Abstract
The clustering of human papillomavirus (HPV) infections in some individuals is often interpreted as the result of common risk factors rather than biological interactions between different types of HPV. The intraindividual correlation between times-at-risk for all HPV infections is not generally considered in the analysis of epidemiologic studies. We used a deterministic transmission model to simulate cross-sectional and prospective epidemiologic studies measuring associations between 2 HPV types. When we assumed no interactions, the model predicted that studies would estimate odds ratios and incidence rate ratios greater than 1 between HPV types even after complete adjustment for sexual behavior. We demonstrated that this residual association is due to correlation between the times-at-risk for different HPV types, where individuals become concurrently at risk for all of their partners’ HPV types when they enter a partnership and are not at risk when they are single. This correlation can be controlled in prospective studies by restricting analyses to susceptible individuals with an infected sexual partner. The bias in the measured associations was largest in low-sexual-activity populations, cross-sectional studies, and studies which evaluated infection with a first HPV type as the exposure. These results suggest that current epidemiologic evidence does not preclude the existence of competitive biological interactions between HPV types.
Keywords: bias (epidemiology), coinfection, cross-protection, microbial interactions, papillomavirus infections, sexually transmitted diseases, time factors, viral interference
Persons with a sexually transmitted infection (STI) generally have a higher prevalence and incidence rate of infection with other STIs (1–12). These observations have sparked inquiry into the causal effects a first STI might have on the probability that a second STI can establish an active infection upon contact with an infected sexual partner. This causal effect on transmission would entail a biological interaction between the STIs. It is important to ascertain the existence of biological interactions from a public health perspective, because interventions against one STI could also affect the other STIs with which it interacts (13–15). If previous infection with a first STI increases the risk of becoming infected with a new STI (facilitative interaction), then interventions against the first STI could also reduce the incidence of other STIs. For example, if STIs increase the probability of becoming infected with human immunodeficiency virus (HIV), then STI control and management could help control HIV incidence (16). If previous infection with a first STI reduces the risk of becoming infected with other STIs (competitive interaction), then interventions targeted against the first STI could increase the incidence of infections with other STIs due to the reduced competition. For example, human papillomavirus (HPV) vaccination might increase the incidence of infection with nonvaccine HPV types through type replacement (15).
In practice, measures of association between STIs are biased estimates of their causal effects on each other's transmission. It is largely recognized that because both the exposure (the first STI) and the outcome (the second STI) are sexually acquired, the effect estimate will be confounded by common risk factors (17–19). Notably, sexual behaviors and networks which increase the risk of being infected with one STI also increase the risk of being infected with a second STI, so their incidence is expected to be associated.
Furthermore, STIs’ sexual mode of transmission leads to an additional bias caused by the correlation between the times-at-risk for infection with different STIs. To our knowledge, this issue has not been considered when analyzing STI associations. When individuals create and break off sexual partnerships, their at-risk status changes concurrently over time for all STIs. An individual's incidences of multiple STIs will be correlated over time because 1) when an individual has no partners or has uninfected partners, he/she simultaneously is not at risk for any STI and has a null risk of incident infection with any STI and 2) when an individual has partners who are infected with multiple STIs, he/she is simultaneously at risk for all of the partners’ STIs during their partnership. This issue is separate from sexual risk confounding, because adjustments for sexual behavior markers (e.g., recent/lifetime numbers of sexual partners (8, 11, 12)) control for interindividual differences in infection risk that are assumed to remain constant over time. These markers do not control for the intraindividual correlation between the times an individual is at risk for different STIs due to partnership creation. Eliminating the correlation requires restricting analyses to times when individuals are at risk of being infected with the outcome STI due to sexual contacts with infected partners. However, this restriction is not often feasible in studies of STIs.
Mathematical transmission-dynamic models can help us analyze results of epidemiologic studies by explicitly incorporating the dependence of outcomes inherent to STI dynamics. They are thus valuable complements to traditional statistical analyses, which are often not adapted for dependent outcomes (20).
In this paper, we use HPV as an example with which to examine this bias. Antibodies developed against one type of HPV cross-react with related HPV types (21, 22), so infection with a first HPV type might plausibly decrease the subsequent risk of new HPV infections (competitive interaction). However, various cross-sectional and prospective studies have shown that HPV types cluster together (23–28) and that persons currently infected with one type have a higher risk of incident infection with another type, even after adjustment for sexual behaviors (9–12, 26–28). The remaining association is generally interpreted as residual confounding by sexual activity or immune responses, and the existence of competitive interactions and type replacement following HPV vaccination are generally deemed unlikely (26–29). However, none of these analyses have considered the correlation between the times-at-risk for all HPV types. Using a transmission-dynamic model of 2 HPV types, we aim to illustrate how the correlation between the times-at-risk for infection biases the estimation of causal effects between HPV types in cross-sectional and prospective studies even when we assume that they do not interact. We identify which epidemiologic research designs minimize or eliminate this bias to estimate causal effects. We then generalize results to the situations of 1) STIs with different natural histories, 2) STIs in populations with different sexual behaviors, and 3) the assumption that competitive and facilitative interactions do exist.
METHODS
We built a mathematical model to simulate the transmission of 2 generic HPV types in an illustrative population. In this population, we simulated epidemiologic studies which measure the association between 2 HPV types. If the measured association between HPV types in these studies did not match the underlying modeled effect of the first HPV type on the second, we considered the association to be a biased estimate of the biological interaction. Further details on model structure, parameters, and equations can be found in the Web Appendix (available at http://aje.oxfordjournals.org/), including Web Figures 1 and 2 and Web Table 1.
Model structure
The modeled population is heterosexual, open, and stable. Age is not modeled. Individuals spend on average 40 years in the modeled population, representing their most sexually active years (approximately ages 15–54 years). Individuals are stratified into high and low sexual activity levels, which differ according to their rate of sexual partner acquisition. An assortativity parameter (ε) represents the proportion of sexual partnerships individuals make exclusively with members of their same sexual activity level, while the rest of the partnerships (1 − ε) are made proportionately between levels. We model the duration of sexual partnerships between individuals and the rate of sex acts within partnerships.
