Abstract
Adiponectin is an adipocyte-derived adipokine with potent antidiabetic, anti-inflammatory, and antiatherogenic activity. Long-term, high-fat diet results in gain of body weight, adiposity, further inflammatory-based cardiovascular diseases, and reduced adiponectin secretion. Vitamin A derivatives/retinoids are involved in several of these processes, which mainly take place in white adipose tissue (WAT). In this study, we examined adiponectin expression as a function of dietary high-fat and high–vitamin A conditions in mice. A decrease of adiponectin expression in addition to an up-regulation of aldehyde dehydrogenase A1 (ALDH1A1), retinoid signaling, and retinoic acid response element signaling was selectively observed in WAT of mice fed a normal–vitamin A, high-fat diet. Reduced adiponectin expression in WAT was also observed in mice fed a high–vitamin A diet. Adipocyte cell culture revealed that endogenous and synthetic retinoic acid receptor (RAR)α- and RARγ-selective agonists, as well as a synthetic retinoid X receptor agonist, efficiently reduced adiponectin expression, whereas ALDH1A1 expression only increased with RAR agonists. We conclude that reduced adiponectin expression under high-fat dietary conditions is dependent on 1) increased ALDH1A1 expression in adipocytes, which does not increase all-trans-retinoic acid levels; 2) further RAR ligand–induced, WAT-selective, increased retinoic acid response element–mediated signaling; and 3) RAR ligand–dependent reduction of adiponectin expression.—Landrier, J.-F., Kasiri, E., Karkeni, E., Mihály, J., Béke, G., Weiss, K., Lucas, R., Aydemir, G., Salles, J., Walrand, S., de Lera, A. R., Rühl, R. Reduced adiponectin expression after high-fat diet is associated with selective up-regulation of ALDH1A1 and further retinoic acid receptor signaling in adipose tissue.
Keywords: vitamin A, nuclear hormone receptor, obesity, diabetes, retinaldehyde dehydrogenase
Obesity is considered to be one of the most common nutritional disorders of Western society and is characterized by a disproportionate expansion of body fat mass [reviewed in Gasbarrini and Piscaglia (1)]. In addition to being an energy storage site, white adipose tissue (WAT) also functions as a highly active metabolic regulator and major endocrine organ that secretes various adipokines (2–4). Adiponectin is a major adipokine with strong antidiabetic, anti-inflammatory, and antiatherogenic activity, and its expression is decreased in WAT under high dietary fat conditions [reviewed in Ouchi et al. (5)]. With the exception of the putative role of inflammation (6–8), the precise mechanisms that mediate this down-regulation remain to be elucidated.
Retinoids are important regulators of adipogenesis. Diets high in vitamin A (9), dietary excess of retinoic acid (10, 11), and diets high in β-carotene (12–14) result in increased adipocyte apoptosis and inhibition of adipogenesis, while low concentrations of retinoic acid were described to be proadipogenic (reviewed in refs. 9, 11, 15, 16). Retinoids, that is, naturally occurring and synthetic retinol analogues (reviewed in refs. 17, 18), are responsible for activation of specific nuclear receptors: the retinoic acid receptor (RAR) and the retinoid X receptor (RXR). Bioactive retinoic acids are formed from precursor retinaldehydes by the action of retinaldehyde dehydrogenase enzymes (RALDHs/ALDH1A) (19).
RALDH1/ALDH1A1–null adult mice have been shown to be resistant to high-fat (HF) diet–induced weight gain (20, 21), which suggested that ALDH1A1 and its metabolic products are necessary for HF diet–induced obesity (22–24). ALDH1A1 can synthesize retinoic acids (25), such as all-trans-retinoic acid (ATRA), 9-cis-retinoic acid, and, presumably, the newly found endogenous RXR ligand, 9-cis-13,14-dihydroretinoic acid (26). The last two are ligands for both RXRs and RARs, whereas ATRA only binds RAR. Unfortunately, only a few studies have detected retinoic acids in low concentrations in adipose tissue (27, 28), but no study has addressed the presence of retinoic acids in WAT when comparing ALDH1A1+/+ or ALDH1A1−/− mice. Whether retinoic acids, and which retinoic acids, are the major metabolites of ALDH1A1 in WAT is yet unknown. Moreover, ALDH1A1 expression has been shown to be regulated by liver X receptor (LXR) (29) as well as estrogen receptor–mediated pathways (30, 31).
Various nuclear hormone receptor pathways are involved in adipokine secretion and adipocyte differentiation, proliferation, and lipid accumulation (reviewed in refs. 7, 32). In particular, RXRs, the central heterodimer-forming partners, play important roles during obesity (33–35). RXRα as well as RXRγ KO and RXR-antagonist treatment induce resistance to weight gain after HF diet and also promote a higher metabolic rate (36–38). RXRs can also interact with several nuclear receptors, such as RAR, LXR, peroxisome proliferator-activated receptor (PPAR), vitamin D receptor (VDR), or NR4A-orphan nuclear receptors (39, 40), and the activation of various so-called permissive heterodimers (RXR-PPAR, -LXR, -VDR, and -NR4A1/2) by an RXR ligand can initiate heterodimer-mediated signaling (39–41).
The aim of our study was to find out how HF diet reduces adiponectin expression in WAT, focusing primarily on vitamin A–mediated RAR- and RXR-dependent pathways.
MATERIALS AND METHODS
Experimental diets
Manually prepared diets were made with wheat starch (Weizenstärke, Foodstar, Germany; provided by Kröner-Stärke, Ibbenbüren, Germany), saccharose (purchased from a local supermarket in Hungary), casein (Sigma-Aldrich, Budapest, Hungary), cellulose (Vivapur; JRS Pharma GmbH; Rosenberg, Germany), vitamin mix (Vitamin-Vormischung C1000; Altromin GmbH, Lage, Germany), mineral mixture (Mineral-Spurenelemente-Vormischung C100; Altromin GmbH), and sunflower oil (Henry Lamotte, Bremen, Germany).
Animal experiments
Animal experiments were performed in the Laboratory Animal Core Facility of the University of Debrecen. Experiments were performed according to Hungarian ethical guidelines.
Experiment with low-, normal-, or high-fat dietary supplementation
After the acclimatization period, animals received a vitamin A–deficient [0 retinol equivalent (RE)/kg diet] diet for 10 wk that contained 5% sunflower oil as a dietary lipid, which represented a diet with normal fat (NF) content (42). Animals were divided into different feeding groups (n = 6 per group) and were fed for 4 wk with specific diets that contained different amounts of dietary fat and equal amounts of vitamin A (2500 RE/kg diet, normal vitamin A). Sunflower oil was added as dietary fat, which contained either 2% [as weight %; low-fat (LF) diet], 5% (NF diet), or 25% (HF diet). The source of the fat was always sunflower oil in different proportions added to feed. On the basis of the analyzed feed of the NF diet, it contained 11.6% saturated fats, 20% monounsaturated fatty acids, and 68.4% polyunsaturated fatty acids (Weiss et al., in preparation). Furthermore, dietary composition was 180 g/kg casein, 10 g/kg vitamin mix, 45 g/kg mineral mix, and 20 g/kg cellulose for all applied diets (42). As a result of the increased amount of fat in the diet, the carbohydrate proportion was lower; the LF diet contained 29.5% sucrose and 43% starch, the NF diet 28% sucrose and 41.5% starch, and the HF diet 17% sucrose and 32.5% starch (42).
Experiment with normal– or high–vitamin A dietary supplementation
For vitamin content, diets were supplemented with vitamin mix (Vitamin-Vormischung C1000) that contained either 2500 RE/kg as normal–vitamin A diet, or for high–vitamin A diet, an additional retinyl-palmitate (RetPal) supplement (final 326,500 RE/kg; Sigma-Aldrich) was added to the normal–vitamin A diet (42, 43).
After euthanizing mice, blood collection was carried out by cardiac puncture. Blood was centrifuged for 20 min and plasma was stored at −80°C. Mice were anatomized, and WAT samples were immediately frozen in liquid nitrogen after dissection and later stored at −80°C until RNA extraction.
