Abstract
Cells adopt specific shapes that are necessary for specific functions. For example, some neurons extend elaborate arborized dendrites that can contact multiple targets. Epithelial and endothelial cells can form tiny seamless unicellular tubes with an intracellular lumen. Recent advances showed that cells can auto-fuse to acquire those specific shapes. During auto-fusion, a cell merges two parts of its own plasma membrane. In contrast to cell-cell fusion or macropinocytic fission, which result in the merging or formation of two separate membrane bound compartments, auto-fusion preserves one compartment, but changes its shape. The discovery of auto-fusion in C. elegans was enabled by identification of specific protein fusogens, EFF-1 and AFF-1, that mediate cell-cell fusion. Phenotypic characterization of eff-1 and aff-1 mutants revealed that fusogen-mediated fusion of two parts of the same cell can be used to sculpt dendritic arbors, reconnect two parts of an axon after injury, or form a hollow unicellular tube. Similar auto-fusion events recently were detected in vertebrate cells, suggesting that auto-fusion could be a widely used mechanism for shaping neurons and tubes.
Keywords: Auto-fusion, fusogen, dendrite morphogenesis, neurons regeneration, seamless tube
1. Introduction – cell shaping by auto-fusion
Cells adopt specific shapes that are necessary for specific functions. For example, neurons extend elongated axons and arborized dendrites to contact their partners and transmit and receive electro-chemical signals [1]. Epithelial and endothelial cells can form hollow tubules to transport gases and fluids [2]. Pioneering studies in C. elegans showed that cell auto-fusion is an important mechanism that contributes to cell shaping [3-6]. Auto-fusion is the process whereby a cell merges two parts of its own plasma membrane using a mechanism similar to cell-cell fusion. In this review, we describe how cells can fuse their own membrane to acquire specific shapes. We compare auto-fusion to endocytic processes such as macropinocytosis (“cell gulping”), which also contribute to cell shaping.
2. Types of fission, fusion and fusogens
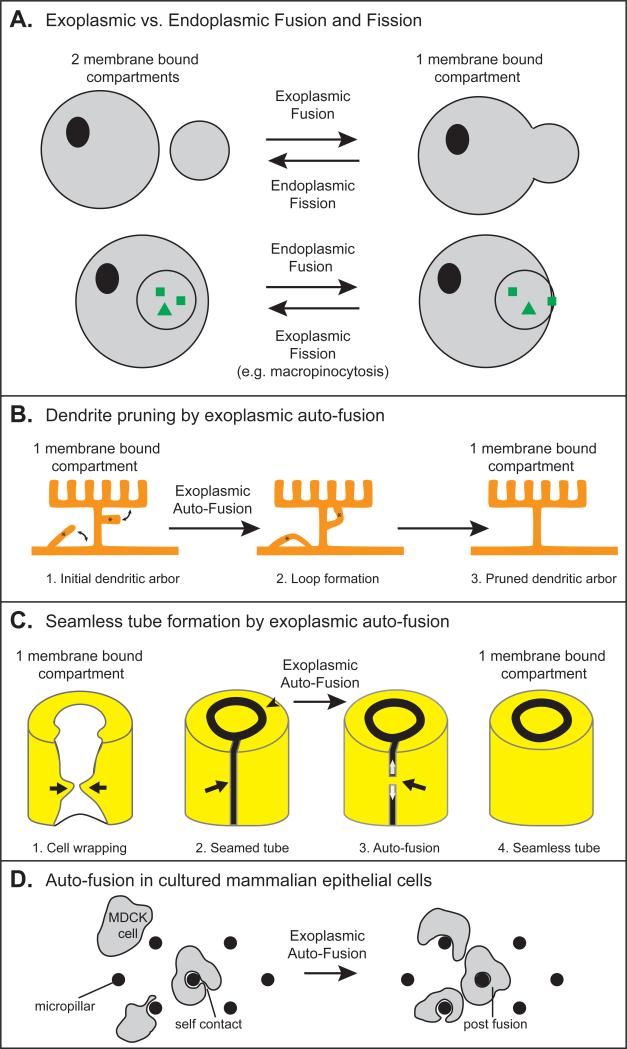
Membrane merging occurs during two different processes, fission and fusion [7] (Fig. 1A). Cell fission converts a single cytosolic compartment into two independent compartments surrounded by lipid bi-layers; examples include cytokinesis, vesicle endocytosis and budding of membrane-bound extracellular vesicles. Cell fusion is traditionally the merging of two compartments into one, as in vesicle fusion and exocytosis during secretion, virus-cell fusion during infection, gamete fusion during fertilization, or cell-cell fusion to form a syncytium. But fusion can also be used cell autonomously to form specific structures, as we will discuss.
