Abstract
Arterial dysfunction has been linked to decline in cardiac function and increased risk of cardiovascular disease events. We calculated the value of arterial function, measured at baseline (2000–2002), in predicting time to first coronary heart disease (CHD) event (median follow-up, 10.2 years) among participants in the Multi-Ethnic Study of Atherosclerosis (MESA). Measures included the following: C1 and C2, derived from diastolic pulse contour analysis from the radial artery blood pressure waveform obtained by tonometry (n = 6,336); carotid distensibility and Young's elastic modulus at the carotid artery, derived from carotid artery ultrasonography (n = 6,531 and 6,528); and aortic distensibility, measured using cardiac magnetic resonance imaging (n = 3,677). After adjustment, the hazard ratio for a CHD event per standard-deviation increment in arterial function was 0.97 (95% confidence interval (CI): 0.86, 1.10) for C1, 0.73 (95% CI: 0.63, 0.86) for C2, 0.98 (95% CI: 0.86, 1.11) for carotid distensibility, 0.99 (95% CI: 0.90, 1.09) for Young's modulus, and 0.90 (95% CI: 0.74, 1.10) for aortic distensibility. We examined the area under the receiver operating characteristic curve for the model with full adjustment plus the addition of each measure individually. C2 provided additional discrimination for the prediction of CHD (area under the curve = 0.736 vs. 0.743; P = 0.04). Lower C2 was associated with a higher risk of future CHD events.
Keywords: arteries, coronary disease, vascular stiffness
An increase in arterial stiffness, or a decrease in elasticity, has been described in the process of vascular aging. Various methods have been developed to estimate increases in arterial stiffness or decreases in arterial elasticity in order to detect early changes in arterial function beyond arterial blood pressure. In this paper, arterial elasticity and stiffness measures are referred to as “arterial function measures.” Measurement techniques have been implemented to identify vascular disease at early stages, before the occurrence of cardiovascular disease (CVD) events. Thus, measures of arterial function may identify people who are likely to progress to clinical events, beyond what can be estimated using only traditional CVD risk factors (1). We hypothesized that a functional derivative from the arterial blood pressure waveform would be more predictive of future coronary events than measures derived from cardiac cycle–dependent changes in cross-sectional area. We examined the predictive value of the following measures of arterial function: 1) derivatives of the arterial blood pressure waveform: C1 and C2; and 2) derivatives of cross-sectional analysis of arteries: aortic distensibility (AD), carotid distensibility (CD), and Young's elastic modulus (YM) at the carotid artery. In this study, we investigated the strength of the association of these arterial function measures with coronary heart disease (CHD) events and whether these measures could predict CHD events beyond traditional CVD risk factors.
METHODS
Study population
The Multi-Ethnic Study of Atherosclerosis (MESA) is an ongoing longitudinal study funded by the National Heart, Lung, and Blood Institute (2). The full MESA cohort contains 6,814 men and women who were between the ages of 45 and 84 years at baseline. Participants did not have clinical CVD at enrollment and were recruited from 6 field centers in the United States. The initial baseline examination was completed during the period of July 2000 to August 2002. This study was approved by the institutional review boards of all MESA study sites, and all participants gave their informed consent.
Data collection and definitions of baseline CVD risk factors
Data on age, race/ethnicity (white, African-American, Hispanic, or Chinese-American), sex, and smoking status were obtained by self-report. Body mass index was calculated as weight (kg) divided by height (m) squared. Resting seated systolic and diastolic blood pressure measurements were made 3 times using a DINAMAP PRO 100 automated oscillometric sphygmomanometer (Critikon Inc., Tampa, Florida); the average of the last 2 measurements was used. Hypertension was defined as systolic blood pressure of at least 140 mm Hg, diastolic blood pressure of at least 90 mm Hg, or current use of antihypertensive medication. Heart rate (beats/minute) was obtained from the record for the 12-lead electrocardiogram at study baseline. Use of antihypertensive medication and use of cholesterol-lowering medication were assessed using standardized questionnaires and by examining medication containers.
A central laboratory (University of Vermont, Burlington, Vermont) measured concentrations of total cholesterol, high-density lipoprotein cholesterol, triglycerides, plasma glucose, and high-sensitivity C-reactive protein in samples obtained after 12-hour fast. Diabetes was defined as having a fasting plasma glucose greater than or equal to 126 mg/dL (7.0 mmol/L) or a history of receiving medical treatment for diabetes.
Parameters derived from diastolic pulse contour analysis (C1 and C2)
During the baseline examination, C1 and C2 were calculated from the diastolic pulse contour derived from the radial artery blood pressure waveform obtained by applanation tonometry using the HDI/PulseWave CR-2000 (Hypertension Diagnostics, Inc., Eagan, Minnesota). Details on these measurements in MESA have been previously published (3). C1 and C2 are each the product of estimated systemic vascular resistance (SVR) and a function of modified third-order windkessel model parameters estimated from the pulse wave form. SVR is estimated from participant characteristics, including age, mean arterial blood pressure, heart rate, height, and weight. C2 multiplied by SVR (C2 × SVR) and C1 multiplied by SVR (C1 × SVR) are each a function of the pulse waveform only.
