ABSTRACT
We tested whether exposure of β cells at reduced glucose leads to mitochondrial adaptions and whether such adaptions modulate effects of hypoxia. Rat islets, human islets and INS-1 832/13 cells were pre-cultured short term at half standard glucose concentrations (5.5 mM for rat islets and cells, 2.75 mM for human islets) without overtly negative effects on subsequently measured function (insulin secretion and cellular insulin contents) or on viability. Culture at half standard glucose upregulated complex I and tended to upregulate complex II in islets and INS-1 cells alike. An increased release of lactate dehydrogenase that followed exposure to hypoxia was attenuated in rat islets which had been pre-cultured at half standard glucose. In INS-1 cells exposure to half standard glucose attenuated hypoxia-induced effects on several viability parameters (MTT, cell number and incremental apoptotic DNA). Thus culture at reduced glucose of pancreatic islets and clonal β cells leads to mitochondrial adaptions which possibly lessen the negative impact of hypoxia on β cell viability. These findings appear relevant in the search for optimization of pre-transplant conditions in a clinical setting.
KEYWORDS: hypoxia, insulin secretion, INS-1, low glucose, mitochondrial complexes, oxygen consumption, rat and human islets, viability
Introduction
It has been demonstrated that resilience to toxic influences can, in many types of cells, be enhanced by mild stress, a phenomenon that has been termed hormesis or preconditioning.1 It has also been proposed that induction of hormesis can be used clinically to alleviate cellular damage due to various types of severe stress.2,3 The success of transplantation of insulin producing β cells to diabetic subjects suffers from the massive death of transplanted pancreatic islets that occurs, in part at least, because of hypoxia4-7 to which β cells may be more sensitive than other cell types because of high energy demands.8 Preconditioning β cells to better withstand hypoxia after transplantation is an attractive idea. The concept has so far been tested with some positive effects following pre-transplant exposure to K+-ATP channel openers.9 However other approaches to preconditioning of β cells have so far not been tested.
Glucose is the prime effector of insulin secretion and biosynthesis, and profoundly affects the rate of β cell metabolism. It is known from other tissues that lowering cell metabolism can offer protection against hypoxia.10 For example culture at low glucose has been shown to protect human fibroblasts against hypoxia.11 Also it has been reported that elevated glucose can give rise to some degree of hypoxia in pancreatic islets even under normoxic conditions.12 We reasoned therefore that a lower rate of metabolism as achieved by a lower glucose concentration preceding hypoxia could offer some degree of protection against damage. A likely prerequisite for any effect would be persisting changes of mitochondrial function incurred by lower availability of glucose, something that has however not been previously documented. The aim of the present study was thus twofold: first to test for mitochondrial adaptions by short-term culture at lower-than-standard glucose concentrations and, second, to test for associated modulating effects on β cell viability and function after exposure to hypoxia. To this end we have investigated rat and human pancreatic islets as well as clonal β cells of rat origin (INS-1 832/13) according to the scheme outlined in Fig. 1.
Figure 1.
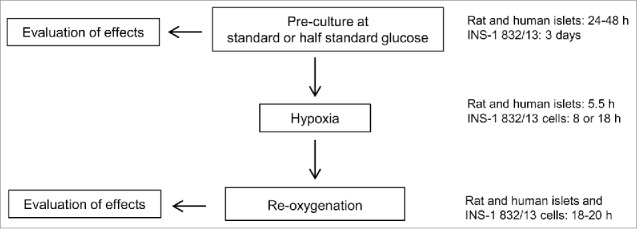
General study design. Standard glucose concentrations in culture media were for rat islets and INS-1 832/13 cells 11 mM (half standard: 5.5 mM) and for human islets 5.5 mM (half standard: 2.75 mM). The media for rat and human islets were renewed to contain standard glucose concentrations before exposure to hypoxia. For INS-1 832/13 cells the media were renewed without changing the glucose concentrations before exposure to hypoxia. Oxygen levels during hypoxia were 0.8 or 2.8% for rat islets and 0.8% for human islets while INS-1 832/13 cells were exposed to 0.3–0.5% oxygen. The media employed in the re-oxygenation period always contained standard glucose concentrations for both islets and cells. (Not indicated are selected experiments in which measurements were made also immediately after hypoxia).
Results
Reduced glucose does not negatively affect function or viability
In rat islets, pre-exposure to half standard glucose (5.5 mM) left both basal and glucose-stimulated insulin secretion unaltered (Table 1). In concordance with rat islets, insulin secretion in human islets was also unaffected by previously reduced glucose (2.75 mM) (Table 1). Insulin contents were unaffected by half standard glucose both in rat and human islets (results not shown). In INS-1 cells exposure to half standard glucose (5.5 mM) enhanced basal insulin secretion when expressed per cellular total protein. Also stimulated secretion induced by 11 or 27 mM glucose was enhanced (Table 1). However, insulin contents in INS-1 cells were higher after culture at 5.5 rather than 11 mM glucose (for basal secretion at 3.3 mM glucose: 3528 ± 474 vs. 2512 ± 289 µU/well, for stimulated secretion at 11 mM glucose: 3309 ± 436 vs. 2375 ± 331 µU/well and for stimulated secretion at 27 mM glucose: 3343 ± 426 vs. 2420 ± 878 µU/well. For all 3 conditions, p < 0.05, n = 6). Consequently, glucose stimulated secretion in INS-1 cells was reduced after culture at 5.5 mM glucose when results were expressed per cellular insulin contents (Fig. S1).
Table 1.
Insulin release
| Insulin release (μU/islet and for cells μU/μg protein) | ||||||
|---|---|---|---|---|---|---|
| Rat islets (n = 4) | Inc. G (mM) | Inc. G (mM) | Inc. G (mM) | |||
| Prev. G (mM) | ||||||
| 11 | 0.4 ± 0.0 | 3.3 | 100.3 ± 6.9 | 16.7 | ||
| 5.5 | 0.7 ± 0.2 | 3.3 | 89.3 ± 10.9 | 16.7 | ||
| Human islets (n = 4) | ||||||
| Prev. G (mM) | ||||||
| 5.5 | 1.4 ± 0.8 | 1.6 | 14.1 ± 4.3 | 16.7 | ||
| 2.75 | 1.9 ± 1.1 | 1.6 | 16.0 ± 6.9 | 16.7 | ||
| INS-1 832/13 (n = 5) | ||||||
| Prev. G (mM) | ||||||
| 11 | 0.13 ± 0.01 | 3.3 | 0.35 ± 0.06 | 11 | 0.37 ± 0.06 | 27 |
| 5.5 | 0.26 ± 0.04* | 3.3 | 0.46 ± 0.11* | 11 | 0.64 ± 0.24* | 27 |
Insulin release after previous (Prev.) culture at standard or half standard glucose (G) as investigated in final incubations (Inc.). Data are mean ± SEM, *p< 0.05 for the effect of previous culture at 5.5 vs. 11 mM G in INS-1 cells.