Figure 1. Fission, Fusion and Auto-fusion.
(A) Comparison of different types of fission and fusion, adapted from [7]. Cytoplasmic domains are marked with grey coloring. (B-D) Examples of exoplasmic auto-fusion maintaining one membrane bound compartment. (B) A C. elegans arborized dendrite (“menorah”) has two ectopic branches. Auto-fusion induces loop formation to prune the ectopic branches and maintain only the appropriate right-angled branches [4]. (C) The three steps of seamless tube formation [5, 6, 79]. (1) Cell wrapping. White, apical surface; yellow, basal surface. The arrows show the path of cell wrapping. (2) Formation of a seamed tube. An auto-cellular junction (arrow) maintains the cell in the shape of a tube. A ring junction (arrow-head) seals the cell with its neighbors (not shown) to continue the tube. (3) Proposed model of Auto-fusion. A fusion pore forms (black arrow) and extends (white arrows) to form a seamless tube (4). (D) Model of auto-fusion in cultured mammalian MDCK cells [91].
Fission and fusion events can be further categorized as endoplasmic or exoplasmic, depending on whether initial membrane contacts occur between cytosol-proximal monolayers or external monolayers [7] (Fig. 1A). This distinction is important mechanistically, because each monolayer exposes different sets of lipids and proteins. Endocytosis, cell-cell fusion, and auto-fusion are all exoplasmic events.
2.1. Role of fusogens in membrane merging
A general pre-requisite for all types of membrane merging is that the two membranes involved are brought in very close proximity. In addition to cytoskeletal forces, this often involves specific transmembrane proteins that mediate membrane recognition to confer specificity, and help to overcome repulsive electrostatic and hydration forces to allow direct contact between the bilayers [8]. Upon sufficiently close contact, the exposed lipid leaflets can merge to form a hemi-fusion intermediate, which then is resolved to form a fusion pore that can expand to complete the fusion process [8].
Fusogens are membrane proteins that are both necessary and sufficient to promote membrane merging [8]. Some fusogens are exposed on only one of the fusing membranes and interact with other partners in the opposite membrane, making a heterotypic interaction. Other fusogens must be present on both membranes and require a homotypic interaction to mediate fusion.
Endoplasmic and exoplasmic merging events rely on different classes of fusogens with different orientations in the membrane. In the case of endoplasmic fusion, one major class of fusogens is the well-studied SNARE family; heterotypic interactions between V-SNARES and T-SNARES on different membranes promote fusion [9]. SNAREs have also been implicated in endoplasmic fission [10]. Other endoplasmic fusogens include atlastin, involved in Endoplasmic Reticulum fusion [11, 12], and mitofusins 1 and 2 and OPA1, involved in mitochondria membrane fusion [13-15]. Many of the known exoplasmic fusogens are viral, and belong to three distinct structural classes [8]. Only a few exoplasmic fusogens have been identified in animals, including mammalian Syncytins and C. elegans Epithelial Fusion Failure-1 (EFF-1) and Anchor cell Fusion Failure-1 (AFF-1) [16-19], which are structurally related to viral class I and class II fusogens, respectively [20-23]. HAP2/GCS1 is a potential fusogen present in gametes of plants, protozoa, amoebae and some invertebrate animals [24-26].