In a previous study among 131 MESA participants, coefficients for correlation between 2 measurements performed on the same day by the same technician were 0.74 for C1, 0.84 for C2, 0.58 for C1 × SVR, and 0.74 for C2 × SVR (4).
Aortic distensibility
AD was measured using 1.5-T whole-body MRI systems: Signa CV/i or Signa LX (GE Medical Systems, Milwaukee, Wisconsin). Descriptions of AD measurement in MESA have been previously published (5, 6). AD was calculated by dividing the relative change in the cross-sectional area of the ascending aorta between systole and diastole by the pulse pressure at the brachial artery (7, 8). Blood pressure was measured immediately before and after the MRI aortic measurements, while the patient was in the supine position on the table inside the MRI machine. The average systolic and diastolic values were used to calculate pulse pressure.
AD obtained by MRI in 20 healthy volunteers aged 20 to 70 years in a non-MESA study showed coefficients of variation for intraobserver and interobserver variability of 1% and 2%, respectively, indicating high reproducibility (9).
Carotid distensibility and Young's elastic modulus
CD and YM were measured using B-mode ultrasound at the distal right common carotid artery with a Logiq 700 ultrasonograph (GE Medical Systems, Milwaukee, Wisconsin). This method has been described previously (10). CD was calculated by the relative change in the cross-sectional area of the carotid artery by the pulse pressure at the brachial artery. The brachial blood pressure measurement was made on the right arm using the automated upper-arm sphygmomanometer (DINAMAP PRO 100; Critikon, Inc.) once at the time of the carotid artery ultrasound. YM was calculated by dividing Peterson's elastic modulus by the wall thickness of the aorta. Peterson's elastic modulus was calculated as pulse pressure divided by the relative change in diameter of the carotid artery between systole and diastole.
In a study of 211 MESA participants, the intraobserver class correlation coefficient was 0.71 for CD and 0.69 for YM. Among 10 interobserver correlations of MESA participants, the interobserver class correlation coefficients were 0.85 for CD and 0.84 for YM. These results indicate good agreement within and between observers for CD and YM measurement (10).
Additional information on the acquisition and calculation of arterial function measures can be found in Web Appendix 1 (available at http://aje.oxfordjournals.org/).
Follow-up
The MESA study recorded new symptomatic and adjudicated CVD events during the follow-up period, from July 2000 through December 2011. For CHD events, the median follow-up time was 10.2 years (standard deviation, 2.6 years; interquartile range, 9.6–10.7 years). All CVD outcomes included in the study were obtained through medical record abstraction by trained personnel and adjudicated by clinicians on the MESA data-review committee. Adjudication procedures in MESA have been previously published and can be found in the MESA Protocol at https://www.mesa-nhlbi.org.
The main outcome of interest—CHD events—included myocardial infarction, resuscitated cardiac arrest, definite angina, probable angina (if followed by revascularization), and CHD death. All CVD events and congestive heart failure (CHF) events were examined as secondary outcomes. “All CVD events” included all outcomes in CHD events plus stroke, CHD death, other atherosclerotic death, and other CVD death. CHF included probable and definite CHF. More detail on the classification of events can be found in the MESA Events Manual of Operations at http://www.mesa-nhlbi.org.
Statistical analysis
Cox proportional hazards regression analysis was used to examine the associations between CHD and each measure of arterial function: C2, C2 × SVR, C1, C1 × SVR, AD, CD, and YM. The hazard ratio per standard-deviation increment in value was calculated for each measure of arterial function. The mean values and standard deviations for the different measures are shown in Table 1.
Table 1.