Bioimaging
Retinoic acid response element (RARE)-Luc female mice (n = 6) were obtained from Cgene (Oslo, Norway) and received LF, NF, or HF diets for 4 wk or the oral retinoid treatments as described before (43, 44).
We conducted ex vivo organ analysis by bioluminescence imaging. All animals were treated with 120 mg/kg d-luciferin (Bioscience, Budapest, Hungary) via intraperitoneal injections 15 min before euthanasia and further organ screening. Mice were euthanized by cervical dislocation. After euthanasia, mouse liver, WAT, intestine, and brain were collected for bioluminescence imaging. Organs were analyzed for bioluminescence signal by using an Andor-Ixon CCD camera (Belfast, United Kingdom), and analysis was performed by Andor-IQ software. After imaging, integrated intensity/area was calculated for liver, WAT, intestine, and brain of each treated animal.
Cell culture
3T3-L1 preadipocytes (American Type Culture Collection, Manassas, VA, USA) were seeded in 3.5-cm-diameter dishes at a density of 15 × 104 cells/well. Cells were grown in DMEM that was supplemented with 10% FBS at 37°C in a 5% CO2 humidified atmosphere, as previously reported (45, 46). To induce differentiation, 2-d postconfluent 3T3-L1 preadipocytes (day 0) were stimulated for 48 h with 0.5 mM isobutylmethylxanthine, 0.25 µM dexamethasone, and 1 µg/ml insulin in DMEM that was supplemented with 10% FBS. Cells were then maintained in DMEM that was supplemented with 10% FBS and 1 µg/ml insulin (47). To examine the effects on gene expression of ATRA (a gift from BASF AG, Ludwigshafen, Germany), an RARα agonist (BMS753), an RARγ agonist (BMS189961; both were prepared in our laboratories as described in the original patents (48, 49)], and an RXR agonist (LG268; gift from Ligand Pharmaceuticals, San Diego, CA, USA), 3T3-L1 adipocytes were incubated with 1 µM of these molecules for 24 h, as previously reported (47). Data presented are the mean of 3 independent experiments each performed in triplicate.
Human adipose biopsies
Eleven lean (body mass index: 22.5 ± 0.5 kg/m2) and 14 obese (body mass index: 31.7 ± 0.9 kg/m2) male participants were recruited, as previously reported (50). Lean and obese volunteers were age 44 ± 7 and 44 ± 5 yr, respectively. Subcutaneous adipose tissue biopsies were performed between 6:30 and 7:30 am after an overnight fast. Biopsies were obtained by needle aspiration in the periumbilical area under local anesthesia. Adipose tissue samples were rinsed in physiologic serum, immediately frozen in liquid nitrogen, and stored at −80°C until RNA extraction. The experimental protocol was performed in accordance with the guidelines in the Declaration of Helsinki and was approved by the Ethical Committee of the Auvergne Region (agreement No. AU 800, March 2010). Participants gave their written informed consent to participate in the study.
Analysis of mRNA expression
Analysis of total cellular RNA extracted from 3T3-L1 cells was performed in France by using Trizol reagent according to manufacturer instructions. Human adipose tissue sample extraction was also performed in France, whereas WAT and liver sample analysis from mice was done in Hungary.
For the cell culture material in the French laboratory, cDNA was synthesized from 1 µg of total RNA in 20 µl by using random primers and Moloney murine leukemia virus reverse transcriptase. Real-time quantitative RT-PCR analyses for genes were performed by using the Mx3005P Real-Time PCR System (Stratagene, La Jolla, CA, USA) as previously described (51). For each sample, expression was quantified in duplicate and 18S rRNA was used as the endogenous control in the comparative cycle threshold (CT) method.
For WAT and liver tissue analysis of human and murine origin, tissues were homogenized in Tri reagent solution (Thermo Fisher Scientific, Waltham, MA, USA) and total RNA was isolated from tissue according to manufacturer guidelines and as previously described (52). Concentration and purity of RNA was measured by using the NanoDrop spectrophotometer (Thermo Fisher Scientific).
For real-time quantitative PCR, total RNA was reverse transcribed into cDNA by using the Super Script II First-Strand Synthesis System (Thermo Fisher Scientific). Quantitative real-time PCR was carried out in triplicate using predesigned MGB assays (Thermo Fisher Scientific) on an ABI Prism 7900 (Applied Biosystems, Villebon-sur-Yvette, France). Relative mRNA levels were calculated by using the Ct method and were normalized to cyclophilin A mRNA. Sequence Detector Software (v. 2.1; Applied Biosystems) was used for data analysis.
Analytical procedures
WAT samples were collected and stored in dark vials at −80°C until analysis. Sample preparation was performed as previously described for retinoid (53) and eicosanoid/docosanoid (54) analysis. HPLC–tandem mass spectrometry analyses for retinoids as well as eicosanoids and docosanoids, which focused on eicosanoids with known PPAR activation potential, were also performed as previously explained (53, 54).
ELISA assays
To examine the effect of retinoids on adiponectin secretion, 3T3-L1 adipocytes were incubated with 1 µM of the retinoids (ATRA, RARα, RARγ, or RXR ligand) for 48 h. Adiponectin quantification was realized on the culture supernatant by using adiponectin ELISA assay according to manufacturer protocol (Quantikine ELISA; R&D Systems, Lille, France).
Statistics
Data are expressed as means ± sem. Significant differences between control and treated cells/groups were determined by Student’s t test using Statview software (SAS Institute, Cary, NC, USA). Values of P < 0.05 were considered significant.
RESULTS
Effects of HF diet on body weight gain
Body weight gain was observed in animals after 4 wk of HF diet compared with LF or NF dietary supplementation (LF: 1.07 ± 0.08 g; NF: 0.92 ± 0.11 g; HF: 3.24 ± 0.37 g; LF-HF P = 0.03 and NF-HF P = 0.04). Food intake slightly decreased in the HF diet group (LF: 2.93 g/d/animal; NF: 2.70 g/d/animal; HF: 2.25 g/d/animal).
HF dietary supplementation results in up-regulation of ALDH1A1 and down-regulation of adiponectin expression
ALDH1A1 was significantly increased only in WAT (LF: 1 ± 0.80; NF: 1.83 ± 0.36; HF: 5.26 ± 0.23) of HF diet–fed mice compared with LF and NF diet–fed mice, and was tissue selective for WAT (Table 1, WAT), compared with unchanged expression in the liver (Table 1, liver). ALDH1A2 expression remained unchanged in liver and WAT (Table 1). ALDH1A3 was also significantly increased in adipose tissue of animals fed HF and NF diets compared with LF diet (Table 1, WAT). In addition, expression of RAR pathway target genes, such as CYP26A1 and CYP26B1, remained unchanged (Table 2), whereas expression of the highly sensitive common RAR/RXR pathway target gene transglutaminase 2 (TG2) was strongly increased (Table 1; LF: 1 ± 0.36; NF: 9.56 ± 0.13; HF: 15.36 ± 0.17) in HF diet–fed mice.
TABLE 1.
Relative adiponectin and ALDH1A1 mRNA expression
| Gene | Fold activation |
Significance |
||||
|---|---|---|---|---|---|---|
| LF | NF | HF | LF:NF | NF:HF | LF:HF | |
| WAT | ||||||
| ALDH1A1 | 1 ± 0.80 | 1.83 ± 0.36 | 5.26 ± 0.23 | 0.46 | 0.05 | 0.01 |
| ALDH1A2 | 1 ± 0.11 | 1.05 ± 0.14 | 1.09 ± 0.19 | 0.77 | 0.87 | 0.69 |
| ALDH1A3 | 1 ± 0.17 | 1.65 ± 0.08 | 2.59 ± 0.24 | 0.03 | 0.12 | 0.02 |
| Adiponectin | 1 ± 0.31 | 0.86 ± 0.12 | 0.15 ± 0.37 | 0.72 | <0.01 | 0.04 |
| Liver | ||||||
| ALDH1A1 | 1 ± 0.10 | 1.10 ± 0.11 | 1.39 ± 0.13 | 0.53 | 0.64 | 0.09 |
| ALDH1A2 | 1 ± 0.12 | 0.79 ± 0.09 | 0.74 ± 0.10 | 0.17 | 0.64 | 0.09 |
Expression shown in WAT and liver of LF (set as 1), NF, and HF diet–fed mice with a normal content of vitamin A in the diet. Gene expression (all n = 6) of adiponectin and retinoic acid synthesizing enzymes (ALDH1A1, ALDH1A2, ALDH1A3). Significant values vs. LF are in italics.