2.2. Exoplasmic fission
Exoplasmic fission encompasses many mechanistically- and morphologically-distinct types of endocytosis. Clathrin-dependent endocytosis and related forms of micropinocytosis involve formation of small coated vesicles (~ 100 nm) that internalize materials from the cell surface [27]. In most cases, the small GTPase dynamin is required to pinch off the vesicle neck to separate it from the plasma membrane, and dynamin is sufficient for such scission in vitro [27]. Macropinocytosis and phagocytosis involve formation of larger, uncoated vesicles (>0.2-5 μm) for internalization of bulk membrane, external fluids and particles [27]. Scission of these larger vesicles can be dynamin- independent, but no membrane fusogens are known to be involved.
Macropinocytosis can influence cell shaping during development. For example, macropinocytosis internalizes plasma membrane during neuronal growth cone collapse and axon turning [28, 29]. Macropinocytosis also is one of several proposed mechanisms involved in formation of small capillary tubules in the vertebrate vascular system [30-33] (see below).
2.3. Exoplasmic fusion and the FF family of fusogens
Exoplasmic cell-cell fusion can involve cells with distinct genetic material, as in the fusion of gametes during sexual reproduction or fusion of enveloped viruses to the host cell during infection, or it can involve cells with identical genetic material. Many examples of cell-cell fusion come from development, where it is used to form syncytia that contain multiple nuclei in a common cytoplasm. In mammals, cell-cell fusion occurs to form myoblasts, eye lens, osteoclasts, and syncytiotrophoblasts [34]. Trophoblast fusion requires Syncytins, which are encoded by elements originated from retroviruses, and are related to the class I viral fusogens [16, 35, 36]. With the exception of Syncytins, the mammalian cell-cell fusogens remain unidentified.
Cell-cell fusion is a central mechanism in the development of the nematode C. elegans. Indeed, the adult hermaphrodite harbors 44 syncytia containing 300 of the total 959 nuclei [37]. Many cell-fusions in C. elegans require two related fusogens, EFF-1 and AFF-1 [18, 19], which define the FF fusogen family. Based on sequence analysis, the FF fusogens are only present in nematodes, some arthropods, a ctenophore and a protist [38]. However, FF fusogens are structurally related to class II viral fusogens [22, 23], so it is conceivable that structural homologs exist in other species. During most characterized fusion events, identical FF fusogens are required on each fusing membrane, suggesting a homotypic mode of action [5, 19, 39]. Ectopic expression of EFF-1 or AFF-1 promotes ectopic cell fusion in vivo [19, 40, 41], and each can generate syncytia when expressed in cultured insect or vertebrate cells [19, 38, 39].
In vivo, FF fusogen expression and localization are regulated to ensure that cells fuse only at the right place and time. Various signaling pathways and transcription factors turn on FF fusogen expression in cells that are destined to fuse [5, 19, 42-46]. Other factors appear to influence FF protein localization or fusogenic activity [47-49]. In the hypodermis, RAB-5- and dynamin-dependent endocytosis keep EFF-1 localized predominantly to intracellular vesicles, so that EFF-1 is observed on the plasma membrane only rarely and transiently even in fusing cells [49]. AFF-1 localizes to both intracellular vesicles and the plasma membrane when overexpressed [19], but its endogenous localization has not yet been described.
The last type of exoplasmic fusion is auto-fusion. Phenotypic characterization of C. elegans eff-1 and aff-1 mutants revealed that FF-mediated fusion of two parts of the same cell can be used to sculpt dendritic arbors (Fig. 1B) [4], reconnect two parts of an axon after injury (Fig. 2) [3], or form a hollow tube (Fig. 1C) [5, 6]. Similar auto-fusion events recently were detected in vertebrate cells [50, 51]. These known examples of auto-fusion are described below.