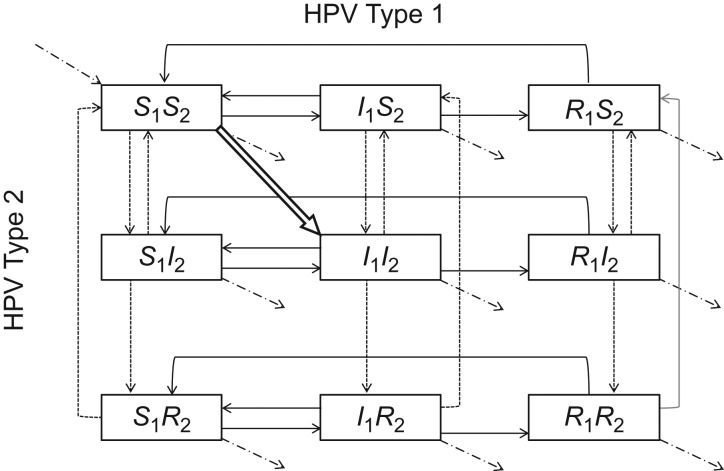
HPV type (1 or 2) is marked by the subscript i. Individuals in the model may be in one of 9 mutually exclusive health states representing the 9 possible combinations of susceptible (Si), infected (Ii), or recovered/immune (Ri) states with both HPV types (Figure 1). We assumed that 1) infections with each HPV type are cleared independently (9, 12); 2) individuals only acquire immunity once they clear an infection; 3) immunity is type-specific and wanes over time; 4) susceptible individuals can only be infected when they are in a sexual partnership with an infected partner; and 5) transmission probabilities of both HPV types are independent per sex act. Individuals susceptible to both HPV types ([S1S2]) who have a coinfected sexual partner ([I1I2]) have a joint probability of being infected with both HPV types (δ1 × δ2), only type 1 (δ1 × (1 − δ2)), or only type 2 ((1 − δ1) × δ2) per sex act. These independence assumptions were made so that in the base case, HPV types do not affect each other's natural history (no interactions). However, the time periods during which individuals are at risk of getting HPV types 1 and 2 are correlated in the model, because they are not at risk for either type unless they are in a partnership with an infected person.
Figure 1.
Different combinations of susceptible (S), infected (I), and recovered/immune (R) statuses for 2 types of human papillomavirus (HPV), designated type 1 and type 2 (indicated by a subscript), with the first letter corresponding to HPV type 1 health status and the second corresponding to HPV type 2 health status. The boxes show mutually exclusive health states, with possible transitions indicated by arrows. Boxes in the left-hand column represent HPV type 1-susceptible individuals; boxes in the middle column represent HPV type 1-infected individuals; and boxes in the right-hand column represent HPV type 1-immune individuals. Boxes in the first row represent HPV type 2-susceptible individuals; boxes in the second row represent HPV type 2-infected individuals; and boxes in the third row represent HPV type 2-immune individuals. Dashed-dotted arrows correspond to population entry and exit points, solid black arrows to HPV type 1 natural history transitions, dashed arrows to HPV type 2 natural history transitions, and the white outlined arrow to natural history transitions affecting both types of HPV. See the Web Appendix for differential equations and parameters for transition rates between health states.
In sensitivity analyses, we allowed HPV types to interact by modifying each other's transmission probability. Individuals currently infected with a first HPV type have their per-sex-act probability of infection with the second HPV type i multiplied by a factor χi. This factor represents the effect of interaction mechanisms hypothetically triggered during the active infection with the first HPV type (e.g., competition for host resources, disrupted epithelial tissues). Individuals immune to a first HPV type have their per-sex-act probability of infection with the second HPV type i multiplied by a factor φi. This factor represents the effect of interaction mechanisms hypothetically caused by long-term immunity to the first HPV type (e.g., cross-immunity). Values of <1, >1, and 1 for these factors correspond to competitive interactions, facilitative interactions, and no interactions, respectively.
Parameterization
For our base-case scenario, which used HPV as an example, we selected model parameters to illustrate HPV epidemiology (Table 1). Parameter values were based on empirical observations (30–35) and on model-based estimates of transmission probabilities (36). Importantly, we assumed no interactions between HPV types (χi = φi = 1). For illustrative purposes, we made the simplifying assumptions that both HPV types had identical natural history parameters and that mixing between sexual activity levels was proportionate (ε = 0). However, because there is high uncertainty surrounding mixing patterns and mixing is probably assortative by sexual behavior (37), we varied this parameter in sensitivity analyses. We defined the low sexual activity level as 2 or fewer sexual partners in the past year (30). We manually set the high sexual activity level's rate of sexual partner change to achieve a type-specific HPV prevalence of 6% (prevalence of HPV-16 generally ranges between 3% and 11% (38–40)).
Table 1.
Base-Case Parameter Values and Sensitivity Analysis Ranges Used in a Model of Partnership Formation, Times-at-Risk for Human Papillomavirus Infection, and Their Interactions
| Parameter | Value | Source of Derivation | Range of Sensitivity Analysis |
|---|---|---|---|
| Proportion of individuals in high sexual activity level | 0.07 | Chandra, 2011 (30)a | |
| Rate of partner change for low sexual activity level (per year) | 0.80 | Chandra, 2011 (30)b | 0.25–2.50 |
| Rate of partner change for high sexual activity level (per year) | 5.00 | —c | |
| Proportion of partnerships made exclusively with persons at the same sexual activity level (degree of assortativity ε) | 0.00 | (Proportionate mixing) | 0.00–1.00 |
| Rate of sex acts in partnerships (per year) | 75.40 | Mercer, 2013 (34) | |
| Proportion of time spent single | 0.25 | Demers, 2012 (35)d | |
| Duration of infection, years | 1.40 | Insinga, 2010 (31)e | 0.25–25.00 |
| Proportion of infections conferring natural immunity | 0.60 | Carter, 2000 (32) | |
| Duration of natural immunity, years | 10.00 | Wang, 2004 (33)f | |
| Probability of infection transmission per sex act with an infected person | 0.13 | Bogaards, 2010 (36)e ,g | 0.001–1.00 |
| Interaction effects (χi and φi) | 1.00 | (No interactions) | 0.05–7.76 |
Abbreviation: HPV, human papillomavirus.
a Based on having had more than 2 sexual partners in the last year.
b Average number among persons with 2 or fewer sexual partners in the last year.
c Manually set to achieve a 6% type-specific prevalence of HPV.
d Based on the proportion of individuals reporting being in a stable partnership.
e Averaged over HPV types.
f We assumed an exponential loss of immunity and found the rate of waning that led to 45% of individuals losing their seropositivity after 6.4 years.
g Calculated so that the average per-infected-partnership probability of infection was 0.79.