For rat and human islets alike viability as tested by MTT was not significantly altered by previous half standard glucose (for rat islets 5.5 mM and for human islets 2.75 mM). Absorbance signals were for rat islets 99.2 ± 5.7% (n = 11) and for human islets 89.9 ± 8.5% (n = 4) of levels at standard glucose. Apoptosis and necrosis was measured in rat islets only and no significant glucose-related effects were seen. Apoptosis levels at 5.5 mM glucose were thus 106 ± 10% and for necrosis 106 ± 27% of levels at 11 mM glucose (n = 16). Also the release of lactate dehydrogenase (LDH) from rat islets was not affected by culture at half standard glucose, levels of LDH being 98 ± 8% of levels at 11 mM glucose (n = 8).
For INS-1 cells viability was measured by cell counting, MTT and quantification of apoptotic and necrotic DNA. Four days of culture at half standard glucose (5.5 mM) did not affect viability as assessed by cell counting (98.5 ± 0.6% viable cells at half standard vs. 98.6 ± 0.7% at standard glucose, n = 4). When normalizing for the decelerated growth of cells cultured at 5.5 mM glucose (which was 42.2 ± 2.3% of growth recorded in cells cultured at 11 mM glucose) there was no negative effect of half standard glucose on the MTT parameter. Further, there was no difference in levels of apoptosis, the signals after half standard glucose being 112 ± 24% of signals at standard glucose (n = 8). Culture at half standard glucose reduced levels of necrosis to 75 ± 7% of levels at standard glucose (p < 0.05 for the glucose effect, n = 8).
Reduced glucose up-regulates mitochondrial complexes
The absence of major functional and viability consequences following half standard glucose suggested mitochondrial adaptions. To test for such adaptations we measured protein subunits of mitochondrial complexes in islets and cells by Western blotting in rat and human islets and in INS-1 832/13 cells. These results are shown in Fig. 2.
Figure 2.
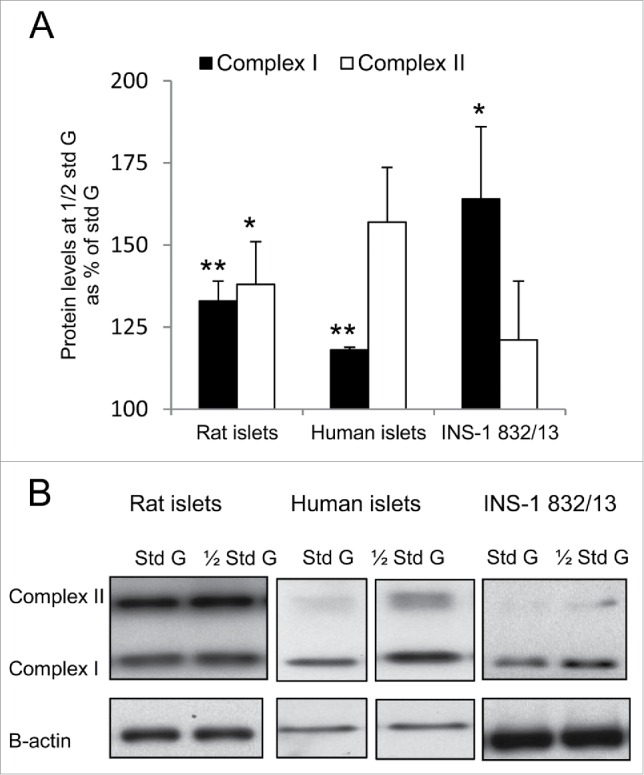
Culture at half standard glucose increases levels of mitochondrial complex proteins. Levels of protein subunits of complexes I and II were measured by Western blotting. (A) shows protein levels after culture at half standard (1/2 Std) vs. standard (Std) glucose (G) (for rat islets and cells 1/2 Std = 5.5 mM G and for human islets 1/2 Std = 2.75 mM G). Data are mean ± SEM of 8 (rat islets), 3 (human islets) and 9 (INS-1 832/13 cells) experiments, **p < 0.002 and *p < 0.03 vs. protein levels after culture at standard glucose. (B) Representative Western blots are shown of levels of complex I and complex II at standard (Std) and half standard (1/2 Std) glucose (G). Band images from human islet experiments were from separate parts of the same gel.
For rat islets pre-culture at half standard glucose (5.5 mM) enhanced proteins of both complex I and II. For complex I the enhancement was 33 ± 6%, p < 0.002, and for complex II 38 ± 13%, p < 0.02 vs. 11 mM glucose (n = 8).
For human islets 5 separate experiments were performed with islets from 3 different donors (one or 2 experiments per donor, the average result of duplicates entered into calculations). Pre-culture at half standard glucose (2.75 mM) enhanced complex I significantly by 18 ± 0.8%, p < 0.002, and complex II non-significantly by 58 ± 16.6%, p < 0.08 (n = 3).
For INS-1 832/13 cells previous half standard glucose (5.5 mM) enhanced protein subunits of complex I significantly by 164 ± 22%, p < 0.01. Also protein subunits of complex II tended to be enhanced, by 121 ± 18% vs. 11 mM glucose (n = 9).
For both islets and cells the protein levels of complex III were not affected by changes in glucose concentration. The same was seen for complex IV (results for complex III and IV are not shown).
Does pre-culture at half standard glucose affect hypoxia-induced cellular damage?
Having tested for functional, viability and adaptive effects by pre-exposure to half standard glucose we next tested for modulation of negative effects by experimental hypoxia. The impact of the hypoxic event was purposely aimed to be moderate, the rationale being that a moderate rather than a severe effect would be necessary in order to observe modulation. Effects of hypoxia were usually measured after a period of re-oxygenation (termed in following hypoxia-reoxygenation) but also, in some experiments, in direct sequence to the hypoxic event. Effects of hypoxia during conditions of standard glucose have been previously published13; the effects of pre-exposure to half standard glucose were here in part compared with the previous results.
Insulin secretion and contents after hypoxia
In rat islets, hypoxia-reoxygenation reduced glucose-induced insulin secretion in islets pre-cultured at 5.5 mM glucose by a mean of 68%. The reduction was similar (60%) in islets pre-cultured throughout at 11 mM glucose (for both conditions p < 0.02 for the effect of hypoxia, n = 4) (Fig. 3A). As to insulin contents pre-culture at half standard glucose significantly attenuated a hypoxia-reoxygenation-induced reduction (−21 ± 5% after pre-culture at 5.5 mM vs. −47 ± 9% after pre-culture at 11 mM glucose, p < 0.02 for difference, n = 4).