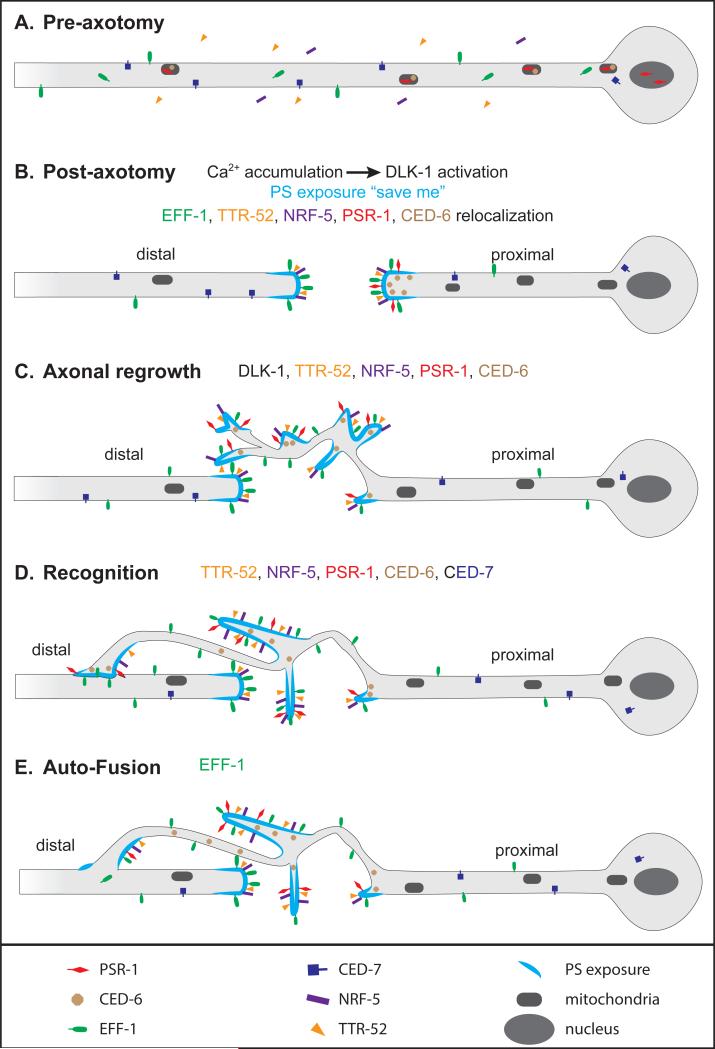
Figure 2. Model of axon reconnection by auto-fusion.
Model based on refs. [3, 69, 71]. (A) Before axotomy, PSR-1 (red) localizes in mitochondria and in the nucleus, and CED-6 (brown) localizes in mitochondria only. EFF-1 (green) is both intracellular and on the plasma membrane. (B) Immediately after axon injury, calcium is released and phosphatidylserine (PS, light blue) is exposed to the external lipid layer. Calcium release induces DLK-1 activation to promote axon regrowth. PSR-1 and CED-6 accumulate at the proximal tip. TTR-52 (orange), NRF-5 (purple) and EFF-1 accumulate on external membranes at both proximal and distal tips. (C) DLK-1 and the phagocytosis pathway promote axonal regrowth. (D) The phagocytosis pathway promotes recognition of distal axon by the proximal growth cone. (E) EFF-1 stimulates auto-fusion to reconnect the proximal part to the distal part of the axon.
3. Auto-fusion during Neural morphogenesis and Regeneration
3.1. Dendrite tiling
The establishment of specific dendritic arborization patterns is essential for the development of neuronal circuits [1]. In C. elegans, the two nociceptive PVD neurons (PVDL and PVDR) have a very specific dendritic pattern [52, 53]. Each PVD is located on one side of the animal with a single axon that extends to the ventral nerve cord. The PVD dendrites have multiple branches and extend along the body axis from the neck to the tail. The primary branches grow from the cell body in anterior and posterior directions, and the secondary, tertiary and quaternary branches form at right angles to one another, resulting in candelabra-like structures called menorahs (Fig. 1B). This precise pattern of dendritic arborization requires dendrite auto-fusion in order to prune ectopic branches [4].