Characteristics of Study Participants According to Arterial Functional Measure Group at the Baseline Examination, Multi-Ethnic Study of Atherosclerosis (MESA), United States, 2000–2002
| Characteristic | C2 a (n = 6,336) | AD (n = 3,677) | CDb (n = 6,531) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No. of Participants | % | Mean (SD) | No. of Participants | % | Mean (SD) | No. of Participants | % | Mean (SD) | |
| Site | |||||||||
| Forsyth County, North Carolina | 1,050 | 16.57 | 623 | 16.94 | 1,051 | 16.09 | |||
| New York, New York | 1,024 | 16.16 | 738 | 20.07 | 1,061 | 16.25 | |||
| Baltimore, Maryland | 867 | 13.68 | 762 | 20.72 | 994 | 15.22 | |||
| St. Paul, Minnesota | 1,000 | 15.78 | 332 | 9.03 | 1,031 | 15.79 | |||
| Chicago, Illinois | 1,111 | 17.53 | 735 | 19.99 | 1,111 | 17.01 | |||
| Los Angeles, California | 1,284 | 20.27 | 487 | 13.24 | 1,283 | 19.64 | |||
| Race/ethnicity | |||||||||
| White | 2,412 | 38.07 | 1,575 | 42.83 | 2,517 | 38.54 | |||
| Chinese-American | 769 | 12.14 | 399 | 10.85 | 783 | 11.99 | |||
| African-American | 1,723 | 27.19 | 1,075 | 29.24 | 1,779 | 27.24 | |||
| Hispanic | 1,432 | 22.60 | 628 | 17.08 | 1,452 | 22.23 | |||
| Cigarette smoking statusc | |||||||||
| Never smoker | 3,215 | 50.85 | 1,877 | 51.24 | 3,287 | 50.48 | |||
| Former smoker | 2,297 | 36.33 | 1,303 | 35.57 | 2,379 | 36.53 | |||
| Current smoker | 810 | 12.81 | 483 | 13.19 | 846 | 12.99 | |||
| Use of antihypertensive medicationc | 2,348 | 37.08 | 1,286 | 35.00 | 2,410 | 36.92 | |||
| Use of cholesterol-lowering medicationc | 1,007 | 15.90 | 562 | 15.30 | 1,047 | 16.04 | |||
| Diabetes mellitusc , d | 802 | 12.70 | 374 | 10.20 | 816 | 12.53 | |||
| Age, years | 62.02 (10.23) | 60.55 (9.97) | 62.18 (10.24) | ||||||
| Weight, pounds | 173.40 (38.13) | 171.17 (35.79) | 172.88 (37.79) | ||||||
| Height, cm | 166.42 (10.03) | 166.90 (9.86) | 166.35 (10.03) | ||||||
| Heart rate, beats/minute | 63.09 (9.62) | 62.96 (9.47) | 63.09 (9.63) | ||||||
| Systolic blood pressure, mm Hg | 126.46 (21.33) | 124.81 (21.11) | 126.55 (21.50) | ||||||
| Diastolic blood pressure, mm Hg | 72.00 (10.25) | 71.96 (10.32) | 71.94 (10.26) | ||||||
| Total cholesterol, mg/dL | 194.15 (35.66) | 194.05 (34.62) | 194.08 (35.77) | ||||||
| High-density lipoprotein cholesterol, mg/dL | 50.83 (14.69) | 51.88 (15.21) | 50.91 (14.75) | ||||||
| Triglycerides, mg/dL | 131.91 (87.03) | 126.95 (83.29) | 131.56 (87.67) | ||||||
| C-reactive protein, mg/L | 3.71 (5.77) | 3.64 (5.81) | 3.74 (5.89) | ||||||
| C2, mL/mm Hg × 100 | 4.5 (2.8) | ||||||||
| C2 × SVR, seconds × 10 | 0.5 (0.3) | ||||||||
| C1, mL/mm Hg × 10 | 13.3 (5.6) | ||||||||
| C1 × SVR, seconds | 1.6 (0.6) | ||||||||
| AD, mm Hg−1 × 10−3 | 1.9 (1.3) | ||||||||
| CD, mm Hg−1 | 2.50 × 10−3 (1.10 × 10−3) | ||||||||
| YM, mm Hg/mm | 1,300.5 (645.8) | ||||||||
Abbreviations: AD, aortic distensibility; CD, carotid distensibility; SD, standard deviation; YM, Young's elastic modulus.
a The population that had C1 (mean = 13.3 (SD, 5.6) mL/mm Hg × 10) measured (n = 6,336) was the same as the population that had C2 measured.
b The population of participants that had YM (mean = 1,300.5 (SD, 645.8) mm Hg/mm) measured (n = 6,348) was almost identical to the population that had CD measured (n = 6,351).
c There were participants with missing data for this variable such that the subgroups do not sum to the totals for the groups that had measurements of C2, AD, and CD. Refer to Web Table 11 for numbers of participants with missing data for different variables.
d Diabetes was defined as having a fasting plasma glucose concentration ≥126 mg/dL (≥7.0 mmol/L) or a history of receiving medical treatment for diabetes.
We calculated hazard ratios to assess whether observed trends in the relationships were consistent with the linearity assumption of the model with the corresponding continuous predictor variable. We compared the first, second, and third quartiles of C2, C1, CD, and AD with the fourth quartile of those measures (most elastic), and we compared the second, third, and fourth quartiles of YM with the first quartile of that measure (most elastic).
The minimal model included adjustment for demographic and anthropometric factors, including age, sex, race/ethnicity, clinical center site, and height. Height was included in the minimal adjustment as a measure of frame size related generally to arterial bore. The proportional hazards assumption was satisfied for all covariates in the minimal model. After examination of the proportional hazards assumption, the full-adjustment model stratified the results by diabetes mellitus status (yes/no), and it adjusted for all the covariates listed in the minimal model plus other traditional CVD risk factors, including mean arterial pressure, use of antihypertensive medication, body mass index, smoking status, total cholesterol level, high-density lipoprotein cholesterol, triglyceride level, use of cholesterol-lowering medication, and high-sensitivity C-reactive protein level. For C2 and C1 only, the full-adjustment model included heart rate as a covariate. Heart rate is a potential confounder for models containing C2 and C1, because heart rate is a component of the C2 and C1 formulas and is associated with increased cardiovascular morbidity (11, 12). We examined mean arterial pressure as a potential modifier of the association between arterial function and CHD events by observing whether the 2-sided P value of the interaction term, containing mean arterial pressure multiplied by the arterial function measure, was less than 0.05.