Figure 3.
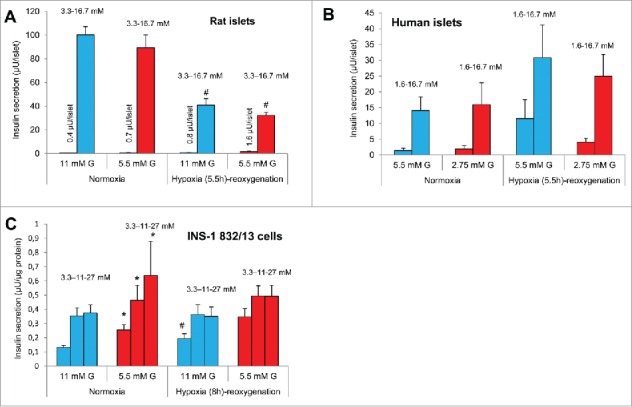
Effects of hypoxia followed by re-oxygenation on insulin secretion after pre-culture at different glucose (G) concentrations. Levels of insulin release after culture at half standard glucose are indicated by red bars and standard glucose by blue bars. (A) Insulin release from rat islets at 3.3 and 16.7 mM G, #p < 0.02 for the effect of hypoxia, n = 4. (B) Insulin release from human islets at 1.6 and 16.7 mM G, n = 4 (one or 3 experiments per donor, 4 donors). (C) Insulin release from INS-1 832/13 cells at 3.3, 11 and 27 mM glucose, *p < 0.05 for the effect of culture at half standard G, #p < 0.05 for the effect of hypoxia, n = 5. Effects of hypoxia at standard G were previously reported.13 All data are mean ± SEM.
In human islets hypoxia-reoxygenation tended to increase basal insulin secretion for islets pre-cultured at half standard glucose (2.75 mM) as well as for islets continuously cultured at standard glucose (5.5 mM) (Fig. 3B). Glucose (16.7 mM)-stimulated insulin secretion was non-significantly increased by hypoxia-reoxygenation for islets cultured at half standard glucose (Fig. 3B) whereas islets cultured at standard glucose displayed a close to significant increased secretion (p < 0.07, n = 4). The glucose stimulation index (GSI, fold increase due to 16.7 mM glucose) was not significantly affected by hypoxia-reoxygenation. Nor were insulin contents affected by hypoxia-reoxygenation (results not shown, n = 4).
For INS-1 832/13 cells basal insulin secretion (at 3.3 mM glucose) was increased by hypoxia-reoxygenation (from 0.13 ± 0.1 to 0.19 ± 0.1 µU/µg protein, p < 0.05, n = 5) after continuous culture at standard glucose (Fig. 3C). The GSI was reduced by hypoxia-reoxygenation for cells cultured at half standard glucose (5.5 mM) (from 1.8 ± 0.2 to 1.5 ± 0.2) as well as standard glucose (11 mM) (from 2.7 ± 0.2 to 1.9 ± 0.2, p <0.05 for effects of hypoxia-reoxygenation after both half standard and standard glucose, n = 5). The difference in glucose concentrations during culture did not significantly alter the impact of hypoxia on GSI. As to insulin contents hypoxia-reoxygenation reduced contents similarly in cells cultured at half standard and standard glucose, i.e. by respectively −8.8 ± 1.3% and −7.3 ± 7.1 (n = 3).
Viability parameters after hypoxia
For rat islets the MTT signal was similarly reduced by hypoxia-reoxygenation after conditions of half standard (5.5 mM) or standard glucose (11 mM) (35.3 ± 3.3% vs. 39.8 ± 4.8%, p < 0.22 for a glucose effect, n = 11) (Fig. 4A). With regard to apoptosis and necrosis results did not reveal significant modulation by half standard glucose on effects by hypoxia-reoxygenation when 0.8% oxygen was present during hypoxia (Table 2). Following exposure to a lesser degree of hypoxia (2.8% oxygen) the effects of hypoxia-reoxygenation on apoptosis and necrosis were much less severe but again not modified by the glucose concentration during pre-culture (Table 2). However, an increase in the release of LDH (another measure of toxicity) after hypoxia-reoxygenation was attenuated in islets pre-cultured overnight at 5.5 mM glucose (9 ± 7% after half standard vs. 28 ± 7% increase after standard glucose, p < 0.03 for difference, n = 8) (Fig. 5).
Figure 4.
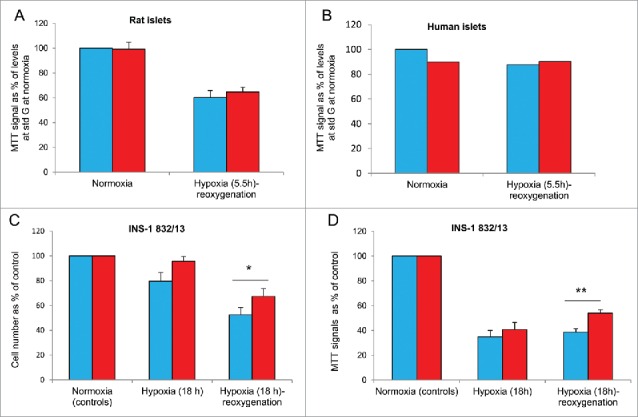
Effects of hypoxia on viability after pre-culture at different glucose (G) concentrations. Blue bars denote pre-culture at standard (std) glucose (for rat islets and cells: 11 mM, for human islets: 5.5 mM), red bars at half standard glucose. (A) Rat islets were exposed to 0.8% oxygen during hypoxia. There was no significant effects due to glucose concentrations on MTT signals, n = 11. (B) Human islets, n = 2 (2 experiments per donor, 2 donors). (C and D) INS-1 832/13 cells. Cell number is depicted in (C) and MTT signals in (D), after 18 h of hypoxia. Attenuating effects on hypoxia-reoxygenation by half vs. standard glucose were significant *p < 0.03 (C) and **p < 0.002 (D), n = 4-5.
Table 2.