TABLE 2.
Relative gene expression of genes involved in RAR and PPAR signaling in mouse WAT
| Gene | Fold activation |
Significance |
||||
|---|---|---|---|---|---|---|
| LF | NF | HF | LF:NF | NF:HF | LF:HF | |
| RAR pathway | ||||||
| CYP26A1 | 1 ± 0.50 | 0.16 ± 0.41 | 0.43 ± 0.49 | 0.13 | 0.26 | 0.33 |
| CYP26B1 | 1 ± 0.63 | 0.58 ± 0.52 | 0.93 ± 0.66 | 0.60 | 0.65 | 0.94 |
| TG2 | 1 ± 0.36 | 9.56 ± 0.13 | 15.36 ± 0.17 | <0.01 | 0.08 | <0.01 |
| PPAR pathway | ||||||
| PPARγ | 1 ± 0.12 | 1.23 ± 0.09 | 0.93 ± 0.09 | 0.18 | 0.07 | 0.67 |
| RETSAT | 1 ± 0.21 | 1.96 ± 0.23 | 1.38 ± 0.26 | 0.09 | 0.37 | 0.37 |
| FABP4 | 1 ± 0.03 | 1.00 ± 0.10 | 1.08 ± 0.10 | 0.99 | 0.57 | 0.47 |
| FADS2 | 1 ± 0.43 | 1.31 ± 0.41 | 1.46 ± 0.48 | 0.71 | 0.88 | 0.64 |
Expression in WAT of LF (set as 1), NF, and HF diet–fed mice with a normal content of vitamin A in diet (all n = 6). Significant values vs. LF are in italics.
In addition to increased retinoid signaling, expression of adiponectin was reduced in HF diet–fed mice (LF: 1 ± 0.31; NF: 0.86 ± 0.12; HF: 0.15 ± 0.37).
Increased ALDH1A1 and reduced adiponectin expression in obese volunteers
Experiments using adipose tissue biopsies from normal-weight and obese human volunteers confirmed increased ALDH1A1 (healthy volunteers were set as 1; 1.20 ± 0.07) and reduced adiponectin (0.85 ± 0.04) expression in the obese volunteers (Table 3).
TABLE 3.
Relative expression from adiponectin and ALDH1A1 in human WAT
| Gene | Fold activation |
Significance | |
|---|---|---|---|
| NV, n = 20 | OB, n = 26 | ||
| ALDH1A1 | 1.00 ± 0.08 | 1.20 ± 0.07 | 0.03 |
| Adiponectin | 1.00 ± 0.04 | 0.85 ± 0.04 | 0.01 |
Expression in WAT of obese (OB) and normal volunteers (NV). Significant values vs. NV are in italics. NV was calculated to be set as 1.
High–vitamin A dietary supplementation results in increased expression of ALDH1A1 and reduced adiponectin expression
Expression of ALDH1A1 increased (NF, normal vitamin A was set as 1: 1 ± 0.19; NF, high vitamin A: 2.32 ± 0.47) in the WAT of mice fed an NF diet with high vitamin A supplementation, whereas adiponectin expression (NF, normal vitamin A was set as 1: 1 ± 0.47; NF, high vitamin A: 0.37 ± 0.21) was decreased in WAT (Table 4).
TABLE 4.
Relative adiponectin and ALDH1A1 mRNA expression levels in WAT depending on vitamin A
| Gene | Fold activation |
Significance | |
|---|---|---|---|
| Normal vitamin A | High vitamin A | ||
| ALDH1A1 | 1 ± 0.19 | 2.32 ± 0.47 | 0.05 |
| Adiponectin | 1 ± 0.47 | 0.37 ± 0.21 | 0.02 |
Expression in WAT of mice fed an NF diet with normal or high vitamin A supplementation (set as 1; n = 6). Significant values vs. NF, normal vitamin A are in italics.
Decreased retinoic acid concentrations present in WAT of animals fed an HF diet do not correspond to increased RARE-mediated signaling in RARE-Luc mice, and PPARγ ligands remain mainly unchanged
Retinol levels remained stable in the WAT of LF, NF, and HF diet–fed animals, whereas ATRA (LF: 2.2 ± 0.1 ng/g; NF: 1.7 ± 0.2 ng/g; HF: 0.6 ± 0.1 ng/g) levels were lower in the WAT of HF diet–fed animals (Table 5).
TABLE 5.
HPLC–tandem mass spectrometry analysis of retinoids and eicosanoids in WAT
| Compound | Level (ng/g) |
Significance |
||||
|---|---|---|---|---|---|---|
| LF | NF | HF | LF:NF | NF:HF | LF:HF | |
| Retinoid | ||||||
| ATRA | 2.2 ± 0.1 | 1.7 ± 0.2 | 0.6 ± 0.1 | 0.19 | 0.01 | <0.01 |
| ROL | 1461 ± 97 | 1591 ± 94 | 1520 ± 50 | 0.35 | 0.38 | 0.41 |
| Eicosanoid | ||||||
| 13-HODE | 557 ± 46 | 605 ± 79 | 803 ± 83 | 0.40 | 0.21 | 0.11 |
| 9-HODE | 186 ± 17 | 157 ± 18 | 211 ± 27 | 0.29 | 0.22 | 0.35 |
| 13-KODE | 228 ± 18 | 674 ± 266 | 433 ± 95 | 0.22 | 0.34 | 0.16 |
| 12-KETE | 10.3 ± 2.4 | 7.6 ± 1.1 | 19.6 ± 4.6 | 0.31 | 0.12 | 0.20 |
| PgJ2 | 0.2 ± 0.0 | 0.4 ± 0.0 | 0.2 ± 0.2 | 0.08 | 0.09 | 0.44 |
| d15d12PgJ2 | UQL | UQL | UQL | |||
| HXB3 | 0.5 ± 0.2 | 2.2 ± 0.5 | 5.3 ± 1.1 | 0.08 | 0.13 | 0.03 |
Analysis of retinoids, ATRA and retinol, as well as the endogenous relevant PPAR ligands, 13-HODE, 9-HODE, 13-ketooctadecadienoic acid (KODE), 12-ketoeicosatetraenoic acid (KETE), PgJ2, d15d12PgJ2, and hepoxilin B3 (HXB3); all in ng/g ± sem of WAT samples from LF, NF, and HF diet–fed mice with a normal content of vitamin A in diet (all n = 4). Significant values vs. LF are in italics. ROL, retinol; UQL, under the quantification limit.
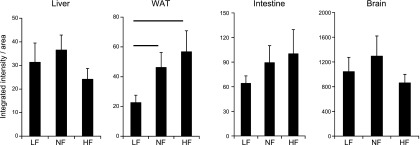
Increased retinoid signaling was confirmed in RARE-Luc mice, with increased RARE-mediated signaling detected specifically in adipose tissue of HF compared with LF and NF diet–fed animals, whereas in liver, intestine, and brain, no increased RARE-mediated signaling was observed (Fig. 1).
Figure 1.
Integrated intensity areas of bioluminescence imaging of various organs of RARE-LUC mice (n = 6) that were fed LF, NF, and HF diets, with normal vitamin A content in the diet. The line over the bars indicates statistical significance.
Endogenous PPAR ligands [9-hydroxyoctadecadienoic acid (9-HODE), 13-HODE, 13-ketooctadecadienoic acid, 12-ketoeicosatetraenoic acid, PgJ2, and d15d12PgJ2] were mainly unchanged, except the adipose tissue–specific PPARγ ligand, hepoxilin B3, which is increased in adipose tissue of HF diet–fed animals (Table 5).