To assess the ability of each measure of arterial function to discriminate between participants who did and did not have events, we used receiver operating characteristic curves and areas under the receiver operating characteristic curves (AUC). We obtained the predicted risks of CHD from the minimal- and full-adjustment models with and without the arterial function measures. Then we treated CHD as uncensored and binary to estimate and test differences in AUCs from models with and without each arterial function measure. Differences in AUCs from models with and without each arterial function measure were compared using an algorithm suggested by DeLong et al. (13) and implemented using the roccomp command in STATA, version 11.2 (StataCorp LP, College Station, Texas). We also calculated Harrell's C statistic for censored data (14).
The same analysis steps were used for the secondary outcomes: all CVD events and CHF. The full-adjustment model for all CVD events was stratified by using separate baseline hazards for racial/ethnic groups and diabetes mellitus status. The full-adjustment model for CHF was stratified by using separate baseline hazards for tertiles of total cholesterol level. All analyses were performed using STATA 11.2.
RESULTS
Study cohort
Participants in the MESA study chose whether to participate in the radial tonometry, cardiac magnetic resonance imaging, and carotid artery ultrasonography procedures used to obtain the arterial function measures. Thus, 3 overlapping subgroups of MESA participants were examined in this analysis, and their characteristics are shown in Table 1. The groups that underwent carotid ultrasound (n = 6,531) and radial tonometry (n = 6,336) were very similar in terms of total participants and the distributions of covariates. A much smaller group (n = 3,677) underwent aortic MRI than underwent the other procedures. Compared with the other 2 groups, the group that had aortic MRI had a slightly higher proportion of white participants, a slightly lower proportion taking medications for hypertension, a slightly lower proportion of participants with diabetes, a slightly lower mean age, and a slightly lower mean triglyceride concentration. Due to missing data for 1 or more variables, 1.75% of participants with C2 and C1 measured, 1.39% with AD measured, and 1.33% with YM measured were excluded from the analyses.
Each subgroup had different numbers of CHD events: 417 for those with C1 and C2 measured, 220 for those with AD measured, and 435 for those who had CD and YM measured. The numbers of all CVD and CHF events for the 3 subgroups are listed in Web Table 1.
Association between measures of arterial function and incident CHD, all CVD events, and CHF events
In the minimal model, with adjustment for demographic and anthropometric factors only, higher values of C2, C2 × SVR, and C1 were associated with lower predicted risk of CHD (Table 2). After adjustment for demographic, anthropometric, and traditional CVD risk factors in the full-adjustment model, only higher values of C2 and C2 × SVR showed protective associations with CHD events of statistical significance (P < 0.05). The hazard ratio for a CHD event per standard-deviation increment of each arterial function measure was 0.73 (95% confidence interval (CI): 0.63, 0.86) for C2 and 0.78 (95% CI: 0.69, 0.91) for C2 × SVR.
Table 2.
Hazard Ratios for Coronary Heart Disease Events per Standard-Deviation Increment in Measures of Arterial Function, Multi-Ethnic Study of Atherosclerosis (MESA), United States, 2000–2011
| Measure of Arterial Function and Modela , b | No. of Participants | |||
|---|---|---|---|---|
| HR | 95% CI | P Valuec | ||
| C2 | ||||
| Minimal-adjustment model |
6,308 | 0.69 | 0.60, 0.80 | <0.001 |
| Full-adjustment model | 6,205 | 0.73 | 0.63, 0.86 | <0.001 |
| C2 × SVR | ||||
| Minimal-adjustment model | 6,308 | 0.73 | 0.64, 0.83 | <0.001 |
| Full-adjustment model | 6,241 | 0.78 | 0.69, 0.91 | <0.001 |
| C1 | ||||
| Minimal-adjustment model | 6,308 | 0.87 | 0.77, 0.98 | 0.026 |
| Full-adjustment model | 6,205 | 0.97 | 0.86, 1.10 | 0.638 |
| C1 × SVR | ||||
| Minimal-adjustment model | 6,308 | 0.93 | 0.84, 1.04 | 0.196 |
| Full-adjustment model | 6,205 | 0.99 | 0.89, 1.10 | 0.844 |
| AD | ||||
| Minimal-adjustment model | 3,660 | 0.81 | 0.66, 1.01 | 0.064 |
| Full-adjustment model | 3,612 | 0.90 | 0.74, 1.10 | 0.315 |
| CD | ||||
| Minimal-adjustment model | 6,500 | 0.89 | 0.79, 1.01 | 0.074 |
| Full-adjustment model | 6,421 | 0.98 | 0.86, 1.11 | 0.700 |
| YM at carotid artery | ||||
| Minimal-adjustment model | 6,497 | 1.03 | 0.94, 1.12 | 0.585 |
| Full-adjustment model | 6,418 | 0.99 | 0.90, 1.09 | 0.809 |
Abbreviations: AD, aortic distensibility; CD, carotid distensibility; CI, confidence interval; HR, hazard ratio; YM, Young's modulus.
a Minimal-adjustment model: age, sex, clinical center site, height, and race/ethnicity.
b Full-adjustment model: minimal-adjustment model covariates plus heart rate (beats/minute) (C2 and C1 only), mean arterial pressure (mm Hg), use of antihypertensive medication, weight, smoking (never smoker, former smoker, current smoker), total cholesterol level, high-density lipoprotein cholesterol level, triglyceride level, use of cholesterol-lowering medication, and high-sensitivity C-reactive protein level; stratified by diabetes mellitus status.
c P value for statistical significance of the arterial-function measure using Cox regression modeling.
