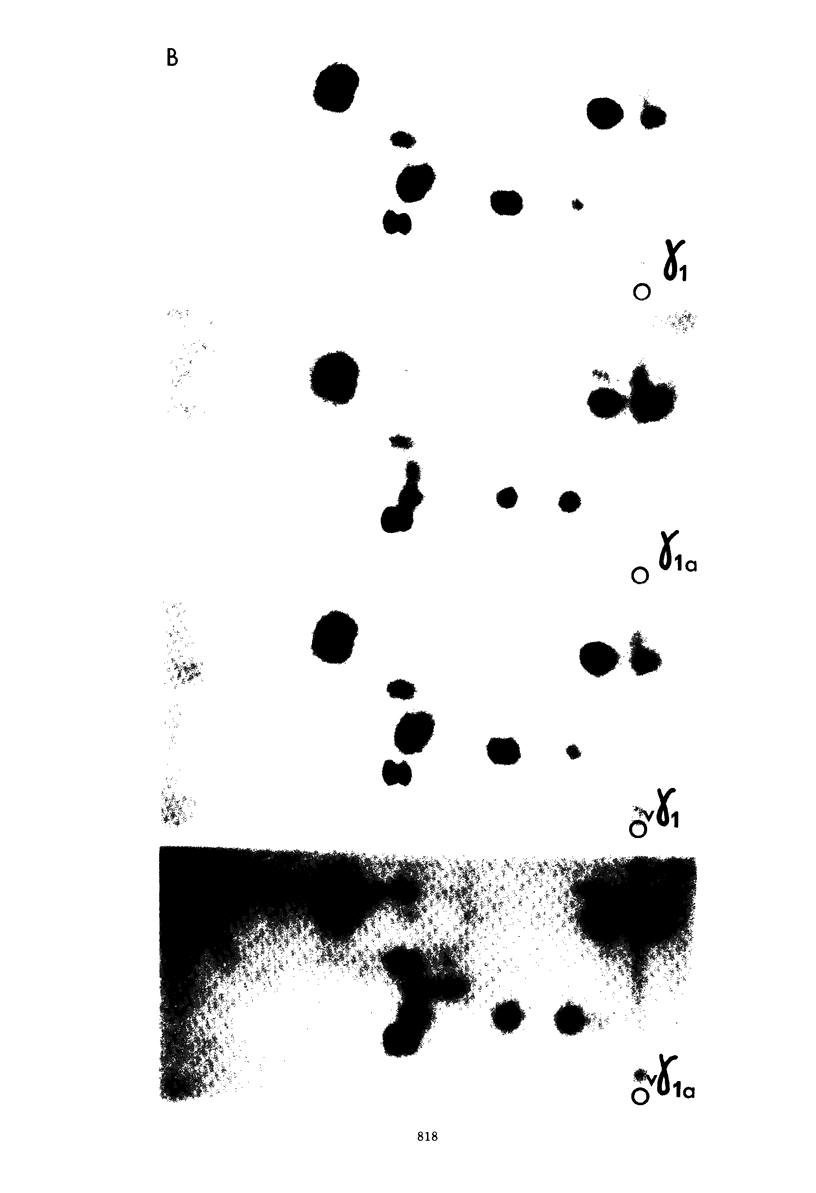
An investigation of virus-specific protein synthesis in infectious pancreatic necrosis virus (IPNV)-infected rainbow trout gonad cells was undertaken to find a relationship between the coding capacity of the virus genome (two segments of double-stranded RNA of 2.5 × 106 and 2.3 × 106 molecular weight) and the sizes and relative amounts of virus-specific proteins. Using polyacrylamide slabgel electrophoresis and autoradiography, eight distinct virus-specific polypeptides were detected in infected, [35S]methionine-labeled cells. These proteins may be grouped into three size classes on the basis of molecular weight: (i) large, α (90,000); (ii) medium, β1 (59,000), β2 (58,000), and β3 (57,000); and (iii) small, γ1 (29,000), γ1A (28,000), γ2 (27,000), and γ3 (25,000). The combined molecular weight of these polypetides (373,000) is beyond the coding capacity of the virus genome. Purified IPNV contained polypeptides α, β3, γ1, and γ1A. Pulse-chase experiments and tryptic peptide map comparisons revealed that only four of the eight intracellular proteins were primary gene products, namely, α, β1, γ1, and β2, with a combined molecular weight of 205,000. Of these primary gene products only the α polypeptide was found to be stable, whereas the other three underwent intracellular proteolytic cleavage during virus morphogenesis. Polypeptide β1 was cleaved to generate β2 and β3; γ1 was trimmed to produce γ1A, and the only nonstructural primary gene product, γ2, was found to be a precursor of γ3. These results suggest that IPNV possesses a unique mechanism to synthesize three size classes of proteins using mRNA transcripts from two high-molecular-weight double-stranded RNA genome segments.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alayse A. M., Cohen J., Scherrer J. Etude de la synthèse des ARN viraus dans les cellules FHM et RTG-2 infectées par le virus de la nécrose pancréatique infectieuse (NPI) Ann Microbiol (Paris) 1975 Dec;126(4):471–483. [PubMed] [Google Scholar]
- Both G. W., Lavi S., Shatkin A. J. Synthesis of all the gene products of the reovirus genome in vivo and in vitro. Cell. 1975 Feb;4(2):173–180. doi: 10.1016/0092-8674(75)90124-5. [DOI] [PubMed] [Google Scholar]
- Cohen J., Poinsard A., Scherrer R. Physico-chemical and morphological features of infectious pancreatic necrosis virus. J Gen Virol. 1973 Dec;21(3):485–498. doi: 10.1099/0022-1317-21-3-485. [DOI] [PubMed] [Google Scholar]
- Cohen J. Ribonucletic acid polymerase activity in purified infectious pancreatic necrosis virus of trout. Biochem Biophys Res Commun. 1975 Feb 3;62(3):689–695. doi: 10.1016/0006-291x(75)90454-4. [DOI] [PubMed] [Google Scholar]