NF and HF dietary supplementation does not result in altered PPARγ-mediated signaling
Expression of PPARγ and PPARγ target genes retinol saturase (RETSAT)/fatty acid binding protein 4 (FABP4)/FADS2 in mouse WAT remained unaffected by NF and HF dietary supplementation compared with LF diet (Table 2).
Adiponectin expression is reduced by RAR and RXR agonists using 3T3-L1 adipocytes cell culture
Treatment of cultured adipocytes with synthetic RARα-selective ligands [control (CTRL) set as 1; adiponectin: 0.22 ± 0.01 and ALDH1A1 2.37 ± 0.04], RARγ-selective ligands (CTRL set as 1; adiponectin: 0.22 ± 0.01 and ALDH1A1 2.64 ± 0.01), and the natural RAR ligand ATRA (CTRL set as 1; adiponectin: 0.25 ± 0.03 and ALDH1A1 3.19 ± 0.07), in addition to a synthetic RXR agonist (LG268; CTRL set as 1; adiponectin: 0.51 ± 0.04 and ALDH1A1 1.04 ± 0.04), resulted in increased ALDH1A1 expression for RAR agonists, whereas adiponectin expression was reduced for all RAR and RXR ligands. In addition, these results were confirmed at the protein level in cell culture supernatants where adiponectin secretion was reduced for all administered RAR and RXR ligands, except for the RARγ-selective ligand, which displayed a nonsignificant decrease (Table 6).
TABLE 6.
Relative adiponectin concentrations and relative adiponectin and ALDH1A1 mRNA expression in 3T3-adipocytes
| Adiponectin | Significance | |||||
|---|---|---|---|---|---|---|
| Retinoid | ELISA | REX | ALDH1A1 REX | ELISA | Adiponectin REX | ALDH1A1 REX |
| ATRA | 0.57 ± 0.14 | 0.25 ± 0.03 | 3.19 ± 0.07 | 0.02 | <0.01 | <0.01 |
| RARα-LIG | 0.67 ± 0.04 | 0.22 ± 0.01 | 2.37 ± 0.04 | 0.05 | <0.01 | 0.01 |
| RARγ-LIG | 0.81 ± 0.04 | 0.22 ± 0.01 | 2.64 ± 0.01 | 0.20 | <0.01 | <0.01 |
| RXR-LIG | 0.64 ± 0.04 | 0.51 ± 0.04 | 1.04 ± 0.04 | 0.05 | 0.03 | 0.14 |
Expression after 24 h in cultured 3T3-L1 adipocytes with ATRA (1 μM), an RARα-specific agonist BMS753/RARα-LIG (1 μM), an RARγ-specific agonist BMS189961/RARγ-LIG (1 μM), and an RXR ligand RXR-LIG/LG268 (1 μM) calculated with CTRL treatments set as 1. Significance and sem are based on n = 6 parallel treatments. Significant values vs. CTRL are in italics. LIG, ligand. REX, relative expression.
DISCUSSION
Obesity is classically associated with a decrease of adiponectin plasma level in humans and rodents, as well as a decreased expression in adipose tissue (5). This relationship between obesity and decreased adiponectin expression is suspected to be linked to the increased inflammatory status of adipose tissue, as TNF-α, one of the main inflammatory markers produced by adipose tissue (55), is known to reduce adiponectin expression (56). However, this mechanism is probably not exclusive, and other pathways—RAR signaling among them—could be involved in this regulation.
In this study, we reported that, in mice, reduced adiponectin expression in WAT after HF dietary supplementation was associated with an increase of ALDH1A1 expression. Similar results were also obtained by comparing lean vs. obese WAT biopsies. Surprisingly, increased ALDH1A1 expression in mice does not result in increased ATRA levels in WAT.
ALDH1A1, the major enzyme for retinoic acid synthesis using retinaldehyde as a substrate, is highly likely to play an important role in the relationship between retinoid signaling and obesity (20, 21, 57). Indeed, its expression is increased in WAT during HF-induced obesity (58). In ALDH1A1−/−-deficient adipocytes as well as in ALDH1A1−/− mice, adipogenesis is impaired and mice are resistant to HF diet–induced obesity (20), which is suggested to be related to altered retinoid signaling [(25) and reviewed in refs. 9, 11, 15]. Retinoic acids, the products from ALDH1A1 metabolism, are the endogenous activators of RARs and RXRs. Reduced retinaldehyde and retinol levels were measured in adipose tissue of animals fed an HF diet, and ATRA levels were speculated to be increased upon ALDH1A1 activity (20). However, the detection and quantification of retinoic acid levels in adipose tissue have been scarcely examined (27, 28) and, unfortunately, the connection of retinoic acids in response to ALDH1A1 expression in adipose tissue has not been studied before. In the present study, we report that increased ALDH1A1 expression in mice does not result in increased ATRA levels in WAT. On the contrary, ATRA levels were even lower in WAT of HF vs. LF or NF diet–fed animals. Similar reduced levels of ATRA were confirmed in serum and adipose tissue of obese volunteers compared with obese volunteers after a weight-loss diet (unpublished data), which indicates that obesity is related to reduced local and systemic retinoid levels in humans. These findings of reduced local retinoid levels in adipose tissue of obese animals fit well with previous studies on animals fed a vitamin A–deficient diet, which were found to become obese upon reduced ATRA synthesis, levels, and ATRA-mediated signaling [reviewed in Bonet et al. (11)]. In addition, it is well established that retinoids, and especially ATRA, as signaling ligands, have the ability to inhibit proliferation of adipocytes; enhance up-regulation of genes involved in lipid oxidation, energy dissipation, and insulin response; and thereby prevent obesity and insulin resistance [reviewed in Bonet et al. (11)], probably by targeting adipocyte oxidative phosphorylation and mitochondriobiogenesis (59).
As a result of this unclear evidence and inconclusive determination of retinoic acids levels in WAT, we opted, like others [(21, 57) plus follow-up reviews in refs. 15, 22], for an indirect method of detection of retinoid signaling by using a RARE-reporter mouse model (44) and we confirmed increased WAT-selective, RARE-mediated signaling in the WAT of HF vs. LF diet–fed mice (57). Previous experimental studies claimed, without any analytical proof, the involvement of ALDH1A1-synthesized ATRA in adipose tissue and, only on the basis of increased RARE signaling (21, 57), that ATRA is the metabolite of ALDH1A1 in adipose tissue and that the described effects of ALDH1A1, by consequence, are mediated by ATRA-RAR signaling. Furthermore, they claimed that the ALDH1A1 product ATRA must be involved in the ALDH1A1-mediated increase of adipose tissue expansion and diet-induced obesity. Our solid data, generated by using HPLC–tandem mass spectrometry quantification of ATRA in adipose tissue, contradicts these claims and warns about the common obtainment of false-positive data from RARE-Luc activation models (60).
We concluded that either a still-uncharacterized endogenous RAR ligand must be synthesized in the WAT of HF diet–fed mice to induce WAT-selective, RARE-mediated signaling or alternative mechanisms that possibly involve transporter protein–mediated signaling (reviewed in ref. 61 and speculated upon in ref. 62) or post-translational modifications in adipocytes [(21) and reviewed in ref. 63] must be taken into consideration. With regard to ligands other than ATRA, it is still unknown which RAR- and/or RXR-activating ligands could be synthesized upon ALDH1A1 expression in WAT, and we could not conclusively suggest a possible structure using our current analytical expertise (26, 53). However, several known and unknown candidates, including 9-cis- and all-trans-13,14-dihydroretinoic acid, retinal, apo-lycopenoic acids, apo-13′-carotenone, apo-10′-carotenoic acid, and apo-14′-carotenoic acid (20, 26, 44, 47, 64–72), were recently identified and could constitute potential endogenous retinoids.