Assessment of apoptosis and necrosis in rat islets and INS-1 832/13 cells.
| Hypoxia-reoxygenation-induced increase in DNA fragments (for islets fold increased DNA and for cells incremental DNA). | ||||
|---|---|---|---|---|
| Rat islets | Subjected to 0.8% O2 for 5.5 h (n = 4) |
Subjected to 2.8% O2 for 5.5 h (n = 4) |
||
| Previous glucose (mM) |
Apoptosis |
Necrosis |
Apoptosis |
Necrosis |
| 11 | 10.00 ± 3.70 | 15.50 ± 2.00 | 3.22 ± 0.42 | 1.65 ± 0.29 |
| 5.5 | 12.20 ± 5.00 | 12.10 ± 0.70 | 2.54 ± 0.61 | 1.31 ± 0.16 |
| INS-1 832/13 cells | Subjected to 0.3-0.5% O2 for 18 h (n = 8) |
|||
| Previous glucose (mM) |
Apoptosis |
Necrosis |
|
|
| 11 | 2.42 ± 0.19 | 2.31 ± 0.26 | ||
| 5.5 | 1.52 ± 0.14* | 1.52 ± 0.27 | ||
For rat islets data are fold increased DNA vs. levels at normoxia, mean ± SEM. For INS-1 832/13 data are mean ± SEM of incremental DNA.
p < 0.02 for the effect of culture at 5.5 vs. 11 mM glucose.
Figure 5.
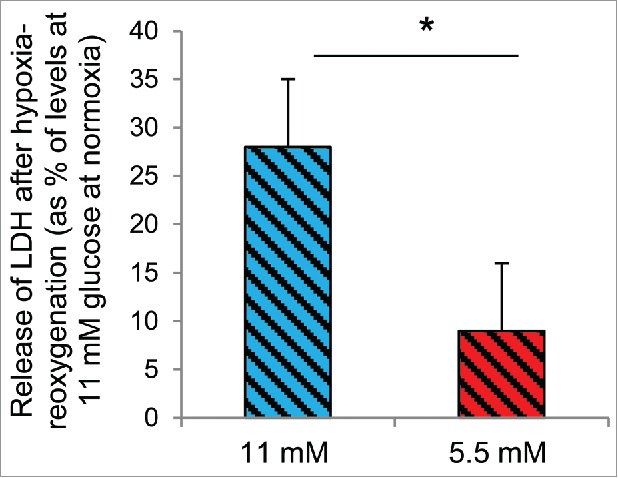
Half standard glucose attenuates hypoxia-induced release of lactate dehydrogenase (LDH). Hypoxia (2.8% oxygen for 5.5 h followed by re-oxygenation) increased the release of LDH from rat islets cultured continuously at 11 mM glucose (blue patterned bar). The release was attenuated in islets pre-cultured at 5.5 mM glucose (red patterned bar, *p < 0.03 for difference). Mean ± SEM of 8 experiments.
Human islets were pre-exposed to 2.75 mM vs. continuously 5.5 mM glucose for 24-48 h. There was no effect of pre-culture at reduced glucose to the reduction of the MTT signals following hypoxia-reoxygenation (Fig. 4B).
For INS-1 cells we tested the effects of both a shorter (8 h) and longer (18 h) period of hypoxia followed by re-oxygenation. Employing the former (8 h of hypoxia, followed by re-oxygenation) protocol the cell number per culture flask of half standard glucose (5.5 mM)-exposed cells was less reduced (reduction 11.6 ± 2.2%) compared to standard glucose (11 mM) (25.8 ± 4.8%, p < 0.02 for difference, n = 7). Similarly, when employing the latter (18 h of hypoxia, followed by re-oxygenation) protocol the decline in cell number was less pronounced in cells cultured at half standard glucose (32.7 ± 6.4% decline vs. 47.6 ± 6.1% for cells cultured at standard glucose, p < 0.002, n = 5). A similar tendency was seen when cell numbers were measured in direct sequence to the period of 18 h of hypoxia (8.7 ± 3.9% decline vs. 20.4 ± 7.1%, n = 4, ns) (Fig. 4C). As to MTT, employing the former (8 h of hypoxia, followed by re-oxygenation) protocol, reduced the MTT signal non-significantly both after half standard and standard glucose (results not shown). Employing the 18 h hypoxia-reoxygenation protocol clearly reduced the MTT signal for both conditions of culture (Fig. 4D). However, cells grown at half standard glucose displayed lesser reduction in MTT signal (46 ± 2.7%) compared to cells cultured at standard glucose throughout (62 ± 2.9%, p < 0.002 for difference, n = 4). Also, the negative impact of hypoxia tended to be less after half standard than standard glucose when tested directly after the hypoxic event (p < 0.07, n = 4) (Fig. 4D).
Employing the 18 h hypoxia-reoxygenation protocol markedly increased apoptotic and necrotic DNA. However, these effects were lesser in INS-1 cells exposed to half standard rather than standard glucose (Table 2).
Oxygen consumption after hypoxia
Oxygen consumption was measured in INS-1 cells to further investigate how half standard glucose (5.5 mM) may affect mitochondrial function. Effects on parameters of mitochondrial oxidation have been previously reported using 11 mM glucose13; these effects (which include indications of decreased mitochondrial uncoupling after hypoxia-reoxygenation) were compared here with effects of previous 5.5 mM glucose. Basal respiration was modestly but significantly increased by previous hypoxia in cells pre-cultured with 5.5 but not 11 mM glucose (Fig. 6). A difference in this ratio due to glucose concentrations was not significant. The calculated oligomycin/FCCP ratios were significantly affected by hypoxia-reoxygenation in cells cultured at 11 mM glucose, but not in cells cultured at 5.5 mM glucose. A difference in this ratio due to glucose concentrations was not significant. Also, comparisons of other parameters of mitochondrial oxidation did not reveal any clear differences between previous half vs. standard glucose. Likewise extending the period of hypoxia from 8 to 18 h did not either reveal differences due to the glucose concentration, (results not shown).
Figure 6.