After adjustment for demographic, anthropometric, and traditional CVD risk factors, the hazard ratio for all CVD events per standard-deviation increment of C2 was 0.75 (95% CI: 0.66, 0.86) for C2 (Web Table 2). After adjustment for demographic, anthropometric, and traditional CVD risk factors, the hazard ratio for CHF per standard-deviation increment of arterial function measure was 0.70 (95% CI: 0.56, 0.88) for C2 and 0.77 (95% CI: 0.62, 0.94) for C1 (Web Table 3).
Analysis of CHD, all CVD events, and CHF events over quartiles of the arterial function measures did not suggest a departure from linearity (Web Tables 4–6).
In the full-adjustment model, mean arterial pressure was not found to be a consistent modifier of the association between any of the arterial function measures and CHD, all CVD events, or CHF events. Mean arterial pressure was found to be a modifier only for the association between C1 × SVR and CHD (data not shown).
Change in discrimination for CHD events with addition of arterial function measures
The results based on AUCs for uncensored outcomes and C statistics for censored data were identical to 2 decimal places. Only the AUCs assuming uncensored outcomes are reported.
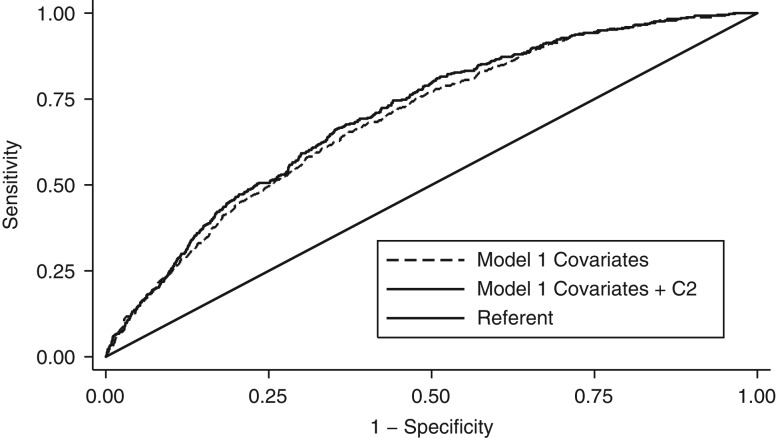
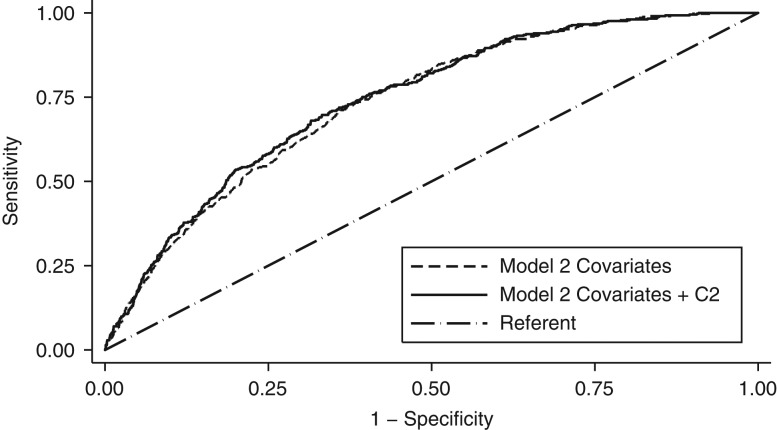
For CHD events, the difference between the AUC with C2 and the AUC with covariates only was statistically significant for both the minimal model (difference = 0.013, P = 0.01) (Figure 1) and full-adjustment model (difference = 0.007, P = 0.04) (Figure 2). Similar results were shown for C2 × SVR. For CHD events, we did not detect a statistically significant difference between the AUC with arterial function measures and the AUC with covariates only in either minimal-adjustment models or full-adjustment models including each of the other arterial function measures. (Web Table 7).
Figure 1.
Receiver operator characteristic curves showing area under the curve for prediction of incident coronary heart disease risk using minimal-adjustment model (model 1) covariates with and without C2 among Multi-Ethnic Study of Atherosclerosis (MESA) participants, United States, 2000–2011. Model 1 was a Cox regression model that included age, sex, clinical center site, height, and race/ethnicity.
Figure 2.