Epidemiologic studies of interactions between HPV types
We simulated 3 types of cross-sectional and prospective epidemiologic studies of HPV infection in the model population. In each study, we measured the association between HPV types 1 and 2. We considered the difference between the measured association and the modeled interaction effects (χi, φi) to be a bias in the estimation of the causal effect. The 3 types of studies differed in their definitions of exposure and outcome:
Prospective studies in which the exposure is defined as being seropositive for HPV type 1 and the outcome is incident infection with HPV type 2 (41–44). We calculated the IRR for HPV type 2 infection by HPV type 1 immunity status (equation 3).
| (3) |
We assumed that prospective analyses are performed in individuals susceptible to HPV type 2 infections.
These crude associations calculated in the overall population are expected to be biased estimates of the interaction effects χ2 and φ2, due to sexual risk confounding and to the correlation between the times-at-risk of infection. To remove these biases, we performed the following analyses. First, we adjusted associations for sexual activity level using Mantel-Haenszel estimators. Because sexual activity level determines all sexual behaviors, adjusted associations have no residual confounding due to sexual risk factors. Second, we restricted analyses to only individuals in sexual partnerships. This partially controls for the correlation by removing immortal person-time from single individuals not at risk for any HPV infection. Third, we restricted analyses to only those individuals who had HPV type 2-infected partners (at-risk partnered individuals). This restriction should completely remove the correlation between the times-at-risk for infection with both HPV types, as it conditions on all individuals being at risk.
Sensitivity analyses
Natural history and sexual behavior
We varied natural history and sexual behavior parameters in sensitivity analyses. Infection prevalences were allowed to vary in response to parameter changes.
Interactions
We varied the interaction parameters χi and φi to evaluate whether the different studies could correctly estimate the effects of hypothetical interactions. We varied interaction parameters separately (φi = 1 when χi is varied and vice versa). HPV prevalences were allowed to vary in response to interactions.
RESULTS
Base-case scenario assuming no interactions between HPV types
When we assumed no interactions between HPV types (χi = φi = 1), the difference between the measured POR/IRRs and 1 constituted the bias in the estimation of the interaction effect.
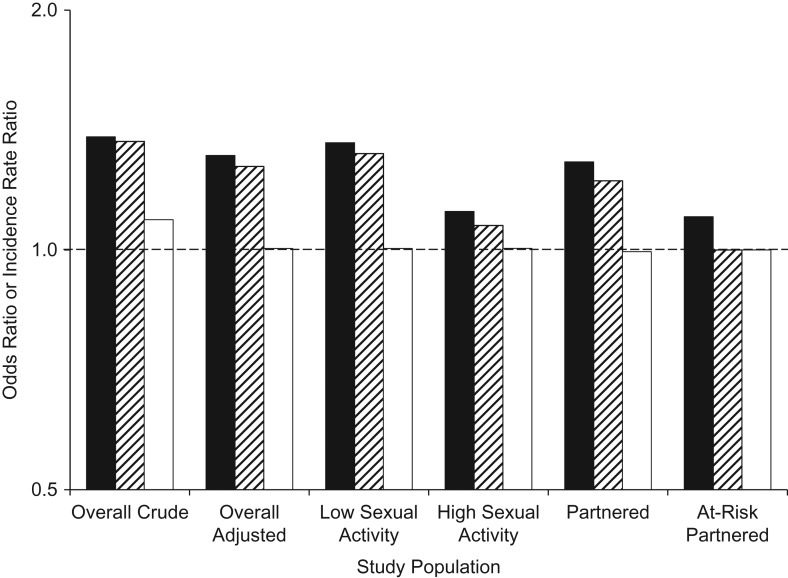
The crude POR and IRRs measured in the overall population were 1.39, 1.37, and 1.09, respectively, in the absence of any interactions (Figure 2, Web Table 2). Adjustment for sexual activity level almost completely removed the bias for the IRR by immunity status (IRR = 1.00) but only slightly reduced the bias for the POR and the IRR by infection status. Residual bias in the POR and IRR by infection status was higher in the low sexual activity level (POR = 1.36, IRR = 1.32) than in the high sexual activity level (POR = 1.12, IRR = 1.07). Restricting the analysis to only individuals with sexual partners did not substantially reduce these associations. When the analysis was restricted to individuals at risk (those in sexual partnerships with HPV type 2-infected partners), both prospective IRRs estimated no association between HPV types 1 and 2 (IRRs = 1.00), but the POR was still greater than 1. This restriction thus eliminates the prospective correlation between the times-at-risk for HPV infections, but it does not control for correlation between the times-at-risk from past sexual partnerships, which affects cross-sectional measures of association.
Figure 2.
Measures of association between 2 types of human papillomavirus (HPV), designated type 1 and type 2, in the absence of interactions. The prevalence odds ratio (POR) (black columns) compares odds of a prevalent HPV type 2 infection in HPV type 1-infected versus -uninfected individuals. The incidence rate ratio (IRR) by infection status (striped columns) compares the HPV type 2 incidence rate in HPV type 1-infected versus -uninfected individuals. The IRR by immunity status (white columns) compares the HPV type 2 incidence rate in HPV type 1-immune versus -nonimmune individuals. The POR and IRRs are calculated in the full population including both single and partnered individuals (“Overall Crude”), in the full population with adjustment for sexual activity level (“Overall Adjusted”), in persons of each sexual activity level (“Low Sexual Activity” and “High Sexual Activity”), only among persons in current sexual partnerships (“Partnered”), and only among persons in current sexual partnerships with HPV type 2-infected partners (“At-Risk Partnered”). All associations (except for the overall crude association) were adjusted for sexual activity level. The y-axis is on a base-2 log scale.