To further exclude the involvement of PPARγ, the key regulator of adipogenesis (7, 73), as a major nuclear receptor responsible for adiponectin reduction after an HF-supplemented diet, endogenous PPAR ligands were determined. Levels in adipose tissue were mainly unaltered after LF, NF, or HF dietary supplementation (74–77). Only the levels of the endogenous and adipose tissue–specific PPARγ ligand, hepoxilin B3, (78, 79) were significantly increased in HF vs. LF diet–fed animals. In addition, our data show no increased expression of PPARγ and known PPARγ target genes, RETSAT, FABP4, and FADS2, in the WAT of HF diet–fed mice, which, in part, contrasts with previous studies. In general, increased PPARγ expression in adipose tissue after HF diet is mainly related to omental and not subcutaneous fat in humans, as reviewed in (80). In mice, increased PPARγ expression is observable just after diets with extreme HF conditions, after strong weight gain, and after a long time of HF dietary supplementation (<8 wk) (81–84). Finally, it is well established that PPARγ signaling activation increases secretion of adiponectin rather than decreases it (85). In summary, all these data strongly imply that PPARγ-mediated signaling in adipose tissue of animals fed an HF-supplemented diet is unlikely to be of major importance for further reduced adiponectin expression.
To evaluate RAR- and RXR-mediated signaling pathways in adipocytes directly, 3T3-L1 adipocyte cell culture models were used, and we determined that ALDH1A1 was increased after administration of ATRA and synthetic RARα- and RARγ-selective RAR ligands, and not by a synthetic RXR ligand, whereas adiponectin expression and secretion in the cell supernatant were decreased after administration of RAR or RXR agonists. We conclude, therefore, that this direct down-regulation of adiponectin is an RAR- or RXR-mediated pathway and that ALDH1A1 expression is just regulated by an RAR ligand.
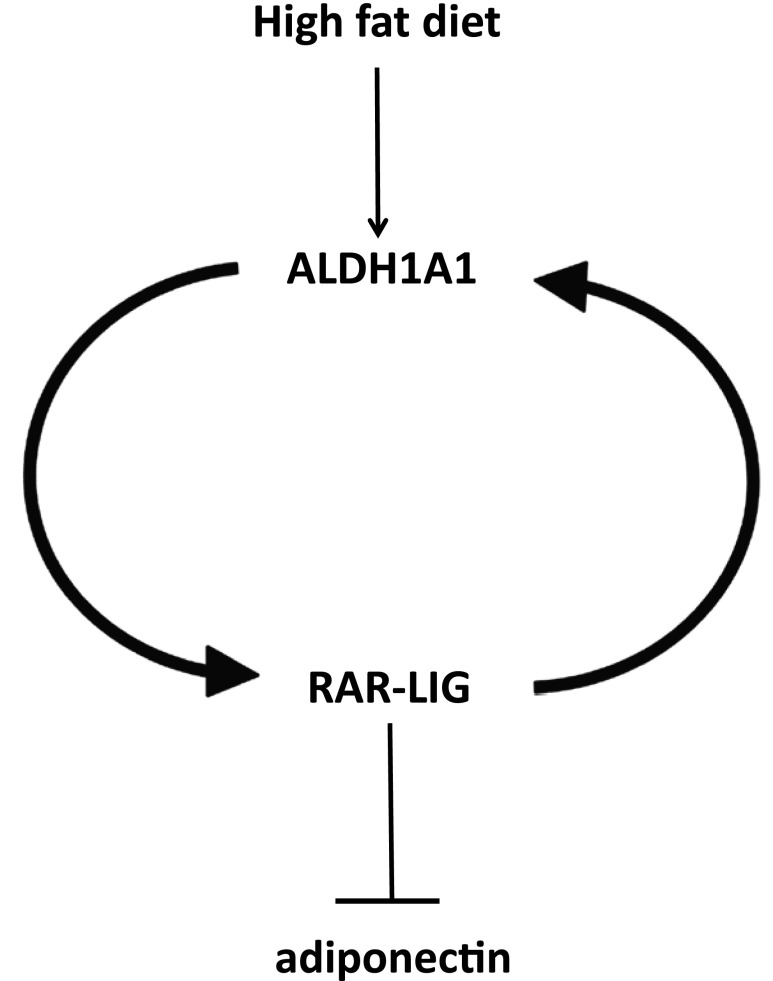
In summary (Fig. 2), we found that reduced adiponectin expression in the WAT of mice is under the control of retinoid-mediated signaling, mainly via RAR-mediated signaling pathways. We suggest that altered retinoid signaling in adipose tissue is an important mechanism of HF diet–induced obesity. In particular, ALDH1A1 seems to be the key enzyme that is responsible for the synthesis of alternative endogenous RAR ligands selectively in WAT. This increased ALDH1A1 and reduced adiponectin expression was also confirmed to occur in adipose tissue from obese human volunteers. Endogenous as well as synthetic RAR ligands were shown to further directly inhibit adiponectin expression in cultured adipocytes. The nature of the endogenous RAR/RXR agonists or antagonists synthesized by ALDH1A1 in WAT remains elusive and is the topic of future studies. Characterization of these novel endogenous retinoids with mainly RAR, as well as potential RXR, ligand activation potential and their metabolic pathways can help clarify the controversy of the altered retinoid signaling in adipose tissue. On the basis of these data, novel strategies can be developed to selectively inhibit distinct retinoid signaling, especially that which involves ALDH1A1 products under HF diet, focused on adipose tissue to enable sufficient beneficial adiponectin expression.
Figure 2.
Simplified scheme showing how HF diet induces ALDH1A1 expression, increased RAR ligand (RAR-LIG), and reduced adiponectin expression selectively in WAT.
Glossary
- 9-HODE
9-hydroxyoctadecadienoic acid
- ALDH1A1
aldehyde dehydrogenase 1A1
- ATRA
all-trans-retinoic acid
- CTRL
control
- FABP4
fatty acid binding protein 4
- HF
high fat
- LF
low fat
- LXR
liver X receptor
- NF
normal fat
- PPAR
peroxisome proliferator-activated receptor
- RALDH
retinaldehyde dehydrogenase
- RAR
retinoic acid receptor
- RARE
retinoic acid response element
- RE
retinol equivalent
- RETSAT
all-trans-retinol 13,14-reductase
- RXR
retinoid X receptor
- TG2
transglutaminase 2
- VDR
vitamin D receptor
- WAT
white adipose tissue
AUTHOR CONTRIBUTIONS
R. Rühl and J.-F. Landrier designed the experiments; E. Kasiri, E. Karkeni, J. Mihály, G. Béke, K. Weiss, R. Lucas, G. Aydemir, J. Salles, and S. Walrand performed the experiments; E. Karkeni, J. Mihály, G. Béke, and G. Aydemir analyzed the data; and A. R. de Lera provided reagents.