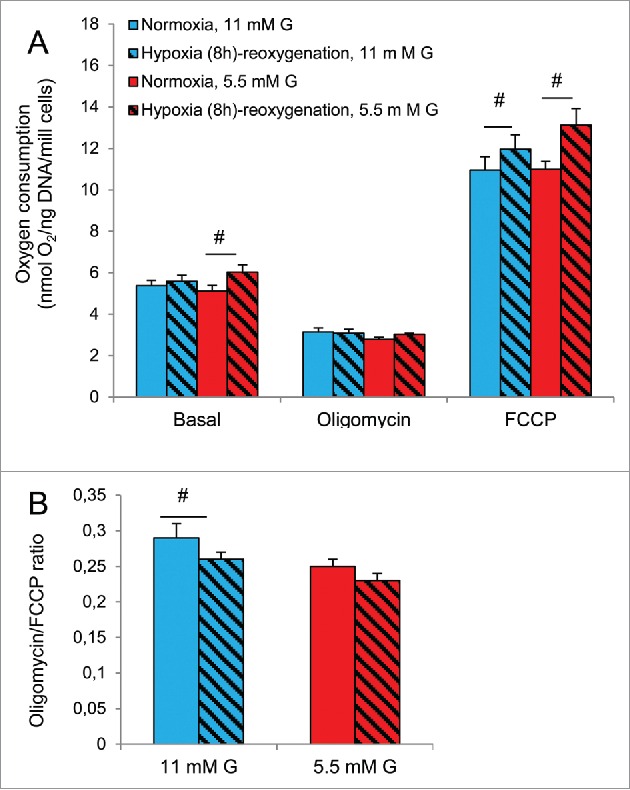
Effects of hypoxia on oxygen consumption in INS-1 832/13 cells after culture at 5.5 or 11 mM glucose (G). Blue bars represent culture at standard G (11 mM), red bars represent culture at half standard G (5.5 mM). Patterned bars indicate exposure to 8 h of hypoxia followed by re-oxygenation. (A) Respiration at basal conditions, followed by conditions of ATP synthase inhibition by oligomycin and finally a non-coupled state achieved by FCCP. (B) Uncoupled (oligomycin) to FCCP-induced (FCCP) respiration ratio. #p < 0.05 or less for effects of hypoxia-reoxygenation. Data are mean ± SEM of 5 separate experiments, each consisting of 2-4 parallel measurements. Effects with culture at 11 mM glucose throughout were previously reported.13
Discussion
The present study documents, to our knowledge for the first time, a flexibility of β cell mitochondria in adapting to a moderate restriction of glucose availability. Adaption was assessed from increased amounts of subunits of mitochondrial complexes I and II after short term culture at below-standard glucose concentrations. The findings were similar in rat and human islets as well as in clonal β cells. These similarities indicate that mitochondrial adaption to nutrient scarcity in β cells is a universal phenomenon.
We previously demonstrated13 in INS-1 cells mitochondrial adaptability of oxygen consumption as manifested by decreased uncoupling, i.e., increased efficiency for ATP formation. Here evidence may additionally suggest a modulating effect of culture at half standard glucose (5.5 mM), such that basal oxygen consumption was significantly increased after hypoxia but not significantly in cells that had been cultured at standard glucose (11 mM). To which extent this particular feature should be regarded as beneficial for over-all function is however unclear.
Given the evidence for mitochondrial flexibility that we find, does it increase the ability of β cells to withstand the damaging effects by hypoxia on function and survival? Some of our findings do indicate increased resilience. Culture at lower-than-standard glucose was thus associated with somewhat lesser hypoxia-induced apoptosis and necrosis in rat islets and in INS-1 cells. Also the effects of hypoxia on other parameters of viability, including MTT, were attenuated in INS-1 cells, whereas the hypoxia-induced effect was non-significantly attenuated in human islets. However, beneficial effects by lower-than-standard glucose were not uniformly found.
Notably the effects of hypoxia per se differed in magnitude between rat and human islets and INS-1 cells. Such differences could be species-dependent (rat vs. human islets), preparation dependent (rat islets vs.INS-1 cells) and/or could relate to non-identical protocols used in this study. We also recognize that the - arbitrary - halving of the standard glucose concentration used for long term culture may have a different impact in human islets (i.e. the reduction from 5.5 to 2.75 mM glucose) than the reduction from 11 to 5.5. mM glucose for rat islets and INS-1 cells.
Prolonged culture at low glucose is well known to irreversibly damage β cells,14-17 as is also prolonged exposure to hypoxia. Any resilient effect of “mitohermesis”18 would then be confined to only mild stress by nutrient scarcity, i.e., short-term culture at moderately reduced glucose concentrations as well as moderate exposure to hypoxia. The presently used protocols were designed to provide conditions favorable for demonstrating “mitohermesis.” However, we recognize that optimal conditions may differ between species, islets and clonal cells and those differences may not have been taken fully into account.
In conclusion we demonstrate, to our knowledge for the first time, that mitochondria of β cells show adaptation to a moderate reduction in nutrient availability. Further we provide evidence compatible with – but not proving – that such adaption can improve the capacity of β cells to withstand hypoxia.
Methods and materials
Procurement, isolation and culture of islets and cells
Islets from male Sprague-Dawley rats (Rattus Norwegicus) were obtained from Scanbur (Sollentuna, Sweden). The rats had free access to water and a standard diet. At the time of experiments, the rats weighed 300-350 g. Islets of Langerhans were isolated by collagenase digestion.19 Islets were cultured free floating, at 37°C at a humidified atmosphere of 5% CO2 in air in RPMI 1640 medium (cat. no. R8758, Sigma) supplemented with 10% fetal calf serum, 1 mM sodium pyruvate, 2 mM L-glutamine, 100 IU/ml penicillin and 100 µg/ml streptomycin.
Human islets were isolated using a modified semi-automated digestion method20 from deceased donors at the islet isolation facility at the Section for Transplantation Surgery at Oslo University Hospital, Oslo, Norway, after appropriate consent was given for multi-organ donation. The islet viability was 80-95%. Islet preparations were those where quantitative insufficiencies had precluded their use for clinical transplantation. Islet preparations were initially cultured in CMRL 1066 medium (cat. no. 99-603-CV, Mediatech Inc.) supplemented with 10% human AB serum (cat. no. 000084, Milan Analytica), 2 mM L-glutamine, 100 IU/ml penicillin and 100 µg/ml streptomycin (all from Life Technologies). Upon arrival in Trondheim human islets were cultured before experiments similarly to rat islets, except for a lower glucose concentration (5.5 mM).
INS-1-derived 832/13 cells were developed by researchers now at Duke University21 and kindly provided by professor Hindrik Mulder, Malmö, Sweden. Cells were continuously tested and proven free from mycoplasma. Cells were grown in monolayer cultures in RPMI-1640 medium with the same composition as for islets, except for supplementation with 50 µM 2-mercaptoethanol (cat. no. 25351.182, VWR). Cells were cultured at a humidified atmosphere of 5% CO2 in air. Cells were sub cultured once a week after detachment with 0.01% trypsin in 0.02% EDTA. The RPMI medium was changed every 3-4 d of culture. RPMI medium with 5.5 mM glucose was obtained by mixing RPMI medium containing 11 mM glucose with an equal amount of glucose-free RPMI medium (cat. no. 11879-020, Invitrogen) (which otherwise contained the same additives as the standard RPMI medium).
Experimental protocols
Standard protocols are schematically outlined in Fig. 1. Rat islets were cultured for 24 h (if not indicated otherwise) at either 11 mM (standard) or 5.5 mM glucose (half standard). For both conditions the media were renewed to contain 11 mM glucose before exposure either to 5.5 h of hypoxia or to continuous normoxia. Hypoxia was induced by placing the islets in a hypoxia chamber (Billups-Rothenberg Inc., Del Mar, CA) together with an oxygen monitor (Dräger Safety AG & Co., KGaA, Lübeck, Germany) and a Petri dish with 5 ml water to uphold humidity. The chamber interior was flushed with nitrogen gas (95% N2, 5% CO2) until the desired O2 concentration was reached (0.8 or 2.8% O2).