Receiver-operator characteristic curves showing area under the curve for prediction of incident coronary heart disease risk using full-adjustment model (model 2) covariates with and without C2 among Multi-Ethnic Study of Atherosclerosis (MESA) participants, United States, 2000–2011. Model 2 was a Cox regression model that included adjustment for age, sex, clinical center site, height, and race/ethnicity, plus heart rate (beats/minute) (for C2 and C1 only), mean arterial pressure (mm Hg), use of antihypertensive medication, weight, smoking status, total cholesterol level, high-density lipoprotein cholesterol, triglyceride level, use of cholesterol-lowering medication, and high-sensitivity C-reactive protein level. Model 2 allowed for different baseline hazards by diabetes mellitus status (yes/no).
We calculated <6%, 6%–20%, and >20% risks of having a CHD event within 10 years with a model including only established CVD risk factors and also with the new model, in which C2 was added to previous risk factors (Web Table 8). There was a small increase in the percentage of noncases that were classified into the lowest risk category and small increase in the percentage of cases that were classified into the highest risk category.
For all CVD events, the difference between the AUC with C2 and the AUC with covariates only was statistically significant in the full-adjustment model (difference = 0.006, P = 0.015) (Web Appendix 2). For CHF events, the difference between the AUC with an arterial function measure added and the AUC with covariates only was not statistically significant for any of the arterial function measures in the full-adjustment model (Web Appendix 2).
Sensitivity analysis
Fewer MESA participants underwent measurement of AD (by cardiac MRI) than had assessment of C1 and C2 (by radial tonometry) or CD and YM (by carotid ultrasonography). As a result, there was a wider confidence interval around the hazard ratio for AD than for the other 4 arterial function measures. As a sensitivity analysis, we repeated the analysis in a subgroup of 3,297 MESA participants who had data on all 5 measures. Similar results for the relationships for each of C2, AD, CD, and YM with CHD were found in this subgroup as were found in the original analysis (Web Table 9). The hazard ratio for CHD per 1-standard-deviation-higher value of C2 was 0.79 (95% CI: 0.63, 0.98) in the subgroup of 3,297 participants versus 0.73 (95% CI: 0.63, 0.86) among all 6,336 participants, after adjustment for demographic, anthropometric, and traditional CVD risk factors. The difference in AUC in the full-adjustment model with C2 and without C2 was 0.003 (P = 0.533) in the subgroup versus 0.007 (P = 0.04) among all participants (Web Table 10).
DISCUSSION
Using data from participants in MESA—a large, multicenter, multiethnic study of a cohort that was free of overt CVD at baseline—we assessed the predictive capability of 5 measures of arterial function and found that C2 (a measure derived from the blood pressure waveform) was more strongly associated with CHD than C1 (another measure derived from the blood pressure waveform) or other elasticity measures derived from cross-sectional measurement of the artery (AD, CD, and YM). Only C2 predicted the risk of CHD events during follow-up after adjustment for demographic, anthropometric, and other CVD risk factors. We also observed a small, statistically significant increase in the discrimination, as measured by the C statistic, of the risk prediction for CHD with the addition of C2. The other measures (C1, AD, CD, and YM) did not increase the discrimination of the model for CHD risk prediction.
We found that C2, a measure designed to estimate the global arterial function of the more distal part of the arterial circulation, was the most useful measure in our analysis for the prediction of CHD. C2 was also an important independent predictor of additional outcomes found in the category “all CVD events”—CHD plus stroke, other atherosclerotic death, and other CVD death—and of CHF. C2 has previously been called distal compliance, oscillatory compliance, and small artery elasticity. Duprez et al. (3) have suggested that C2 represents different activities that occur to produce oscillations during diastole when the left ventricle is filling with blood.
C1 provides useful information for the prediction of CHD events that is largely attenuated when adjusting for other known CVD risk factors. C1 is designed to estimate the global arterial function of the more proximal part of the arterial circulation. C1 has previously been called proximal compliance, capacitive compliance, and large artery elasticity. C1 was an independent predictor of CHF in this analysis. Our finding suggested that C1 might reflect the development of CHF; this is consistent with the theory that increased stiffness of the large arteries increases left ventricular load, eventually leading to left ventricular dysfunction and finally CHF (15, 16).
Our results suggest that measures of arterial function designed to capture the elastic properties at a local arterial site—such as AD, CD, and YM—may have little predictive value for CHD. AD and CD were designed to quantify the elastic properties of the ascending aorta and carotid artery, respectively. YM was designed to capture the elastic properties of the arterial wall material itself at the carotid artery. However, Redheuil et al. (17) found that AD was predictive of “hard CVD events” among MESA participants with low to intermediate risk of CVD based on the Framingham risk category. “Hard CVD events” included myocardial infarction, resuscitated cardiac arrest, stroke and stroke death, and CHD death.