Mutants lacking the fusogen EFF-1 display hyper-branching in the PVD neurons, with excess secondary and tertiary branches that form at odd angles and overlap extensively [4]. Tissue-specific rescue experiments suggest that EFF-1 functions cell autonomously within these neurons, and its over-expression dramatically reduces the number of dendrite branches. Both live imaging and transmission electron microscopy (TEM) data support the model that EFF-1 functions subsequent to dendrite branch growth; in wild type, excess branches are constantly being generated and extended, but EFF-1 induces loop formation by neurite auto-fusion and retraction to maintain only the appropriate right-angled branches (Fig. 1B).
Many other genes that affect PVD branching patterns have been identified. External cues emanating from the hypodermal cells influence dendrite growth and self-avoidance. A basement membrane protein UNC-52/Perlecan is required to pattern the distribution of the transmembane protein SAX-7/Ig CAM in a regular stripe fashion along the hypodermal cell membrane [54]. SAX-7 and another transmembrane protein MNR-1, interact with the Leucine Rich Repeat transmembrane protein DMA-1 on the PVD neurons [55, 56]. Upon its activation by SAX-7 and MNR-1, DMA-1 promotes branch formation and extension [57, 58]. Another signaling pathway, involving the secreted protein UNC-6/Netrin and its PVD receptors UNC-40 and UNC-5, is involved in self-avoidance of PVD dendritic branches [57, 59, 60]. Finally, the claudin-like transmembrane protein HPO-30 promotes dendrite stabilization [61].
Mechanistic links between the known developmental signals and EFF-1-dependent branch pruning are not yet understood. But it is interesting to note that the eff-1 mutant phenotype resembles that caused by overexpression of the PVD transmembrane protein DMA-1 [57], suggesting that EFF-1 could be downregulated by DMA-1 signaling to allow branch outgrowth. It will be interesting to observe the location of EFF-1 at the dendrite membrane and ultimately understand how fusion and pruning are restricted to specific locations.
Could auto-fusion also shape dendritic arbors in other organisms? Studies in Drosophila and in vertebrates showed that dendritic self-avoidance requires the Dscam or protocadherin families of cell surface recognition proteins, respectively [62]. Although these proteins mediate homophilic adhesion in vitro [63, 64], they appear to mediate homophilic repulsion in vivo, through still poorly understood mechanisms. It will be interesting to test if auto-fusion could be involved in this mechanism to resolve self-contact.
3.2. Axon regeneration
Regeneration of injured axons is a great challenge for organisms and requires that axons reconnect with their original target tissues. In most cases, Wallerian degeneration occurs in the distal part of the injured axon, and the proximal part regrows to connect the original target. Another way to achieve such repair is to reconnect severed axon fragments, thus re-establishing the original tract (Fig. 2). This process has been described in several invertebrate species, including crayfish, earthworms, leeches and C. elegans [3, 65-67], and is another example of auto-fusion. However, unlike the other examples of auto-fusion, this example involves the reunification of two distinct compartments of the same cell post-injury, rather than the fusion of a single compartment.
The process of axon regeneration by auto-fusion has been studied in C. elegans mechanosensory neurons ALM and PLM, which are involved in detection of light touch. In wild-type animals, after laser axotomy, most injured ALM and PLM neurons resume axon growth, and many of the re-growing proximal axons contact their respective distal part and fuse with it [3, 68]. In eff-1 mutants, proximal axon outgrowth still occurs, but fusion generally does not [3]. Restoring EFF-1 to the injured neurons rescues the repair defect, suggesting that EFF-1 functions cell autonomously in these neurons for fusion-mediated repair (Fig. 2) [69].
Many of the events leading to axon fusion have been characterized (Fig. 2). The first response after axon injury is a rapid accumulation of Ca2+ in both proximal and distal fragments [3]. Release of Ca2+ results in the activation of the apoptotic pathway components CED-4 and CED-3 [70] and stimulates adenylate cyclase- dependent accumulation of cAMP, which in turn stimulates Protein Kinase A (PKA) [3]. The activation of these pathways finally results in the activation of the mitogen-activated protein kinase kinase kinase (MAPKKK) DLK-1 that is essential for growth cone formation in injured axons [3, 71, 72].