Sensitivity analyses assuming no interactions between HPV types
Natural history
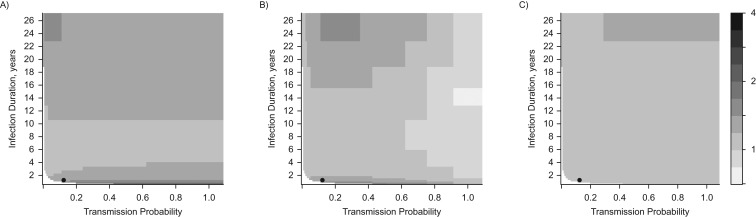
Adjusted measures of association in the overall population were greater than or equal to 1 in the absence of any interactions under most biologically plausible parameter values for infection duration and transmission probability. The bias in the POR was larger when we assumed fairly short (<4 years) or long (>10 years) average durations of infection (Figure 3A). The adjusted IRR by infection status was also greater than or equal to 1 under most parameter values for duration and transmission, but it could be less than 1 when high transmission probabilities (>65%) were combined with infection durations longer than 1.4 years (Figure 3B). This is because at high transmission probabilities HPV types are almost always transmitted during the same sex act from coinfected partners, diminishing the positive prospective correlation. The adjusted IRR by immunity status was very close to 1 under most parameter values and only became noticeably larger than 1 when infection durations were very long (>20 years) (Figure 3C).
Figure 3.
Adjusted measures of association between 2 types of human papillomavirus (HPV), designated type 1 and type 2, under different combinations of infection duration (in years) and transmission probabilities (per sex act with an infected individual). No interactions between HPV types are assumed. A) Prevalence odds ratio (POR); compares HPV type 2 infection prevalence odds in HPV type 1-infected versus -uninfected individuals. B) Incidence rate ratio (IRR) by infection status; compares the HPV type 2 incidence rate in HPV type 1-infected versus -uninfected individuals. C) IRR by immunity status; compares the HPV type 2 incidence rate in HPV type 1-immune versus -nonimmune individuals. All PORs and IRRs are measured in the overall population and adjusted for sexual activity level using Mantel-Haenszel estimators. White areas represent combinations of transmission probabilities and infection durations for which sustained transmission becomes impossible and the infections are eliminated from the model population. The black circle represents the base-case scenario.
Sexual behavior
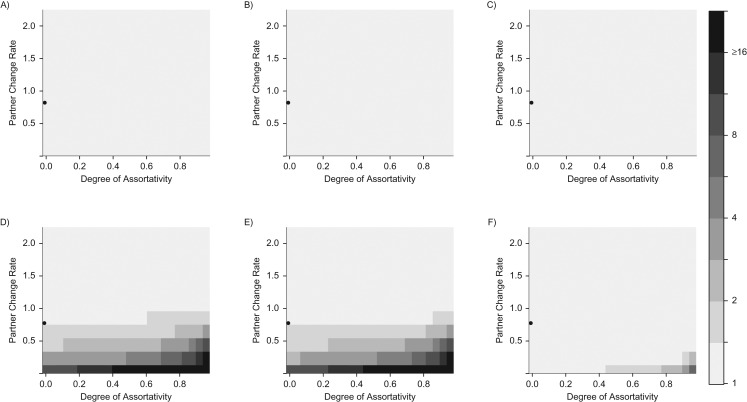
The bias in the POR and IRR by infection status in the low sexual activity level was extremely sensitive to sexual behavior (Figure 4). The bias in the POR and IRR by infection status was larger in the low sexual activity level (Figure 4D–4F) than in the high sexual activity level (Figure 4A–4C), and this difference substantially increased when the sexual mixing became more assortative within sexual activity levels (ε approaches 1) and when the low sexual activity level had lower rates of partner change (<1.0 partner change/year). This is because the decreasing infection prevalence and higher assortativity among low-sexual-activity individuals leads to more of their partners being co-uninfected, which substantially increases the correlation between their times-at-risk of infection with both HPV types. The IRR by immunity status was very close to 1 for both sexual activity levels under most sexual behavior parameter values, and the bias only appreciably increased in the low sexual activity level when there was a very high within-level assortativity combined with a very low rate of partner change (<0.5/year).
Figure 4.
Sexual-activity-level–stratified measures of association between human papillomavirus (HPV) types 1 and 2 under different combinations of the partner change rate (per year) in the low sexual activity level and the degree of assortativity between sexual activity levels. A) Prevalence odds ratio (POR) in the high sexual activity level. B) Incidence rate ratio (IRR) by infection status in the high sexual activity level. C) IRR by immunity status in the high sexual activity level. D) POR in the low sexual activity level. E) IRR by infection status in the low sexual activity level. F) IRR by immunity status in the low sexual activity level. No interactions between HPV types are assumed. Black areas indicate values greater than or equal to 16 for the measured associations. The degree of assortativity corresponds to the proportion of sexual contacts made exclusively with members of the same sexual activity level (0 = proportionate, 1 = completely assortative). The black circle represents the base-case scenario.
Sensitivity analyses assuming interactions between HPV types
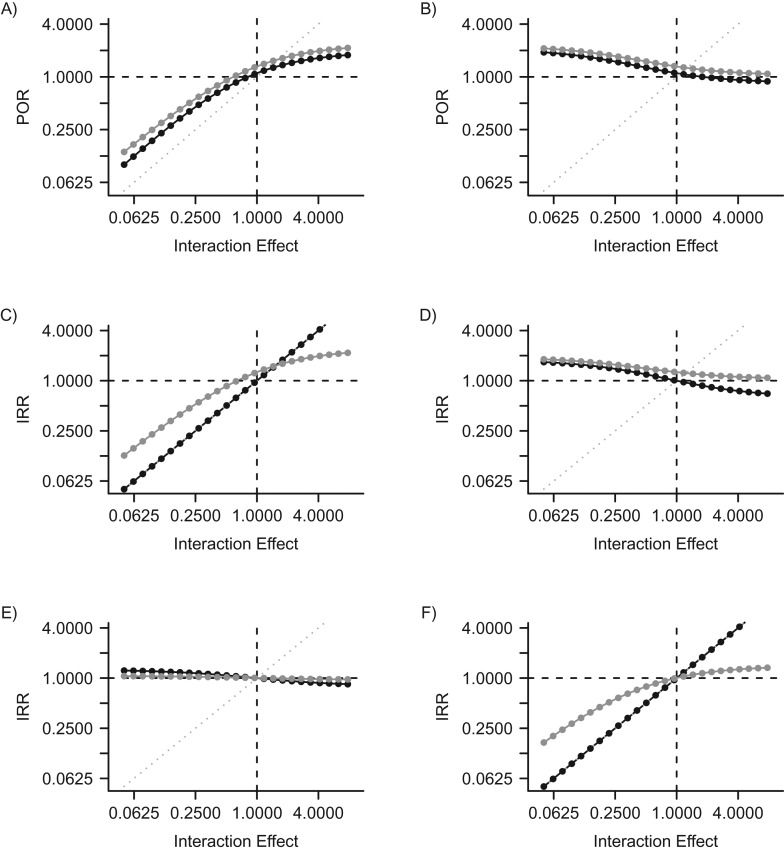
When we assumed interactions between HPV types, the difference between the measured POR/IRRs and the true modeled interaction effects χ2 or φ2 constituted the bias (Figure 5).