REFERENCES
- 1.Gasbarrini A., Piscaglia A. C. (2005) A natural diet versus modern Western diets? A new approach to prevent “well-being syndromes.” Dig. Dis. Sci. 50, 1–6 [DOI] [PubMed] [Google Scholar]
- 2.Ahima R. S. (2006) Adipose tissue as an endocrine organ. Obesity (Silver Spring) 14, 242S–249S [DOI] [PubMed] [Google Scholar]
- 3.Sakurai T., Ogasawara J., Kizaki T., Ishibashi Y., Sumitani Y., Takahashi K., Ishida H., Miyazaki H., Saitoh D., Haga S., Izawa T., Ohno H. (2012) Preventive and improvement effects of exercise training and supplement intake in white adipose tissues on obesity and lifestyle-related diseases. Environ. Health Prev. Med. 17, 348–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maury E., Brichard S. M. (2010) Adipokine dysregulation, adipose tissue inflammation and metabolic syndrome. Mol. Cell. Endocrinol. 314, 1–16 [DOI] [PubMed] [Google Scholar]
- 5.Ouchi N., Parker J. L., Lugus J. J., Walsh K. (2011) Adipokines in inflammation and metabolic disease. Nat. Rev. Immunol. 11, 85–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karki S., Chakrabarti P., Huang G., Wang H., Farmer S. R., Kandror K. V. (2011) The multi-level action of fatty acids on adiponectin production by fat cells. PLoS One 6, e28146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iwaki M., Matsuda M., Maeda N., Funahashi T., Matsuzawa Y., Makishima M., Shimomura I. (2003) Induction of adiponectin, a fat-derived antidiabetic and antiatherogenic factor, by nuclear receptors. Diabetes 52, 1655–1663 [DOI] [PubMed] [Google Scholar]
- 8.Lagishetty V., Nandiwada V. B., Kalashikam R. R., Manchala R. (2007) Effect of maternal vitamin and mineral restrictions on the body fat content and adipocytokine levels of WNIN rat offspring. Nutr. Metab. (Lond.) 4, 21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonet M. L., Ribot J., Felipe F., Palou A. (2003) Vitamin A and the regulation of fat reserves. Cell. Mol. Life Sci. 60, 1311–1321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bonet M. L., Puigserver P., Serra F., Ribot J., Vázquez F., Pico C., Palou A. (1997) Retinoic acid modulates retinoid X receptor alpha and retinoic acid receptor alpha levels of cultured brown adipocytes. FEBS Lett. 406, 196–200 [DOI] [PubMed] [Google Scholar]
- 11.Bonet M. L., Ribot J., Palou A. (2012) Lipid metabolism in mammalian tissues and its control by retinoic acid. Biochim. Biophys. Acta 1821, 177–189 [DOI] [PubMed] [Google Scholar]
- 12.Lobo G. P., Amengual J., Li H. N., Golczak M., Bonet M. L., Palczewski K., von Lintig J. (2010) Beta,beta-carotene decreases peroxisome proliferator receptor gamma activity and reduces lipid storage capacity of adipocytes in a beta,beta-carotene oxygenase 1-dependent manner. J. Biol. Chem. 285, 27891–27899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Canas J. A., Damaso L., Altomare A., Killen K., Hossain J., Balagopal P. B. (2012) Insulin resistance and adiposity in relation to serum β-carotene levels. J. Pediatr. 161, 58–64.e1–2 [DOI] [PubMed] [Google Scholar]
- 14.Amengual J., Gouranton E., van Helden Y. G., Hessel S., Ribot J., Kramer E., Kiec-Wilk B., Razny U., Lietz G., Wyss A., Dembinska-Kiec A., Palou A., Keijer J., Landrier J. F., Bonet M. L., von Lintig J. (2011) Beta-carotene reduces body adiposity of mice via BCMO1. PLoS One 6, e20644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yasmeen R., Jeyakumar S. M., Reichert B., Yang F., Ziouzenkova O. (2012) The contribution of vitamin A to autocrine regulation of fat depots. Biochim. Biophys. Acta 1821, 190–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marcotorchino J., Tourniaire F., Landrier J. F. (2013) Vitamin D, adipose tissue, and obesity. Horm. Mol. Biol. Clin. Investig. 15, 123–128 [DOI] [PubMed] [Google Scholar]
- 17.Rühl R. (2007) Effects of dietary retinoids and carotenoids on immune development. Proc. Nutr. Soc. 66, 458–469 [DOI] [PubMed] [Google Scholar]
- 18.Blomhoff R., Blomhoff H. K. (2006) Overview of retinoid metabolism and function. J. Neurobiol. 66, 606–630 [DOI] [PubMed] [Google Scholar]
- 19.Napoli J. L. (1999) Interactions of retinoid binding proteins and enzymes in retinoid metabolism. Biochim. Biophys. Acta 1440, 139–162 [DOI] [PubMed] [Google Scholar]
- 20.Ziouzenkova O., Orasanu G., Sharlach M., Akiyama T. E., Berger J. P., Viereck J., Hamilton J. A., Tang G., Dolnikowski G. G., Vogel S., Duester G., Plutzky J. (2007) Retinaldehyde represses adipogenesis and diet-induced obesity. Nat. Med. 13, 695–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reichert B., Yasmeen R., Jeyakumar S. M., Yang F., Thomou T., Alder H., Duester G., Maiseyeu A., Mihai G., Harrison E. H., Rajagopalan S., Kirkland J. L., Ziouzenkova O. (2011) Concerted action of aldehyde dehydrogenases influences depot-specific fat formation. Mol. Endocrinol. 25, 799–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petrosino J. M., Disilvestro D., Ziouzenkova O. (2014) Aldehyde dehydrogenase 1A1: friend or foe to female metabolism? Nutrients 6, 950–973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mcilroy G. D., Delibegovic M., Owen C., Stoney P. N., Shearer K. D., McCaffery P. J., Mody N. (2013) Fenretinide treatment prevents diet-induced obesity in association with major alterations in retinoid homeostatic gene expression in adipose, liver, and hypothalamus. Diabetes 62, 825–836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang M., Liu C., Hu M. Y., Zhang J., Xu P., Li F., Zhong Z. Y., Liu L., Liu X. D. (2015) High-fat diet enhanced retinal dehydrogenase activity, but suppressed retinol dehydrogenase activity in liver of rats. J. Pharmacol. Sci. 127, 430–438 [DOI] [PubMed] [Google Scholar]
- 25.Gagnon I., Duester G., Bhat P. V. (2003) Enzymatic characterization of recombinant mouse retinal dehydrogenase type 1. Biochem. Pharmacol. 65, 1685–1690 [DOI] [PubMed] [Google Scholar]
- 26.Rühl R., Krzyżosiak A., Niewiadomska-Cimicka A., Rochel N., Szeles L., Vaz B., Wietrzych-Schindler M., Álvarez S., Szklenar M., Nagy L., de Lera A. R., Krężel W. (2015) 9-cis-13,14-dihydroretinoic acid is an endogenous retinoid acting as RXR ligand in mice. PLoS Genet. 11, e1005213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Obrochta K. M., Kane M. A., Napoli J. L. (2014) Effects of diet and strain on mouse serum and tissue retinoid concentrations. PLoS One 9, e99435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kane M. A., Folias A. E., Wang C., Napoli J. L. (2008) Quantitative profiling of endogenous retinoic acid in vivo and in vitro by tandem mass spectrometry. Anal. Chem. 80, 1702–1708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huq M. D., Tsai N. P., Gupta P., Wei L. N. (2006) Regulation of retinal dehydrogenases and retinoic acid synthesis by cholesterol metabolites. EMBO J. 25, 3203–3213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rühl R., Fritzsche B., Vermot J., Niederreither K., Neumann U., Schmidt A., Schweigert F. J., Dollé P. (2006) Regulation of expression of the retinoic acid-synthesising enzymes retinaldehyde dehydrogenases in the uteri of ovariectomised mice after treatment with oestrogen, gestagen and their combination. Reprod. Fertil. Dev. 18, 339–345 [DOI] [PubMed] [Google Scholar]