In addition to EFF-1, contact and fusion between the proximal and distal axon fragments involves many components of a phagocytic pathway that is also involved in apoptotic cell corpse engulfment [69] (Fig. 2). After axon injury, phosphatidylserine (PS) appears to relocate from the cytoplasmic leaflet to the outer leaflet of the plasma membrane, and serve as a “save me” signal. The PS binding protein TTR-52, the lipid binding protein NRF-5, the PS receptor PSR-1, and the adaptor CED-6/GULP all promote axon regrowth and fusion, although none is individually essential. The ATP binding cassette transporter CED-7 also promotes fusion. Over-expression of eff-1 can bypass the fusion requirement for TTR-52 or PSR-1, suggesting that EFF-1 functions downstream of the phagocytic pathway.
It is not yet known if DLK-1 signaling or the phagocytic pathway influence EFF-1 localization or activity, or simply direct the growth cone to grow toward and contact the distal fragment so that EFF-1-mediated fusion can occur. However, proximal and distal axon fragments accumulate EFF-1 at their growth cone membrane and injured tip, respectively [69] (Fig. 2). The significance of the distal tip localization is still unclear, since earlier observations showed that a majority of reconnecting axons make an “end to side” connection instead of an “end to end” connection [3]. A recent study of EFF-1 localization in the hypodermis showed that EFF-1 is rather randomly and dynamically exposed on the membranes of pre-fusing cells [49]. The same kind of widespread and random EFF-1 exposure on the distal axon fragment would maximize the surface area of fusion-ready membrane to allow fusion at any contact point. On the other hand, concentrating EFF-1 exposure at the proximal growth cone could explain why fusion consistently involves this region.
Axon regeneration in mammals is much less efficient than in invertebrates, and the distal axon fragments tend to degenerate rapidly [73]. However, in experiments inspired by the invertebrate literature, Polyethylene glycol (PEG) treatment has been used to fuse and successfully repair severed axons in the sciatic nerve of the Rat [74]. Such PEG-induced axon fusion is mechanistically different from axon fusion in C. elegans, in that it requires a Ca2+ free environment to prevent sealing of the severed axon ends and involves re-connection of severed axon ends that remain open. Nevertheless, recent success of this method in animal models suggests that fusion-based clinical therapies might eventually be developed to repair nerve damage in human patients [73].
4. Formation of seamless tubes by auto-fusion
Tubes are essential structures in most organs, and can have different sizes and shapes [2]. Larger tubes have multiple cells surrounding the lumen, while the smallest tubes, such as many vertebrate capillaries, are only one cell in diameter [75, 76]. These unicellular tubes can be “seamed”, having an autocellular adherens junction along the lumen, or “seamless”, having a continuous membrane without an auto-junction. Both seamed and seamless tubes typically have ring-shaped adherens junctions where they connect to other tubes. Seamless tubes can be toroidal and open at both ends (Fig. 1C) or they can be branched and terminated by dead ends, as observed for the excretory canal cell in C. elegans or the tracheal terminal cell in Drosophila [77, 78]. Both endocytic and exocytic trafficking mechanisms have been implicated in generating the internal apical domain of seamless tubes [77]. Another mechanism for forming a seamless tube involves converting a seamed tube to a seamless tube via auto-fusion (Fig. 1C).