- Dobos P., Hallett R., Kells D. T., Sorensen O., Rowe D. Biophysical studies of infectious pancreatic necrosis virus. J Virol. 1977 Apr;22(1):150–159. doi: 10.1128/jvi.22.1.150-159.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobos P., Martin E. M. Virus-specific polypeptides in ascites cells infected with encephalomyocarditis virus. J Gen Virol. 1972 Nov;17(2):197–212. doi: 10.1099/0022-1317-17-2-197. [DOI] [PubMed] [Google Scholar]
- Dobos P. Size and structure of the genome of infectious pancreatic necrosis virus. Nucleic Acids Res. 1976 Aug;3(8):1903–1924. doi: 10.1093/nar/3.8.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobos P. Virus-specific protein synthesis in cells infected by infectious pancreatic necrosis virus. J Virol. 1977 Jan;21(1):242–258. doi: 10.1128/jvi.21.1.242-258.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A. S., Baltimore D., Stampfer M. Ribonucleic acid synthesis of vesicular stomatitis virus. 3. Multiple complementary messenger RNA molecules. Virology. 1970 Dec;42(4):946–957. doi: 10.1016/0042-6822(70)90343-0. [DOI] [PubMed] [Google Scholar]
- Kelly R. K., Loh P. C. Electron microscopical and biochemical characterization of infectious pancreatic necrosis virus. J Virol. 1972 Oct;10(4):824–834. doi: 10.1128/jvi.10.4.824-834.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lodish H. F. Independent translation of the genes of bacteriophage f2 RNA. J Mol Biol. 1968 Mar 28;32(3):681–685. doi: 10.1016/0022-2836(68)90351-3. [DOI] [PubMed] [Google Scholar]
- Macdonald R. D., Yamamoto T. The structure of infectious pancreatic necrosis virus RNA. J Gen Virol. 1977 Feb;34(2):235–247. doi: 10.1099/0022-1317-34-2-235. [DOI] [PubMed] [Google Scholar]
- Malsberger R. G., Cerini C. P. Multiplication of infectious pancreatic necrosis virus. Ann N Y Acad Sci. 1965 Aug 10;126(1):320–327. doi: 10.1111/j.1749-6632.1965.tb14283.x. [DOI] [PubMed] [Google Scholar]
- Morrison T. G. Site of synthesis of membrane and nonmembrane proteins of vesicular stomatitis virus. J Biol Chem. 1975 Sep 10;250(17):6955–6962. [PubMed] [Google Scholar]
- Schlesinger M. J., Schlesinger S., Burge B. W. Identification of a second glycoprotein in Sindbis virus. Virology. 1972 Feb;47(2):539–541. doi: 10.1016/0042-6822(72)90298-x. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- Zweerink H. J., Joklik W. K. Studies on the intracellular synthesis of reovirus-specified proteins. Virology. 1970 Jul;41(3):501–518. doi: 10.1016/0042-6822(70)90171-6. [DOI] [PubMed] [Google Scholar]