Human islets when entering the experimental protocol were cultured for 24 h (if not indicated otherwise) at either 5.5 or 2.75 mM glucose. For both conditions the media were changed to contain 5.5 mM glucose before islets were exposed either to 5.5 h of hypoxia (0.8% O2) or to continuous normoxia.
For both rat and human islets the period of hypoxia was followed by a 18-20 h period of re-oxygenation. During re-oxygenation after hypoxia all media contained 11 mM glucose (rat islets) or 5.5 mM glucose (human islets).
For cell counting and oxygen consumption experiments INS-1-derived 832/13 cells were seeded in 25 cm2 flasks. For other experiments they were seeded in multi-well plates. Cells (passage 46-56) were cultured for 3 d in media containing either 11 or 5.5 mM glucose. The media were renewed without changing the glucose concentration before exposing cells to hypoxia for 8 or 18 h. Control cells were grown in parallel at continuous normoxia. In experiments that included a re-oxygenation period (the majority of experiments) the renewed media after hypoxia all contained 11 mM glucose.
With cells the duration of hypoxia was longer and the degree of hypoxia more pronounced than for islets. These conditions were arrived at from results of a pilot study. The interior of the hypoxia chamber containing the cells was flushed with nitrogen gas (95% N2, 5% CO2 ) until an O2 concentration of 0.1% was reached. After 8 or 18 h of incubation the oxygen concentration inside the chamber had risen to 0.3-0.5%.
Cell counting
INS-1 832/13 cells were seeded in 25 cm2 culture flasks (7.5 × 105 cells per flask for 11 mM and 106 cells per flask for 5.5 mM glucose) and cultured as detailed above. Trypan blue stain (0.4%) and a sample of the cell suspension were mixed (1:1) and viable and dead cells counted in a Countess automatic cell counter (Invitrogen, Carlsbad, CA).
MTT
Rat islets. For the 3-(4,5-diethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) (cat. no. M5655, Sigma) assay22 islets were handpicked into each of 4 dishes and cultured for 24-48 h in RPMI medium containing either 11 or 5.5 mM glucose. When testing for effects of hypoxia the dishes were exposed to 5.5 h of hypoxia in a hypoxia camber with an atmosphere of 0.8% O2. In all experiments islets were finally transferred to a 24 well plate (20-30 islets/well, 2-5 parallels for each experimental condition) for 4 h of MTT exposure. The MTT reagent in the media was removed by washing the islets several times in 0.9% NaCl. Islets were then incubated for 1 h in 400 µl DMSO (cat. no. 317275, Merck Millipore) per well at 37°C for color development. Fifty µl/well of 0.1 M NaCl in 0.1 M Glycin, pH 10.5, was added for color extraction. Two parallel aliquots per well were secured for absorbance measurements.
Human islets. For human islets the RPMI culture medium contained 5.5 or 2.75 mM glucose. The procedure for the MTT assay was the same as described for rat islets, except that 30-40 islets/well were used for each experimental condition.
Cells. INS-831/13 cells were seeded in 96-well plates (1.2 × 104 cells/well for 11 mM glucose and 1.5 × 104 cells/well for 5.5 mM glucose) and cultured as described above. When testing for effects of hypoxia the MTT assay was performed either directly after the hypoxia exposure or after re-oxygenation.
Apoptosis/necrosis
Rat islets were cultured in dishes overnight in RPMI-medium containing 11 or 5.5 mM glucose. When testing for effects of hypoxia the medium was renewed to contain 11 mM glucose before the islets were exposed to continuous normoxia or 5.5 h of 0.8 or 2.8 % O2 followed by re-oxygenation. Necrotic and apoptotic DNA fragments was quantified by the Cell Death Detection ELISAplus kit (cat. no. 11774425001, Roche Diagnostics Gmbh,).
Cells. INS-1 832/13 cells were seeded in 96 well plates (8 × 103 cells/well for 11 mM glucose and 104 cells/well for 5.5 mM glucose) and cultured as described above. Quantification of apoptotic and necrotic DNA was performed using the same ELISA kit as for rat islets.
Lactate dehydrogenase
Batches of 30 islets from rats per well were cultured with 11 mM glucose or 5.5 mM glucose as described in Experimental protocols, after which aliquots of culture media were collected for measurements of lactate dehydrogenase (LDH) (Cyto ToX 96® Non-Radioactive Cytotoxicity Assay, cat. no. G1780, Promega). The level of oxygen during hypoxia was 2.8%.
Insulin secretion and insulin content
Rat islets. Islets were cultured as described for measurements of apoptosis/necrosis except that for hypoxia the oxygen level was always 0.8%. At the end of experiments islets were pre-incubated with 3.3 mM glucose Krebs-Ringer bicarbonate buffer (KRB). Pre-incubation was followed by batch incubations with 3.3 or 16.7 mM glucose for 1 h. Insulin accumulated in incubation media as well as islet insulin contents were measured by RIA.23,24
Human islets. Islets were pre-incubated for 30 min (in Petri-dishes) in KRB (containing 0.5% BSA and 10 mM Hepes at pH 7.4) together with 1.6 mM glucose. For each experimental condition, groups of 4 or 6 islets (equal number of islets for all conditions in each experiment) were then placed in each of 5-6 parallel wells in a 24 well plate and incubated for another 60 min in KRB (0.5 ml/well) containing 1.6 mM glucose (basal secretion). The same islets were transferred into a new 24-well plate for stimulated insulin secretion by incubation for 90 min in KRB (0.5 ml/well) containing 16.7 mM glucose. Aliquots of incubation media were secured. Intracellular insulin was extracted by adding acid ethanol, as described.24 Samples were kept at -20°C pending insulin measurements by RIA kit for human insulin (cat. no. HI-14K, Millipore).
Cells. At the end of experiments INS-1 832/13 cells were cultured for 2-3 h in RPMI without glucose, supplemented with 20 mM Hepes and 1% FCS. Cells were then pre-incubated for 30 min in KRBH with 10 mM HEPES and 0.1% BSA in the absence of glucose. Final incubations were carried out in 0.5 ml of KRBH per well for 60-90 min with 3.3, 11 or 27 mM glucose (5 parallels per condition). Aliquots of media were secured for insulin assay. Intracellular insulin was extracted by adding acid ethanol (580 µl HCl in 50 ml 95% ethanol), 0.5 ml/well, for overnight at 4°C. Immunoreactive insulin was analyzed by RIA as for rat islets.23
Protein quantification
The Micro BSA™ Protein Assay Kit (cat.no. 23235, Thermo Scientific) was used for the quantification of protein in lysates from INS-1 832/13 cells and human islets. Protein content in rat islets was measured by the Bradford protein assay.