4.1. C. elegans hemi-vulva toroids
The first evidence for auto-fusion came from observations of the C. elegans vulva, an epithelial tube used for egg laying. The vulva consists of 22 cells that interact to form seven stacked rings of two or four cells each. Five of these rings become seamless toroids as a result of cell-cell fusion [79] involving FF fusogens [18, 19]. However, after laser ablation of some vulval precursors, a hemi-vulva can be built from just 11 cells forming seven stacked rings of one or two cells each [79]. In this case, a single cell can reach around the lumen to form its own ring and auto-fuse to form a seamless toroid. Auto-fusion has also been observed in several mutants with altered numbers of vulval cells [45, 80]. Even though such auto-fusion does not occur during normal vulva development, it demonstrated the basic steps of auto-fusion-dependent seamless tube formation: 1) cell wrapping to initiate self-contact; 2) formation of an autocellular junction; and 3) fusogen-mediated auto-fusion to remove the autocellular junction and form a seamless toroid (Fig. 1C).
4.2. C. elegans pharyngeal-intestinal valve
The second example of auto-fusion to form a seamless tube in C. elegans involves two cells that connect the pharynx and intestine [5]. The pharynx, or foregut, is a monolayered myoepithelial tube. The posterior-most pharyngeal muscle, pm8, and the adjacent intestinal valve cell, vpi1, are each seamless toroids (Fig. 3A,B). These cells require AFF-1 and EFF-1, respectively, to become seamless tubes [5]. It appears that, by utilizing different fusogens, pm8 and vpi1 avoid fusing with each other. Notch signaling is important for pm8 identity, and both upregulates aff-1 and downregulates eff-1 expression to promote auto-fusion and prevent cell-cell fusion [5].
Figure 3. Seamless tubes formed by auto-fusion can have complex shapes.
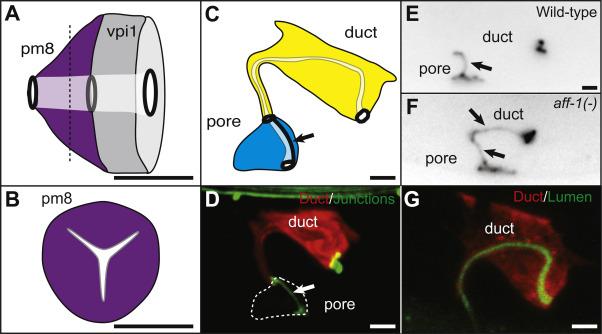
(A) Lateral view of C. elegans pharyngeal-intestinal valve cells (pm8 and vpi1), which are both seamless tubes. The tubes are linked to neighbors by ring-shaped junctions (black ovals). (B) A transverse section of pm8 (dashed line in A) shows the tri-radiate shape of the lumen [5]. (C) The C. elegans excretory duct is a seamless tube, in contrast to the adjacent cell, the excretory pore, which maintains an auto-junction (arrow). Both tubes are linked to neighbors by ring-shaped junctions (black ovals). (D) The excretory duct has an elongated shape. Red, duct cytoplasmic marker lin-48pro::mRFP [85]. Green, junction marker AJM-1::GFP [99]. (E, F) AFF-1 is required for duct seamlessness. Cell junctions marked by AJM-1::GFP. (E) Wild-type excretory duct is a seamless tube. (F) In an aff-1 loss of function mutant, the excretory duct is a seamed tube with an auto-junction (arrows) [6]. (G) The excretory duct has an elongated lumen. Red, duct cytoplasmic marker lin-48pro::mRFP. Green, luminal marker let-653pro::LET-653::GFP [100]. Scale bars. 2 μm.
The cell wrapping behavior of pm8 has been followed by live imaging [5, 81]. Prior to forming toroids, both pm8 and vpi1 are part of a multicellular primordial cyst; these cells are located dorsally within the cyst, with their apical sides facing the cyst lumen and basal sides contacting a basal lamina. The first step of pm8 toroid formation involves detachment from the basal lamina and extension of an apical lamellar process toward the ventral side of the cyst. The migration of pm8 follows a tract of laminin and results in wrapping around finger-like projections from the neighboring pharyngeal marginal cells. After intercalating between other cells in the cyst and surrounding the lumen, pm8 makes contact with itself and auto-fuses. It was not determined whether a transient auto-junction forms prior to fusogen-mediated auto-fusion. However, in eff-1 or aff-1 mutant embryos, the unfused vpi1 or pm8 do have an auto-cellular junction, suggesting a transient auto-junction does form.