Figure 5.
Estimated adjusted measures of association between 2 types of human papillomavirus (HPV), type 1 and type 2, when interactions are modeled. The y-axis corresponds to associations measured in simulated epidemiologic studies. The x-axis represents the actual modeled underlying interactions. A) Prevalence odds ratio (POR) for HPV type 2 infection in HPV type 1-infected versus -uninfected individuals according to interaction effects caused by current infection (χ2). B) POR for HPV type 2 infection in HPV type 1-infected versus -uninfected individuals according to interaction effects caused by immunity (φ2). C) Incidence rate ratio (IRR) for HPV type 2 infection in HPV type 1-infected versus -uninfected individuals according to interaction effects caused by current infection (χ2). D) IRR for HPV type 2 infection in HPV type 1-infected versus -uninfected individuals according to interaction effects caused by immunity (φ2). E) IRR for HPV type 2 infection in HPV type 1-immune versus -nonimmune individuals according to interaction effects caused by current infection (χ2). F) IRR for HPV type 2 infection in HPV type 1-immune versus -nonimmune individuals according to interaction effects caused by immunity (φ2). Gray points correspond to associations measured in the overall population, adjusted for sexual activity level. Black points correspond to associations measured in analyses restricted to at-risk partnered individuals (persons in partnerships with HPV type 2-infected partners). Left-hand panels (A, C, and E) show the influence of interaction effect χ2—the relative probability of HPV type 2 infection per sex act with an infected partner among persons who are HPV type 1-infected compared with persons who are HPV type 1-susceptible. Right-hand panels (B, D, and F) show the influence of interaction effect φ2—the relative probability of HPV type 2 infection per sex act with an infected partner among persons who are HPV type 1-immune compared with persons who are HPV type 1-susceptible. Values to the left of 1.0000 on the x-axis correspond to modeled competitive interactions, while values to the right of 1.0000 correspond to modeled facilitative interactions. The dotted gray line represents the theoretical line of equality, where epidemiologic measures of association would validly estimate the actual modeled interactions. Both axes use a base-2 log scale.
If we assumed that current infection with HPV type 1 strongly increased the probability of being infected with HPV type 2, the adjusted POR and IRR by infection status in the overall population correctly measured the direction of the interaction (>1) but could underestimate the facilitative effect (Figure 5A and 5C; interaction effect χ2 > 1). This underestimation occurs because, as facilitative interactions become stronger, the duration of partnerships and the time spent single become limiting factors in acquiring new HPV types, reversing the direction of the bias. When we assumed that current infection with HPV type 1 reduced the probability of being infected with HPV type 2 (Figure 5A and 5C; interaction effect χ2 < 1), the POR and IRR by infection status estimated a less protective association than the actual modeled effect. The IRR by immunity status was not a valid estimator of interaction effects caused by current infection (Figure 5E).
If immunity to HPV type 1 reduced the probability of being infected with HPV type 2 (Figure 5B and 5D; interaction effect φ2 < 1), the POR and the IRR by infection status in the overall population estimated a strongly positive association of more than 1 even though current infection was assumed to have no effect (χ2 = 1). Persons immune to HPV type 1 [R1S2] are in the unexposed category for these ratios and contribute cases and person-time to the denominator (equation 2). If there is cross-immunity, they will thus contribute to a lower-than-expected rate of HPV type 2 infection in HPV type 1-uninfected individuals. The IRR by immunity status in the overall population correctly estimated the direction of the modeled interaction effect φ2 but underestimated its magnitude (Figure 5F, gray dots).
When we restricted analyses to at-risk individuals in sexual partnerships with HPV type 2-infected partners, the IRR by infection status correctly estimated the modeled interaction effect χ2 caused by infection (Figure 5C, black dots), and the IRR by immunity status correctly estimated the modeled interaction effect φ2 caused by immunity (Figure 5F, black dots).
DISCUSSION
In this study, we found that the correlation between the times-at-risk for HPV infections can substantially bias the estimation of interactions between HPV types in cross-sectional and prospective studies. Relative risk measures in most cases estimated associations between HPV types greater than 1 in the absence of any interaction. This correlation persisted after perfect adjustment for sexual activity. The infection IRR by immunity status was much less affected by this correlation than the POR and IRR by infection status, probably because immunity markers are correlated with past infection risk and are less strongly correlated with current risk for new HPV infections. Interactions between HPV types could not be validly estimated in our cross-sectional analyses. When analyses were restricted to time periods in which individuals were at risk for the outcome HPV type infection due to sexual contact with an infected partner, then the IRR by infection status validly estimated interaction effects caused by current infection with a first HPV type, and the prospective IRR by immunity status validly estimated interaction effects caused by immunity to a first HPV type. When we assumed that immunity with a first HPV type reduced the risk of being infected with a second HPV type per infected contact (competitive interaction), the POR and IRR by infection status measured a strong association (>1) between HPV type incidences.
The observed clustering of HPVs in cross-sectional studies is often interpreted by epidemiologists as evidence against competitive cross-immune interactions that could lead to type replacement. However, our results indicate that cross-immunity could contribute to the clustering of HPV types because cross-immune individuals are protected against both types and would be included in the “co-uninfected” category in cross-sectional and prospective studies. Prospective epidemiologic studies which use HPV seropositivity rather than infection as the exposure are likely to provide more valid estimates of antibody-mediated cross-protective interactions between HPV types. In marked contrast with studies using other designs, prospective studies using seropositivity as the exposure generally find that persons who are seropositive for a first HPV type have no significantly increased risk of new infections with related HPV types or have a decreased risk of such infections (41, 44–46). Therefore, the possibility of competitive cross-immune interactions between HPV types cannot be dismissed, as they could be masked or underestimated in these studies due to residual confounding and the correlation between times-at-risk for infection.
To our knowledge, this study is the first to identify and illustrate the correlation between times-at-risk of infection as a source of bias in epidemiologic data and to suggest study designs which reduce the bias. Researchers in previous studies had also found that PORs greater than 1 can be measured when assuming no interactions between HPV types and that competitive HPV interactions could reproduce epidemiologic data (47, 48), but they had not explored the source of this bias or included sexual risk heterogeneity. Our results suggest that integrating sexual risk heterogeneity and mixing based on empirical data would be necessary for transmission models to validly estimate the interactions between HPV types from epidemiologic data.