Some of our results were similar to those of previous epidemiologic studies, which evaluated the arterial function in different study cohorts. In the Rotterdam Study (n = 2,835), Mattase-Raso et al. (18) also showed a lack of independent association between CD and CHD. In the Hoorn Study (n = 579), van Sloten et al. (19) reported an association (not robust to adjustment for CV risk factors) between decreased CD and CVD events that was larger than what we observed (22% increase in CV event per 1-standard-deviation decrease in CD; 95% CI: −5, 56). Hoorn investigators reported that increased Young's modulus at the carotid artery was associated with increased risk of CV events (19). The outcomes assessed are not completely comparable, because “CV events” in the Hoorn Study included cerebrovascular disease and heart failure, whereas our “CHD events” outcome did not. The Hoorn Study participants also included nearly twice the proportion of persons with diabetes are present in MESA (19). It is possible that the predictive value of an arterial function measure is dependent on the risk factor profile of the group being studied, so studies that demonstrate associations can be difficult to generalize to broader populations (20).
Our study had some limitations. Blood pressure was assessed in the brachial artery in the calculation of AD, CD, and YM. This is the typical approach in observational studies, because direct measurement of aortic and carotid artery pressures, although ideal, requires invasive procedures. It has been observed that the brachial measurement can result in differential misclassification of CD among subjects who developed CVD events compared with those who did not, leaving open the possibility that there is bias in these findings (18).
The assessment of multiple arterial function measures raises concern about the possibility of false-positive findings due to multiple comparisons. Because our goal was to examine the relationship between arterial function and CHD events using different measures, we did not use a statistical correction for multiple comparisons. Each measure represents a distinct and different aspect of the arterial system's functional behavior.
Because of the small number of participants who were reclassified into the correct category for both cases and noncases, our observations may not support use of C2 or the other measures in a clinical setting to predict CVD outcomes.
A strength of this study is that it featured a large, multiethnic, community-based population. Another strength of this study is that it was one of the few studies that have looked at the association between multiple, different measures of arterial function and CHD events within a single population that was free of overt CVD at baseline.
Only C2 was predictive of subsequent CHD events after adjustment for demographic, anthropometric, and other traditional CVD risk factors. C1, AD, CD, and YM at the carotid artery were not predictive of subsequent CHD events after full covariate adjustment. There appears to be slight improvement in the risk prediction model for CHD events with the addition of C2 but not for other arterial function measures.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Department of Epidemiology, School of Public Health, University of Washington, Seattle, Washington (Elizabeth K. Hom, Joel D. Kaufman); Department of Environmental and Occupational Health Sciences, School of Public Health, University of Washington, Seattle, Washington (Elizabeth K. Hom, Joel D. Kaufman); Department of Medicine, Medical School, University of Minnesota, Minneapolis, Minnesota (Daniel A. Duprez); Division of Epidemiology and Community Health, School of Public Health, University of Minnesota, Minneapolis, Minnesota (David R. Jacobs, Jr.); Department of Radiology and Imaging Sciences, National Institutes of Health Clinical Center, Bethesda, Maryland (David A. Bluemke); Department of Biostatistics, School of Public Health, University of Washington, Seattle, Washington (Lyndia C. Brumback); Department of Radiology, Tufts Medical Center, School of Medicine, Tufts University, Boston, Massachusetts (Joseph F. Polak); Department of Medicine, School of Medicine, University of California San Francisco, San Francisco, California (Carmen A. Peralta); Department of Medicine, Feinberg School of Medicine, Northwestern University, Chicago, Illinois (Philip Greenland); Department of Preventive Medicine, Feinberg School of Medicine, Northwestern University, Chicago, Illinois (Philip Greenland); Department of Environmental and Radiological Health Sciences, College of Veterinary Medicine and Biomedical Sciences, Colorado State University, Fort Collins, Colorado (Sheryl L. Magzamen); Department of Medicine, School of Medicine, Johns Hopkins University, Baltimore, Maryland (João A. C. Lima); Department of Radiology, School of Medicine, Johns Hopkins University, Baltimore, Maryland (João A. C. Lima); Sorbonne Universités, Université Pierre et Marie Curie, Cardiovascular Imaging, La Pitié Salpêtrière, Paris, France (Alban Redheuil); Laboratoire d'Imagerie Biomédicale, Institut National pour la Santé et la Recherche Médicale (INSERM UMRS) 1146/Unité Mixte de Recherche 7371, Institute of Cardiometabolism and Nutrition, Paris, France (Alban Redheuil); Department of Cardiology, School of Medicine, Wake Forest University, Winston-Salem, North Carolina (David M. Herrington); Department of Medicine, School of Medicine and Public Health, University of Wisconsin, Madison, Wisconsin (James H. Stein); Department of Medicine, School of Medicine, Johns Hopkins University, Baltimore, Maryland (Dhananjay Vaidya, Pamela Ouyang); and Department of Medicine, School of Medicine, University of Washington, Seattle, Washington (Joel D. Kaufman).
MESA was supported by the National Heart, Lung, and Blood Institute (contracts HSN268201500003I, N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168, and R01-HL-098382). This work was also supported in part by the Environmental Protection Agency (award RD831697) and the National Institute of Environmental Health Sciences (grants P50ES015915, T32ES015459, and K24ES013195). This publication was developed under a STAR research assistance agreement awarded by the Environmental Protection Agency (award RD831697). This publication was supported by the National Institute of Environmental Health Sciences (grants P50ES015915, T32ES015459, and K24ES013195).