- 31.Vermot J., Fraulob V., Dollé P., Niederreither K. (2000) Expression of enzymes synthesizing (aldehyde dehydrogenase 1 and reinaldehyde dehydrogenase 2) and metabolizaing (Cyp26) retinoic acid in the mouse female reproductive system. Endocrinology 141, 3638–3645 [DOI] [PubMed] [Google Scholar]
- 32.Masoodi M., Kuda O., Rossmeisl M., Flachs P., Kopecky J. (2015) Lipid signaling in adipose tissue: connecting inflammation & metabolism. Biochim. Biophys. Acta 1851, 503–518 [DOI] [PubMed] [Google Scholar]
- 33.Desvergne B. (2007) RXR: from partnership to leadership in metabolic regulations. Vitam. Horm. 75, 1–32 [DOI] [PubMed] [Google Scholar]
- 34.Szanto A., Narkar V., Shen Q., Uray I. P., Davies P. J., Nagy L. (2004) Retinoid X receptors: X-ploring their (patho)physiological functions. Cell Death Differ. 11, S126–S143 [DOI] [PubMed] [Google Scholar]
- 35.Shulman A. I., Mangelsdorf D. J. (2005) Retinoid X receptor heterodimers in the metabolic syndrome. N. Engl. J. Med. 353, 604–615 [DOI] [PubMed] [Google Scholar]
- 36.Yamauchi T., Waki H., Kamon J., Murakami K., Motojima K., Komeda K., Miki H., Kubota N., Terauchi Y., Tsuchida A., Tsuboyama-Kasaoka N., Yamauchi N., Ide T., Hori W., Kato S., Fukayama M., Akanuma Y., Ezaki O., Itai A., Nagai R., Kimura S., Tobe K., Kagechika H., Shudo K., Kadowaki T. (2001) Inhibition of RXR and PPARgamma ameliorates diet-induced obesity and type 2 diabetes. J. Clin. Invest. 108, 1001–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Imai T., Jiang M., Chambon P., Metzger D. (2001) Impaired adipogenesis and lipolysis in the mouse upon selective ablation of the retinoid X receptor alpha mediated by a tamoxifen-inducible chimeric Cre recombinase (Cre-ERT2) in adipocytes. Proc. Natl. Acad. Sci. USA 98, 224–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Metzger D., Imai T., Jiang M., Takukawa R., Desvergne B., Wahli W., Chambon P. (2005) Functional role of RXRs and PPARgamma in mature adipocytes. Prostaglandins Leukot. Essent. Fatty Acids 73, 51–58 [DOI] [PubMed] [Google Scholar]
- 39.Mangelsdorf D. J., Thummel C., Beato M., Herrlich P., Schütz G., Umesono K., Blumberg B., Kastner P., Mark M., Chambon P., Evans R. M. (1995) The nuclear receptor superfamily: the second decade. Cell 83, 835–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mangelsdorf D. J., Evans R. M. (1995) The RXR heterodimers and orphan receptors. Cell 83, 841–850 [DOI] [PubMed] [Google Scholar]
- 41.Perlmann T., Jansson L. (1995) A novel pathway for vitamin A signaling mediated by RXR heterodimerization with NGFI-B and NURR1. Genes Dev. 9, 769–782 [DOI] [PubMed] [Google Scholar]
- 42.Weiss K., Mihály J., Liebisch G., Marosvölgyi T., Garcia A. L., Schmitz G., Decsi T., Rühl R. (2014) Effect of high versus low doses of fat and vitamin A dietary supplementation on fatty acid composition of phospholipids in mice. Genes Nutr. 9, 368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mihály J., Gericke J., Aydemir G., Weiss K., Carlsen H., Blomhoff R., Garcia J., Rühl R. (2012) Reduced retinoid signaling in the skin after systemic retinoid-X receptor ligand treatment in mice with potential relevance for skin disorders. Dermatology (Basel) 225, 304–311 [DOI] [PubMed] [Google Scholar]
- 44.Aydemir G., Carlsen H., Blomhoff R., Rühl R. (2012) Lycopene induces retinoic acid receptor transcriptional activation in mice. Mol. Nutr. Food Res. 56, 702–712 [DOI] [PubMed] [Google Scholar]
- 45.Landrier J. F., Gouranton E., El Yazidi C., Malezet C., Balaguer P., Borel P., Amiot M. J. (2009) Adiponectin expression is induced by vitamin E via a peroxisome proliferator-activated receptor gamma-dependent mechanism. Endocrinology 150, 5318–5325 [DOI] [PubMed] [Google Scholar]
- 46.Marcotorchino J., Gouranton E., Romier B., Tourniaire F., Astier J., Malezet C., Amiot M. J., Landrier J. F. (2012) Vitamin D reduces the inflammatory response and restores glucose uptake in adipocytes. Mol. Nutr. Food Res. 56, 1771–1782 [DOI] [PubMed] [Google Scholar]
- 47.Gouranton E., Aydemir G., Reynaud E., Marcotorchino J., Malezet C., Caris-Veyrat C., Blomhoff R., Landrier J. F., Rühl R. (2011) Apo-10′-lycopenoic acid impacts adipose tissue biology via the retinoic acid receptors. Biochim. Biophys. Acta 1811, 1105–1114 [DOI] [PubMed] [Google Scholar]
- 48.Swann R. T., Smith D. E., Tramposch K. M., Zusi F. C. (1996) Preparation and RARg-specific retinoic receptor transacivation of retinobenzoic acid derivatives. U.S. Patent: 5624957 A. Washington, DC, April 29, 1997 [Google Scholar]
- 49.Zusi F. C., Reczek P. R., Ostrowski J. (1998) Preparation of 5-substituted-1,1,3,3-ttramethyl-2-ketoindanes as retinoid-like compounds. European Patent 98912117.3. Munich, Germany, January 19, 2000 [Google Scholar]
- 50.Tourniaire F., Romier-Crouzet B., Lee J. H., Marcotorchino J., Gouranton E., Salles J., Malezet C., Astier J., Darmon P., Blouin E., Walrand S., Ye J., Landrier J. F. (2013) Chemokine expression in inflamed adipose tissue is mainly mediated by NF-κB. PLoS One 8, e66515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Landrier J. F., Malezet-Desmoulins C., Reboul E., Marie Lorec A., Josephe Amiot M., Borel P. (2008) Comparison of different vehicles to study the effect of tocopherols on gene expression in intestinal cells. Free Radic. Res. 42, 523–530 [DOI] [PubMed] [Google Scholar]
- 52.Karkeni E., Marcotorchino J., Tourniaire F., Astier J., Peiretti F., Darmon P., Landrier J. F. (2015) Vitamin D limits chemokine expression in adipocytes and macrophage migration in vitro and in male mice. Endocrinology 156, 1782–1793 [DOI] [PubMed] [Google Scholar]
- 53.Rühl R. (2006) Method to determine 4-oxo-retinoic acids, retinoic acids and retinol in serum and cell extracts by liquid chromatography/diode-array detection atmospheric pressure chemical ionisation tandem mass spectrometry. Rapid Commun. Mass Spectrom. 20, 2497–2504 [DOI] [PubMed] [Google Scholar]
- 54.Szklenar M., Kalkowski J., Stangl V., Lorenz M., Rühl R. (2013) Eicosanoids and docosanoids in plasma and aorta of healthy and atherosclerotic rabbits. J. Vasc. Res. 50, 372–382 [DOI] [PubMed] [Google Scholar]
- 55.Gregor M. F., Hotamisligil G. S. (2011) Inflammatory mechanisms in obesity. Annu. Rev. Immunol. 29, 415–445 [DOI] [PubMed] [Google Scholar]
- 56.Fasshauer M., Klein J., Neumann S., Eszlinger M., Paschke R. (2002) Hormonal regulation of adiponectin gene expression in 3T3-L1 adipocytes. Biochem. Biophys. Res. Commun. 290, 1084–1089 [DOI] [PubMed] [Google Scholar]