Western blotting
Rat islets. Western blotting was performed in rat islets as described.25 Islets were washed twice in ice-cold PBS and denaturated in 0.5 μl loading buffer/islet at room temperature for 20 min. Samples were analyzed on 12% SDS-PAGE gels run for 1 h at 150 V. Samples were then transferred to nitrocellulose membranes for 1 h at 250 mA. Membranes were blocked for 2 h at room temperature with 5% (w/v) fat-free milk, 0.1% Tween 20 in Tris-buffered saline, pH 7.6 and then incubated over night at 4° C with Total OXPHOS Rodent WB antibody Cocktail for oxidative phosphorylation complexes 1-4 (cat. no. MS604, Mito Sciences, diluted 1:5000). Monoclonal mouse anti-β-actin (cat. no. A5441, Sigma) was used as loading control. Secondary antibody incubations employed a HRP-linked anti-mouse antibody (cat.no. 1858413, Thermo Scientific) for 1 h at room temperature. Immunoreactive bands were visualized using chemiluminescence (ECL Western blotting reagent, cat.no. 34095, Thermo Scientific).
Human islets. Islets were washed twice in ice-cold PBS. Lysates were made by adding 1 µl lysis buffer per 5 islets. The lysis buffer contained 150 mM NaCl, 50 mM Tris-HCl (pH 7.4), protease inhibitor cocktail (Complete Mini, cat. no. 11836153001, Roche Diagnostics Gmbh), 1% Nonidet P40 (NP-40), 10% glycerol, 50 mM NaF and 1 mM Na3VO4. Samples were denatured in loading buffer for 20 min at room temperature and further analyzed as described for rat islets.
Cells. Cells were trypsinized and washed 2 times in ice-cold phosphate-buffered saline (PBS). Cells (5 × 105) were lysed on ice for 20-30 min in lysis buffer (as described above for human islets). Protein extracts were denatured in loading buffer at room temperature for 20 min. Samples were further analyzed as described for rat islets.
Oxygen consumption
INS-1 832/13 cells were seeded and cultured/handled as described above under Cell counting including a period of re-oxygenation. Oxygen consumption from these intact cells was measured by Clark-type polarographic oxygen sensors and high-resolution respirometry (Oxygraph-2k, OROBOROS, Innsbruck, Austria). Samples of 106 cells/cm3 suspended in cell culture medium were added to a chamber with magnetic stirring and allowed to equilibrate with air for 2 min before closing the chamber and recording oxygen uptake at basal respiration. Uncoupled respiration was assessed by measuring oxygen consumption after adding the ATP synthase inhibitor oligomycin (2 µg/ml). After that, the protonophore carbonyl cyanide p-trifluoromethoxyphenylhydrazone (FCCP) was added and titrated (up to 5 µM) to achieve a state of maximum respiratory capacity. Finally, rotenone (0.5 µM) and antimycin (2.5 µM), inhibitors of complex I and III, were added in order to assess residual oxygen consumption (ROX). Oxygen consumption rates were calculated as the negative time derivate of the oxygen concentration present in the chamber (pmol/s/mill cells). All values were corrected for ROX.
DNA quantification
DNA in samples (from oxygen consumption experiments in INS-1 832/13 cells) was quantified by the Fluorescent DNA Quantification kit (cat. no. 1702480, Biorad).
Statistics
Results were expressed as mean ± SEM. Significance testing was carried out using Student's t-test (paired or unpaired differences as appropriate) or Wilcoxon's rank test. A p-value < 0.05 (2-sided) was considered as significant.
Ethics
The protocols used with rat islets were approved by the Ethical Committee for Animal Research in Stockholm (Permit Number N88/11). The ethical guidelines of the Karolinska Institutet for the care and use of laboratory animals was followed. Human pancreata were obtained from brain-dead donors after verbal informed consent from relatives. Written consent is not sought, nor required according to the Health Authorities and Ethics Committees in Norway. The consent to donate was documented in the hospital record of the donor. The Regional Committee for Medical and Health Research Ethics Central in Norway approved the verbal consent procedure and the procedure of human islets and use of the tissue for research (permit no 2011/782).
Supplementary Material
Abbreviations
- CI-IV
mitochondrial complexes I-IV
- ETS
electron transfer system
- GSI
glucose stimulation index
- KRB
Krebs Ringer Bicarbonate
- LDH
lactate dehydrogenase
- MTT
3-(4,5-diethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
The assistance by Kari Slørdahl in performing RIA and the DNA quantifications is greatly acknowledged.
Funding
I.K. Hals and R. Singh are supported by the Liaison Committee between the Central Norway Regional Health Authority (RHA) and the Norwegian University of Science and Technology (NTNU). V. Grill received support from the Norwegian Diabetes Association.