Our sensitivity analyses suggest that the correlation between the times-at-risk for infection can be generalized to other STIs, such as HIV or herpes simplex virus, which have low transmission probabilities but long durations of infection (49, 50). Ulcerative STIs could plausibly increase HIV acquisition by disrupting the host's mucosal integrity and increasing the availability of HIV-susceptible cells (6); however, the magnitude of this interaction may not have been correctly estimated in epidemiologic studies due to bias (17). An overestimation of the effect of STIs on HIV acquisition might partly explain why clinical trials of interventions against STIs have shown disappointing effectiveness against HIV incidence (51).
Our study shows that the correlation between the times-at-risk for infection in prospective studies can be controlled by restricting analyses to times at which individuals are at risk for the outcome STI due to sexual contacts with an infected partner. However, this restriction is very challenging, as it requires detailed information on individuals’ sexual partners. This control could be achieved with a discordant couples’ study (52), but this design is often unfeasible due to the large sample sizes required for adequate statistical power and the ethical need to provide treatment for nonviral STIs. Ascertainment of the real-time STI status of individuals is also challenging because of test measurement errors and the need to frequently retest people (18).
Interestingly, we found that associations between HPV types were consistently larger in persons with lower rates of partner change. Persons with low rates of partner change have lower rates of infection, but their infections tend to be more strongly correlated over time because they spend more time not at risk between partners and stay longer in partnerships. This heterogeneity by sexual activity has been observed empirically, with individuals or populations with lower levels of sexual activity showing higher associations between STIs (1–3, 9, 23, 27).
Our study presents some limitations. Firstly, we used perfect measures of infection incidence to isolate the bias due to correlation between times-at-risk of infection with different HPV types. However, there will be information/confounding biases in empirical data which should be considered (17–19, 53). Secondly, we made the simplifying assumption that immunity is acquired after an individual clears infection. However, people may seroconvert while they are still infected with a type of HPV (32). We could not evaluate the influence of simultaneously infected/seropositive individuals in studies. This classification issue did not affect our main results with no interactions because infected and immune individuals have the same rates of infection with new types, but it would affect interaction sensitivity analyses. However, because the temporal overlap is short relative to the duration of seropositivity (only 3%–10% of seropositives are infected) (54), this should not have substantially affected our results. Thirdly, in order to eliminate the influence of stochastic error and truly isolate the systematic bias due to correlation between the times-at-risk, we chose to use a simple deterministic model. However, the magnitude of the bias was extremely sensitive to sexual partner change rates and mixing patterns. Base-case results are therefore not quantitative predictions of the empirical magnitude of bias in epidemiologic studies. To confirm that our results were not a result of our simple model structure, we calculated the POR for 2 HPV types using an individual-based stochastic model of HPV transmission, calibrated to the Canadian population (55), which had 4 levels of sexual activity, assortative mixing by age and sexual activity, and no interactions between HPV types (Web Figure 3). Results were qualitatively similar between models: Estimated PORs were substantially superior to 1 without interactions after adjustment for sexual activity, and they were highest in lower sexual activity levels and at ages with lower partner change rates. The PORs were much larger, however, in a stochastic model with more sexual risk heterogeneity. Our base-case results thus likely underestimate the true magnitude of the bias in empirical data.
There are many important challenges in measuring biological interactions between STIs (17–19). These interactions should be evaluated in epidemiologic studies because of their importance for the success of public health interventions against STIs. However, associations between STIs suffer from various biases which make the estimation and interpretation of causal effects problematic. We showed that both cross-sectional and prospective measures of association between STIs are likely to be substantially biased due to the correlation between the times-at-risk for STIs. While this bias cannot be controlled in cross-sectional studies, prospective studies can be designed to validly measure interactions if analyses are restricted to time periods in which individuals are at risk for the outcome STI. Realization of such studies nonetheless presents significant practical challenges and may not be feasible in most circumstances. Therefore, using STI transmission models to interpret results from epidemiologic studies could help us better understand biological interactions between STIs.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Department of Social and Preventive Medicine, Faculty of Medicine, Laval University, Quebec City, Quebec, Canada (Talía Malagón, Philippe Lemieux-Mellouki, Marc Brisson); Population Health and Optimal Health Care Practices, CHU de Québec Research Center, Saint-Sacrament Hospital, Quebec City, Quebec, Canada (Talía Malagón, Philippe Lemieux-Mellouki, Jean-François Laprise, Marc Brisson); and Department of Infectious Disease Epidemiology, School of Public Health, Faculty of Medicine, Imperial College London, London, United Kingdom (Marc Brisson).
This work was supported by the Canada Research Chairs program (M.B.), an operating grant from the Canadian Institutes of Health Research (CIHR) (grant MOP-119427 to M.B.), a team grant from the CIHR (grant CRN-83320 to M.B.), a foundation scheme grant from the CIHR (grant FDN-143283 to M.B.), and a research grant from the Fondation du Centre hospitalier universitaire de Québec (to T.M.).
Computations were performed on the Colosse supercomputer at Laval University, managed by Compute Québec and Compute Canada. The operation of Colosse is funded by the Canada Foundation for Innovation, NanoQuébec, the Réseau de médecine génétique appliquée, and the Fonds de recherche du Québec–Nature et technologies.
We thank Drs. Marie-Claude Boily, Jacques Brisson, and Eduardo Franco for their valuable comments and insights.
In the past 3 years, M.B. has received an unrestricted grant from Merck Frosst related to zoster burden of illness.