- 57.Yasmeen R., Reichert B., Deiuliis J., Yang F., Lynch A., Meyers J., Sharlach M., Shin S., Volz K. S., Green K. B., Lee K., Alder H., Duester G., Zechner R., Rajagopalan S., Ziouzenkova O. (2013) Autocrine function of aldehyde dehydrogenase 1 as a determinant of diet- and sex-specific differences in visceral adiposity. Diabetes 62, 124–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kiefer F. W., Vernochet C., O’Brien P., Spoerl S., Brown J. D., Nallamshetty S., Zeyda M., Stulnig T. M., Cohen D. E., Kahn C. R., Plutzky J. (2012) Retinaldehyde dehydrogenase 1 regulates a thermogenic program in white adipose tissue. Nat. Med. 18, 918–925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tourniaire F., Musinovic H., Gouranton E., Astier J., Marcotorchino J., Arreguin A., Bernot D., Palou A., Bonet M. L., Ribot J., Landrier J. F. (2015) All-trans retinoic acid induces oxidative phosphorylation and mitochondria biogenesis in adipocytes. J. Lipid Res. 56, 1100–1109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Napoli J. L. (2012) Physiological insights into all-trans-retinoic acid biosynthesis. Biochim. Biophys. Acta 1821, 152–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Frey S. K., Vogel S. (2011) Vitamin A metabolism and adipose tissue biology. Nutrients 3, 27–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Noy N. (2013) The one-two punch: Retinoic acid suppresses obesity both by promoting energy expenditure and by inhibiting adipogenesis. Adipocyte 2, 184–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ahmadian M., Suh J. M., Hah N., Liddle C., Atkins A. R., Downes M., Evans R. M. (2013) PPARγ signaling and metabolism: the good, the bad and the future. Nat. Med. 19, 557–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Aydemir G., Kasiri Y., Bartók E. M., Birta E., Fröhlich K., Böhm V., Mihaly J., Rühl R. (2016) Lycopene supplementation restores vitamin A deficiency in mice and possesses thereby partial pro-vitamin A activity transmitted via RAR signaling. [E-pub ahead of print] Mol. Nutr. Food Res. doi: 10.1002/mnfr.201600031 [DOI] [PubMed] [Google Scholar]
- 65.Moise A. R., Kuksa V., Blaner W. S., Baehr W., Palczewski K. (2005) Metabolism and transactivation activity of 13,14-dihydroretinoic acid. J. Biol. Chem. 280, 27815–27825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sun J., Narayanasamy S., Curley R. W. Jr., Harrison E. H. (2014) β-Apo-13-carotenone regulates retinoid X receptor transcriptional activity through tetramerization of the receptor. J. Biol. Chem. 289, 33118–33124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang C. X., Jiang H., Yuen J. J., Lee S. A., Narayanasamy S., Curley R. W. Jr., Harrison E. H., Blaner W. S. (2015) Actions of β-apo-carotenoids in differentiating cells: differential effects in P19 cells and 3T3-L1 adipocytes. Arch. Biochem. Biophys. 572, 2–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Eroglu A., Hruszkewycz D. P., dela Sena C., Narayanasamy S., Riedl K. M., Kopec R. E., Schwartz S. J., Curley R. W. Jr., Harrison E. H. (2012) Naturally occurring eccentric cleavage products of provitamin A β-carotene function as antagonists of retinoic acid receptors. J. Biol. Chem. 287, 15886–15895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bonet M. L., Canas J. A., Ribot J., Palou A. (2015) Carotenoids and their conversion products in the control of adipocyte function, adiposity and obesity. Arch. Biochem. Biophys. 572, 112–125 [DOI] [PubMed] [Google Scholar]
- 70.Sima A., Manolescu D. C., Bhat P. (2011) Retinoids and retinoid-metabolic gene expression in mouse adipose tissues. Biochem. Cell Biol. 89, 578–584 [DOI] [PubMed] [Google Scholar]
- 71.Aydemir G., Kasiri Y., Birta E., Béke G., Garcia A. L., Bartók E. M., Rühl R. (2013) Lycopene-derived bioactive retinoic acid receptors/retinoid-X receptors-activating metabolites may be relevant for lycopene’s anti-cancer potential. Mol. Nutr. Food Res. 57, 739–747 [DOI] [PubMed] [Google Scholar]
- 72.De Lera A. R., Krezel W., Rühl R. (2016) An endogenous mammalian retinoid X receptor ligand, at last! ChemMedChem 11, 1027–1037 [DOI] [PubMed] [Google Scholar]
- 73.Tishinsky J. M., Ma D. W., Robinson L. E. (2011) Eicosapentaenoic acid and rosiglitazone increase adiponectin in an additive and PPARγ-dependent manner in human adipocytes. Obesity (Silver Spring) 19, 262–268 [DOI] [PubMed] [Google Scholar]
- 74.Dozsa A., Mihaly J., Dezso B., Csizmadia E., Keresztessy T., Marko L., Rühl R., Remenyik E., Nagy L. (2016) Decreased peroxisome proliferator-activated receptor γ level and signalling in sebaceous glands of patients with acne vulgaris. Clin. Exp. Dermatol. 41, 547–551 [DOI] [PubMed] [Google Scholar]
- 75.Dozsa A., Dezso B., Toth B. I., Bacsi A., Poliska S., Camera E., Picardo M., Zouboulis C. C., Bíró T., Schmitz G., Liebisch G., Rühl R., Remenyik E., Nagy L. (2014) PPARγ-mediated and arachidonic acid-dependent signaling is involved in differentiation and lipid production of human sebocytes. J. Invest. Dermatol. 134, 910–920 [DOI] [PubMed] [Google Scholar]
- 76.Nagy L., Tontonoz P., Alvarez J. G., Chen H., Evans R. M. (1998) Oxidized LDL regulates macrophage gene expression through ligand activation of PPARgamma. Cell 93, 229–240 [DOI] [PubMed] [Google Scholar]
- 77.Flachs P., Rossmeisl M., Bryhn M., Kopecky J. (2009) Cellular and molecular effects of n-3 polyunsaturated fatty acids on adipose tissue biology and metabolism. Clin. Sci. 116, 1–16 [DOI] [PubMed] [Google Scholar]
- 78.Hallenborg P., Jørgensen C., Petersen R. K., Feddersen S., Araujo P., Markt P., Langer T., Furstenberger G., Krieg P., Koppen A., Kalkhoven E., Madsen L., Kristiansen K. (2010) Epidermis-type lipoxygenase 3 regulates adipocyte differentiation and peroxisome proliferator-activated receptor gamma activity. Mol. Cell. Biol. 30, 4077–4091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hallenborg P., Petersen R. K., Kouskoumvekaki I., Newman J. W., Madsen L., Kristiansen K. (2016) The elusive endogenous adipogenic PPARγ agonists: lining up the suspects. Prog. Lipid Res. 61, 149–162 [DOI] [PubMed] [Google Scholar]
- 80.Larsen T. M., Toubro S., Astrup A. (2003) PPARgamma agonists in the treatment of type II diabetes: is increased fatness commensurate with long-term efficacy? Int. J. Obes. Relat. Metab. Disord. 27, 147–161 [DOI] [PubMed] [Google Scholar]
- 81.Jones J. R., Barrick C., Kim K. A., Lindner J., Blondeau B., Fujimoto Y., Shiota M., Kesterson R. A., Kahn B. B., Magnuson M. A. (2005) Deletion of PPARgamma in adipose tissues of mice protects against high fat diet-induced obesity and insulin resistance. Proc. Natl. Acad. Sci. USA 102, 6207–6212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Inoue M., Ohtake T., Motomura W., Takahashi N., Hosoki Y., Miyoshi S., Suzuki Y., Saito H., Kohgo Y., Okumura T. (2005) Increased expression of PPARgamma in high fat diet-induced liver steatosis in mice. Biochem. Biophys. Res. Commun. 336, 215–222 [DOI] [PubMed] [Google Scholar]
- 83.Gao M., Ma Y., Liu D. (2015) High-fat diet-induced adiposity, adipose inflammation, hepatic steatosis and hyperinsulinemia in outbred CD-1 mice. PLoS One 10, e0119784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kubota N., Terauchi Y., Miki H., Tamemoto H., Yamauchi T., Komeda K., Satoh S., Nakano R., Ishii C., Sugiyama T., Eto K., Tsubamoto Y., Okuno A., Murakami K., Sekihara H., Hasegawa G., Naito M., Toyoshima Y., Tanaka S., Shiota K., Kitamura T., Fujita T., Ezaki O., Aizawa S., Kadowaki T., et al. (1999) PPAR gamma mediates high-fat diet-induced adipocyte hypertrophy and insulin resistance. Mol. Cell 4, 597–609 [DOI] [PubMed] [Google Scholar]
- 85.Maeda N., Takahashi M., Funahashi T., Kihara S., Nishizawa H., Kishida K., Nagaretani H., Matsuda M., Komuro R., Ouchi N., Kuriyama H., Hotta K., Nakamura T., Shimomura I., Matsuzawa Y. (2001) PPARgamma ligands increase expression and plasma concentrations of adiponectin, an adipose-derived protein. Diabetes 50, 2094–2099 [DOI] [PubMed] [Google Scholar]