References
- [1].Calabrese EJ, Baldwin LA. Defining hormesis. Hum Exp Toxicol 2002; 21:91-7; PMID:12102503; http://dx.doi.org/ 10.1191/0960327102ht217oa [DOI] [PubMed] [Google Scholar]
- [2].Stranahan AM, Mattson MP. Recruiting adaptive cellular stress responses for successful brain ageing. Nat Rev Neurosci 2012; 13:209-16; PMID:22251954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Verges S, Chacaroun S, Godin-Ribuot D, Baillieul S. Hypoxic conditioning as a new therapeutic modality. Front Pediatr 2015; 3:58; PMID:26157787; http://dx.doi.org/ 10.3389/fped.2015.00058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Shapiro AM. State of the art of clinical islet transplantation and novel protocols of immunosuppression. Curr Diab Rep 2011; 11:345-54; PMID:21830042; http://dx.doi.org/ 10.1007/s11892-011-0217-8 [DOI] [PubMed] [Google Scholar]
- [5].Deters NA, Stokes RA, Gunton JE. Islet transplantation: factors in short-term islet survival. Arch Immunol Ther Exp (Warsz) 2011; 59:421-9; PMID:21984594 [DOI] [PubMed] [Google Scholar]
- [6].Carlsson PO, Palm F, Andersson A, Liss P. Markedly decreased oxygen tension in transplanted rat pancreatic islets irrespective of the implantation site. Diabetes 2001; 50:489-95; PMID:11246867; http://dx.doi.org/ 10.2337/diabetes.50.3.489 [DOI] [PubMed] [Google Scholar]
- [7].Carlsson PO, Palm F, Mattsson G. Low revascularization of experimentally transplanted human pancreatic islets. J Clin Endocrinol Metab 2002; 87:5418-23; PMID:12466329; http://dx.doi.org/ 10.1210/jc.2002-020728 [DOI] [PubMed] [Google Scholar]
- [8].Dionne KE, Colton CK, Yarmush ML. Effect of hypoxia on insulin secretion by isolated rat and canine islets of Langerhans. Diabetes 1993; 42:12-21; PMID:8420809; http://dx.doi.org/ 10.2337/diab.42.1.12 [DOI] [PubMed] [Google Scholar]
- [9].Ma Z, Moruzzi N, Catrina SB, Hals I, Oberholzer J, Grill V, Bjorklund A. Preconditioning with associated blocking of Ca2+ inflow alleviates hypoxia-induced damage to pancreatic beta-cells. PLoS One 2013; 8:e67498; PMID:23935835; http://dx.doi.org/ 10.1371/journal.pone.0067498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Hochachka PW, Lutz PL. Mechanism, origin, and evolution of anoxia tolerance in animals. Comp Biochem Physiol B Biochem Mol Biol 2001; 130:435-59; PMID:11691622; http://dx.doi.org/ 10.1016/S1096-4959(01)00408-0 [DOI] [PubMed] [Google Scholar]
- [11].Baracca A, Sgarbi G, Padula A, Solaini G. Glucose plays a main role in human fibroblasts adaptation to hypoxia. Int J Biochem Cell Biol 2013; 45:1356-65; PMID:23538299; http://dx.doi.org/ 10.1016/j.biocel.2013.03.013 [DOI] [PubMed] [Google Scholar]
- [12].Bensellam M, Duvillie B, Rybachuk G, Laybutt DR, Magnan C, Guiot Y, Pouyssegur J, Jonas JC. Glucose-induced O(2) consumption activates hypoxia inducible factors 1 and 2 in rat insulin-secreting pancreatic beta-cells. PLoS One 2012; 7:e29807; PMID:22235342; http://dx.doi.org/ 10.1371/journal.pone.0029807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hals IK, Bruerberg SG, Ma Z, Scholz H, Bjorklund A, Grill V. Mitochondrial Respiration in Insulin-Producing beta-Cells: General Characteristics and Adaptive Effects of Hypoxia. PLoS One 2015; 10:e0138558; PMID:26401848; http://dx.doi.org/ 10.1371/journal.pone.0138558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Van de Casteele M, Kefas BA, Cai Y, Heimberg H, Scott DK, Henquin JC, Pipeleers D, Jonas JC. Prolonged culture in low glucose induces apoptosis of rat pancreatic beta-cells through induction of c-myc. Biochem Biophys Res Commun 2003; 312:937-44; PMID:14651961; http://dx.doi.org/ 10.1016/j.bbrc.2003.11.013 [DOI] [PubMed] [Google Scholar]
- [15].Elouil H, Bensellam M, Guiot Y, Vander Mierde D, Pascal SM, Schuit FC, Jonas JC. Acute nutrient regulation of the unfolded protein response and integrated stress response in cultured rat pancreatic islets. Diabetologia 2007; 50:1442-52; PMID:17497122; http://dx.doi.org/ 10.1007/s00125-007-0674-4 [DOI] [PubMed] [Google Scholar]
- [16].Bensellam M, Van Lommel L, Overbergh L, Schuit FC, Jonas JC. Cluster analysis of rat pancreatic islet gene mRNA levels after culture in low-, intermediate- and high-glucose concentrations. Diabetologia 2009; 52:463-76; PMID:19165461; http://dx.doi.org/ 10.1007/s00125-008-1245-z [DOI] [PubMed] [Google Scholar]
- [17].Sarre A, Gabrielli J, Vial G, Leverve XM, Assimacopoulos-Jeannet F. Reactive oxygen species are produced at low glucose and contribute to the activation of AMPK in insulin-secreting cells. Free Radic Biol Med 2012; 52:142-50; PMID:22064362; http://dx.doi.org/ 10.1016/j.freeradbiomed.2011.10.437 [DOI] [PubMed] [Google Scholar]
- [18].Yun J, Finkel T. Mitohormesis. Cell Metab 2014; 19:757-66; PMID:24561260; http://dx.doi.org/ 10.1016/j.cmet.2014.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Lacy PE, Kostianovsky M. Method for the isolation of intact islets of Langerhans from the rat pancreas. Diabetes 1967; 16:35-9; PMID:5333500; http://dx.doi.org/ 10.2337/diab.16.1.35 [DOI] [PubMed] [Google Scholar]
- [20].Goto M, Eich TM, Felldin M, Foss A, Kallen R, Salmela K, Tibell A, Tufveson G, Fujimori K, Engkvist M, et al.. Refinement of the automated method for human islet isolation and presentation of a closed system for in vitro islet culture. Transplantation 2004; 78:1367-75; PMID:15548977; http://dx.doi.org/ 10.1097/01.TP.0000140882.53773.DC [DOI] [PubMed] [Google Scholar]
- [21].Hohmeier HE, Mulder H, Chen G, Henkel-Rieger R, Prentki M, Newgard CB. Isolation of INS-1-derived cell lines with robust ATP-sensitive K+ channel-dependent and -independent glucose-stimulated insulin secretion. Diabetes 2000; 49:424-30; PMID:10868964; http://dx.doi.org/ 10.2337/diabetes.49.3.424 [DOI] [PubMed] [Google Scholar]
- [22].Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 1983; 65:55-63; PMID:6606682; http://dx.doi.org/ 10.1016/0022-1759(83)90303-4 [DOI] [PubMed] [Google Scholar]
- [23].Herbert V, Lau KS, Gottlieb CW, Bleicher SJ. Coated charcoal immunoassay of insulin. J Clin Endocrinol Metab 1965; 25:1375-84; PMID:5320561; http://dx.doi.org/ 10.1210/jcem-25-10-1375 [DOI] [PubMed] [Google Scholar]
- [24].Bjorklund A, Grill V. B-cell insensitivity in vitro: reversal by diazoxide entails more than one event in stimulus-secretion coupling. Endocrinology 1993; 132:1319-28; PMID:7679978 [DOI] [PubMed] [Google Scholar]
- [25].Ma Z, Portwood N, Brodin D, Grill V, Bjorklund A. Effects of diazoxide on gene expression in rat pancreatic islets are largely linked to elevated glucose and potentially serve to enhance beta-cell sensitivity. Diabetes 2007; 56:1095-106; PMID:17229937; http://dx.doi.org/ 10.2337/db06-0322 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