REFERENCES
- 1.Chen L, Jha P, Stirling B, et al. . Sexual risk factors for HIV infection in early and advanced HIV epidemics in sub-Saharan Africa: systematic overview of 68 epidemiological studies. PLoS One. 2007;2(10):e1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Looker KJ, Garnett GP. A systematic review of the epidemiology and interaction of herpes simplex virus types 1 and 2. Sex Transm Infect. 2005;81(2):103–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alberts CJ, Schim van der Loeff MF, Papenfuss MR, et al. . Association of Chlamydia trachomatis infection and herpes simplex virus type 2 serostatus with genital human papillomavirus infection in men: the HPV in Men Study. Sex Transm Dis. 2013;40(6):508–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oakeshott P, Aghaizu A, Reid F, et al. . Frequency and risk factors for prevalent, incident, and persistent genital carcinogenic human papillomavirus infection in sexually active women: community based cohort study. BMJ. 2012;344:e4168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Plummer M, Vaccarella S, Franceschi S. Multiple human papillomavirus infections: the exception or the rule. J Infect Dis. 2011;203(7):891–893. [DOI] [PubMed] [Google Scholar]
- 6.Fleming DT, Wasserheit JN. From epidemiological synergy to public health policy and practice: the contribution of other sexually transmitted diseases to sexual transmission of HIV infection. Sex Transm Infect. 1999;75(1):3–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ward H, Ronn M. Contribution of sexually transmitted infections to the sexual transmission of HIV. Curr Opin HIV AIDS. 2010;5(4):305–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rositch AF, Hudgens MG, Backes DM, et al. . Vaccine-relevant human papillomavirus (HPV) infections and future acquisition of high-risk HPV types in men. J Infect Dis. 2012;206(5):669–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liaw KL, Hildesheim A, Burk RD, et al. . A prospective study of human papillomavirus (HPV) type 16 DNA detection by polymerase chain reaction and its association with acquisition and persistence of other HPV types. J Infect Dis. 2001;183(1):8–15. [DOI] [PubMed] [Google Scholar]
- 10.Moscicki AB, Ma Y, Jonte J, et al. . The role of sexual behavior and human papillomavirus persistence in predicting repeated infections with new human papillomavirus types. Cancer Epidemiol Biomarkers Prev. 2010;19(8):2055–2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mendez F, Munoz N, Posso H, et al. . Cervical coinfection with human papillomavirus (HPV) types and possible implications for the prevention of cervical cancer by HPV vaccines. J Infect Dis. 2005;192(7):1158–1165. [DOI] [PubMed] [Google Scholar]
- 12.Rousseau MC, Pereira JS, Prado JC, et al. . Cervical coinfection with human papillomavirus (HPV) types as a predictor of acquisition and persistence of HPV infection. J Infect Dis. 2001;184(12):1508–1517. [DOI] [PubMed] [Google Scholar]
- 13.Singer M. Pathogen-pathogen interaction: a syndemic model of complex biosocial processes in disease. Virulence. 2010;1(1):10–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Read AF, Taylor LH. The ecology of genetically diverse infections. Science. 2001;292(5519):1099–1102. [DOI] [PubMed] [Google Scholar]
- 15.Elbasha EH, Galvani AP. Vaccination against multiple HPV types. Math Biosci. 2005;197(1):88–117. [DOI] [PubMed] [Google Scholar]
- 16.Kurth AE, Celum C, Baeten JM, et al. . Combination HIV prevention: significance, challenges, and opportunities. Curr HIV/AIDS Rep. 2011;8(1):62–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Korenromp EL, de Vlas SJ, Nagelkerke NJ, et al. . Estimating the magnitude of STD cofactor effects on HIV transmission: how well can it be done. Sex Transm Dis. 2001;28(11):613–621. [DOI] [PubMed] [Google Scholar]
- 18.Mertens TE, Hayes RJ, Smith PG. Epidemiological methods to study the interaction between HIV infection and other sexually transmitted diseases. AIDS. 1990;4(1):57–65. [DOI] [PubMed] [Google Scholar]
- 19.Tota JE, Ramanakumar AV, Jiang M, et al. . Epidemiologic approaches to evaluating the potential for human papillomavirus type replacement postvaccination. Am J Epidemiol. 2013;178(4):625–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Halloran ME, Struchiner CJ. Causal inference in infectious diseases. Epidemiology. 1995;6(2):142–151. [DOI] [PubMed] [Google Scholar]
- 21.Combita AL, Touze A, Bousarghin L, et al. . Identification of two cross-neutralizing linear epitopes within the L1 major capsid protein of human papillomaviruses. J Virol. 2002;76(13):6480–6486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scherpenisse M, Schepp RM, Mollers M, et al. . Characteristics of HPV-specific antibody responses induced by infection and vaccination: cross-reactivity, neutralizing activity, avidity and IgG subclasses. PLoS One. 2013;8(9):e74797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Villa LL, Franco EL. Epidemiologic correlates of cervical neoplasia and risk of human papillomavirus infection in asymptomatic women in Brazil. J Natl Cancer Inst. 1989;81(5):332–340. [DOI] [PubMed] [Google Scholar]
- 24.Chaturvedi AK, Myers L, Hammons AF, et al. . Prevalence and clustering patterns of human papillomavirus genotypes in multiple infections. Cancer Epidemiol Biomarkers Prev. 2005;14(10):2439–2445. [DOI] [PubMed] [Google Scholar]
- 25.Mejlhede N, Pedersen BV, Frisch M, et al. . Multiple human papilloma virus types in cervical infections: competition or synergy. APMIS. 2010;118(5):346–352. [DOI] [PubMed] [Google Scholar]
- 26.Chaturvedi AK, Katki HA, Hildesheim A, et al. . Human papillomavirus infection with multiple types: pattern of coinfection and risk of cervical disease. J Infect Dis. 2011;203(7):910–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mollers M, Vriend HJ, van der Sande MA, et al. . Population- and type-specific clustering of multiple HPV types across diverse risk populations in the Netherlands. Am J Epidemiol. 2014;179(10):1236–1246. [DOI] [PubMed] [Google Scholar]
- 28.Thomas KK, Hughes JP, Kuypers JM, et al. . Concurrent and sequential acquisition of different genital human papillomavirus types. J Infect Dis. 2000;182(4):1097–1102. [DOI] [PubMed] [Google Scholar]
- 29.Plummer M, Schiffman M, Castle PE, et al. . A 2-year prospective study of human papillomavirus persistence among women with a cytological diagnosis of atypical squamous cells of undetermined significance or low-grade squamous intraepithelial lesion. J Infect Dis. 2007;195(11):1582–1589. [DOI] [PubMed] [Google Scholar]