We thank the MESA investigators and staff.
A full list of investigators can be found at http://www.mesa-nhlbi.org.
This publication has not been formally reviewed by the EPA. The views expressed in this document are solely those of the authors, and the EPA does not endorse any products or commercial services mentioned in this publication.
D.V. is a consultant for Consumable Science, Inc. The other authors report no conflicts.
REFERENCES
- 1.Cohn JN, Duprez DA. Time to foster a rational approach to preventing cardiovascular morbid events. J Am Coll Cardiol. 2008;52(5):327–329. [DOI] [PubMed] [Google Scholar]
- 2.Bild DE, Bluemke DA, Burke GL, et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156(9):871–881. [DOI] [PubMed] [Google Scholar]
- 3.Duprez DA, Jacobs DR, Lutsey PL, et al. Association of small artery elasticity with incident cardiovascular disease in older adults: the Multi-Ethnic Study of Atherosclerosis. Am J Epidemiol. 2011;174(5):528–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brumback LC, Jacobs DR Jr, Dermond N, et al. Reproducibility of arterial elasticity parameters derived from radial artery diastolic pulse contour analysis: the Multi-Ethnic Study of Atherosclerosis. Blood Press Monit. 2010;15(6):312–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheung N, Sharrett AR, Klein R, et al. Aortic distensibility and retinal arteriolar narrowing: the Multi-Ethnic Study of Atherosclerosis. Hypertension. 2007;50(4):617–622. [DOI] [PubMed] [Google Scholar]
- 6.Stacey RB, Bertoni AG, Eng J, et al. Modification of the effect of glycemic status on aortic distensibility by age in the Multi-Ethnic Study of Atherosclerosis. Hypertension. 2010;55(1):26–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O'Rourke MF, Staessen JA, Vlachopoulos C, et al. Clinical applications of arterial stiffness; definitions and reference values. Am J Hypertens. 2002;15(5):426–444. [DOI] [PubMed] [Google Scholar]
- 8.Malayeri AA, Natori S, Bahrami H, et al. Relation of aortic wall thickness and distensibility to cardiovascular risk factors (from the Multi-Ethnic Study of Atherosclerosis [MESA]). Am J Cardiol. 2008;102(4):491–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nelson AJ, Worthley SG, Cameron JD, et al. Cardiovascular magnetic resonance-derived aortic distensibility: validation and observed regional differences in the elderly. J Hypertens. 2009;27(3):535–542. [DOI] [PubMed] [Google Scholar]
- 10.Blaha MJ, Budoff MJ, Rivera JJ, et al. Relationship of carotid distensibility and thoracic aorta calcification: Multi-Ethnic Study of Atherosclerosis. Hypertension. 2009;54(6):1408–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Finkelstein SM, Cohn JN. First- and third-order models for determining arterial compliance. J Hypertens Suppl. 1992;10(6):S11–S14. [PubMed] [Google Scholar]
- 12.Perret-Guillaume C, Joly L, Benetos A. Heart rate as a risk factor for cardiovascular disease. Prog Cardiovasc Dis. 2009;52(1):6–10. [DOI] [PubMed] [Google Scholar]
- 13.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837–845. [PubMed] [Google Scholar]
- 14.Harrell FE Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15(4):361–387. [DOI] [PubMed] [Google Scholar]
- 15.Gosse P, Jullien V, Jarnier P, et al. Reduction in arterial distensibility in hypertensive patients as evaluated by ambulatory measurement of the QKD interval is correlated with concentric remodeling of the left ventricle. Am J Hypertens. 1999;12(12):1252–1255. [DOI] [PubMed] [Google Scholar]
- 16.Roman MJ, Devereux RB. The relation of large arterial structure and function to cardiac hypertrophy in hypertension In: Safar M, O'Rourke M, eds. Arterial Stiffness in Hypertension. Vol. 23 Amsterdam, the Netherlands: Elsevier; 2006:295–308. [Google Scholar]
- 17.Redheuil A, Wu CO, Kachenoura N, et al. Proximal aortic distensibility is an independent predictor of all-cause mortality and incident CV events: the MESA study. J Am Coll Cardiol. 2014;64(24):2619–2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mattace-Raso FU, van der Cammen TJ, Hofman A, et al. Arterial stiffness and risk of coronary heart disease and stroke: the Rotterdam Study. Circulation. 2006;113(5):657–663. [DOI] [PubMed] [Google Scholar]
- 19.van Sloten TT, Schram MT, van den Hurk K, et al. Local stiffness of the carotid and femoral artery is associated with incident cardiovascular events and all-cause mortality: the Hoorn study. J Am Coll Cardiol. 2014;63(17):1739–1747. [DOI] [PubMed] [Google Scholar]
- 20.Henry RMA, Kostense PJ, Spijkerman AM, et al. Arterial stiffness increases with deteriorating glucose tolerance status: the Hoorn study. Circulation. 2003;107(16):2089–2095. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.