- 30.Chandra A, Mosher WD, Copen C, et al. . Sexual behavior, sexual attraction, and sexual identity in the United States: data from the 2006–2008 National Survey of Family Growth. Natl Health Stat Report. 2011(36):1–36. [PubMed] [Google Scholar]
- 31.Insinga RP, Perez G, Wheeler CM, et al. . Incidence, duration, and reappearance of type-specific cervical human papillomavirus infections in young women. Cancer Epidemiol Biomarkers Prev. 2010;19(6):1585–1594. [DOI] [PubMed] [Google Scholar]
- 32.Carter JJ, Koutsky LA, Hughes JP, et al. . Comparison of human papillomavirus types 16, 18, and 6 capsid antibody responses following incident infection. J Infect Dis. 2000;181(6):1911–1919. [DOI] [PubMed] [Google Scholar]
- 33.Wang SS, Schiffman M, Herrero R, et al. . Determinants of human papillomavirus 16 serological conversion and persistence in a population-based cohort of 10 000 women in Costa Rica. Br J Cancer. 2004;91(7):1269–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mercer CH, Tanton C, Prah P, et al. . Changes in sexual attitudes and lifestyles in Britain through the life course and over time: findings from the National Surveys of Sexual Attitudes and Lifestyles (Natsal). Lancet 2013;382(9907):1781–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Demers AA, Shearer B, Severini A, et al. . Distribution of human papillomavirus types, cervical cancer screening history, and risk factors for infection in Manitoba. Chronic Dis Inj Can. 2012;32(4):177–185. [PubMed] [Google Scholar]
- 36.Bogaards JA, Xiridou M, Coupe VM, et al. . Model-based estimation of viral transmissibility and infection-induced resistance from the age-dependent prevalence of infection for 14 high-risk types of human papillomavirus. Am J Epidemiol. 2010;171(7):817–825. [DOI] [PubMed] [Google Scholar]
- 37.Garnett GP, Hughes JP, Anderson RM, et al. . Sexual mixing patterns of patients attending sexually transmitted diseases clinics. Sex Transm Dis. 1996;23(3):248–257. [DOI] [PubMed] [Google Scholar]
- 38.Mollers M, Boot Hein J, Vriend Henrike J, et al. . Prevalence, incidence and persistence of genital HPV infections in a large cohort of sexually active young women in the Netherlands. Vaccine. 2013;31(2):394–401. [DOI] [PubMed] [Google Scholar]
- 39.Moore RA, Ogilvie G, Fornika D, et al. . Prevalence and type distribution of human papillomavirus in 5,000 British Columbia women—implications for vaccination. Cancer Causes Control. 2009;20(8):1387–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wheeler CM, Hunt WC, Cuzick J, et al. . A population-based study of human papillomavirus genotype prevalence in the United States: baseline measures prior to mass human papillomavirus vaccination. Int J Cancer. 2013;132(1):198–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Malik ZA, Hailpern SM, Burk RD. Persistent antibodies to HPV virus-like particles following natural infection are protective against subsequent cervicovaginal infection with related and unrelated HPV. Viral Immunol. 2009;22(6):445–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Palmroth J, Merikukka M, Paavonen J, et al. . Occurrence of vaccine and non-vaccine human papillomavirus types in adolescent Finnish females 4 years post-vaccination. Int J Cancer. 2012;131(12):2832–2838. [DOI] [PubMed] [Google Scholar]
- 43.Viscidi RP, Snyder B, Cu-Uvin S, et al. . Human papillomavirus capsid antibody response to natural infection and risk of subsequent HPV infection in HIV-positive and HIV-negative women. Cancer Epidemiol Biomarkers Prev. 2005;14(1):283–288. [PubMed] [Google Scholar]
- 44.Wilson L, Pawlita M, Castle PE, et al. . Seroprevalence of 8 oncogenic human papillomavirus genotypes and acquired immunity against reinfection. J Infect Dis. 2014;210(3):448–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lu B, Hagensee ME, Lee JH, et al. . Epidemiologic factors associated with seropositivity to human papillomavirus type 16 and 18 virus-like particles and risk of subsequent infection in men. Cancer Epidemiol Biomarkers Prev. 2010;19(2):511–516. [DOI] [PubMed] [Google Scholar]
- 46.Viscidi RP, Ahdieh-Grant L, Schneider MF, et al. . Serum immunoglobulin A response to human papillomavirus type 16 virus-like particles in human immunodeficiency virus (HIV)-positive and high-risk HIV-negative women. J Infect Dis. 2003;188(12):1834–1844. [DOI] [PubMed] [Google Scholar]
- 47.Durham DP, Poolman EM, Ibuka Y, et al. . Reevaluation of epidemiological data demonstrates that it is consistent with cross-immunity among human papillomavirus types. J Infect Dis. 2012;206(8):1291–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pons-Salort M, Letort V, Favre M, et al. . Exploring individual HPV coinfections is essential to predict HPV-vaccination impact on genotype distribution: a model-based approach. Vaccine. 2013;31(8):1238–1245. [DOI] [PubMed] [Google Scholar]
- 49.Boily MC, Baggaley RF, Wang L, et al. . Heterosexual risk of HIV-1 infection per sexual act: systematic review and meta-analysis of observational studies. Lancet Infect Dis. 2009;9(2):118–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mahiane S-G, Legeai C, Taljaard D, et al. . Transmission probabilities of HIV and herpes simplex virus type 2, effect of male circumcision and interaction: a longitudinal study in a township of South Africa. AIDS. 2009;23(3):377–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ng BE, Butler LM, Horvath T, et al. . Population-based biomedical sexually transmitted infection control interventions for reducing HIV infection. Cochrane Database Syst Rev. 2011(3):CD001220. [DOI] [PubMed] [Google Scholar]
- 52.Mbulawa ZZ, Johnson LF, Marais DJ, et al. . The impact of human immunodeficiency virus on human papillomavirus transmission in heterosexually active couples. J Infect. 2013;67(1):51–58. [DOI] [PubMed] [Google Scholar]
- 53.Boily MC, Anderson RM. Human immunodeficiency virus transmission and the role of other sexually transmitted diseases. Measures of association and study design. Sex Transm Dis. 1996;23(4):312–332. [DOI] [PubMed] [Google Scholar]
- 54.Wang SS, Schiffman M, Shields TS, et al. . Seroprevalence of human papillomavirus-16, -18, -31, and -45 in a population-based cohort of 10 000 women in Costa Rica. Br J Cancer. 2003;89(7):1248–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Van de Velde N, Boily MC, Drolet M, et al. . Population-level impact of the bivalent, quadrivalent, and nonavalent human papillomavirus vaccines: a model-based analysis. J Natl Cancer Inst. 2012;104(22):1712–1723. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.