4.3. C. elegans excretory duct tube
The last example of auto-fusion dependent seamless tube formation in C. elegans occurs in the excretory duct cell (Fig. 3C, D). The excretory duct cell is part of the excretory system, a simple epithelial tube network involved in osmoregulation and fluid waste expulsion [78, 82, 83]. The excretory system consists of three tandemly arranged unicellular tubes, with the smaller excretory duct and pore tubes connecting the larger excretory canal cell tube to the outside environment for excretion [78]. The canal cell is a seamless tube proposed to form via pinocytic hollowing and/or exocytic mechanisms [78, 84]. Initially, the excretory duct and pore are both seamed tubes formed by cell wrapping, but the duct uses AFF-1 to auto-fuse and become a seamless tube (Fig. 3 E, F) [6, 85].
Duct auto-fusion is stimulated by EGF-Ras-ERK signaling. Prior to forming tubes, the presumptive duct and pore cells migrate from lateral positions in the embryo to contact each other at the ventral midline. These cells appear to compete for access to the excretory canal cell, which expresses LIN-3/EGF; the left cell reaches the canal cell first and signaling through an EGF Receptor-Ras-ERK pathway specifies it as the duct, while the right cell takes the default pore fate [85]. In let-60/Ras or aff-1 loss of function mutants, both cells wrap to form seamed tubes (Fig. 3F), whereas in let-60/Ras constitutive mutants, both cells fuse to make a binucleate seamless duct tube [85]. We showed recently that Ras signaling upregulates aff-1 gene expression in the duct (F. Soulavie and M. Sundaram, unpublished data).
4.4. Transient seamless tubes during pruning of the zebrafish vascular system
The vertebrate vascular system contains many unicellular capillaries, including both seamed and seamless tubes [75, 76]. Studies in zebrafish suggest that the seamless tubes can form by a variety of mechanisms, one of which is auto-fusion [86]. Specifically, auto-fusion has been observed in the context of vascular pruning [50].
Similar to dendritic arbors described above, vascular tubes form an orderly network. During pruning of a secondary vascular branch, the cells within the branch initially form a multicellular tube, but then withdraw toward the main vessels until the branch becomes partly unicellular [87]. Live imaging revealed that the last unicellular bridging cell wraps around the lumen, forms an auto-junction, and then auto-fuses to form a seamless tube [50]. After auto-fusion, the lumen splits and retracts, and the bridging cell detaches from its partner on one side and is re-integrated into one of the main vessels.
The factors responsible for endothelial cell auto-fusion are not known yet. Other examples of pruning by capillary regression exist in mice [88, 89] and in chicken [90], though auto-fusion has not been demonstrated in these systems.
4.5. Auto-fusion in cultured mammalian epithelial cells
Auto-fusion can also occur in mammalian cells in culture. Cultured epithelial cells usually assemble and maintain junctions with their neighbors, but these cells usually do not maintain auto-junctions. To understand how self-contacts are inhibited, Yamada and Sumida [91] cultured Madin Darby Canine Kidney (MDCK) epithelial cells on micropillar arrays where each individual pillar would serve as a physical barrier for the extending plasma membrane to wrap around and contact its own membrane (Fig. 1D). They showed that a single epithelial cell is able to contact itself around a pillar and establish an auto-junction, but this auto-junction is rapidly eliminated by auto-fusion creating a seamless toroid.
The mechanism by which MDCK cells distinguish self from non-self is still unknown, and so far there is no fusogen known to be involved in this auto-fusion process. However, the Rho GTPases RhoA, Rac1 and Cdc42 appear to regulate membrane auto-fusion through the regulation of the actin cytoskeleton and cell contractility [51]. RhoA accumulates at the site of self-contact but then quickly dissipates, unlike at a typical cell-cell junction. RhoA and its targets, Rho-associated coiled-coil containing kinase (ROCK) and myosin II, which regulate contractility, are required for efficient self-contact-induced auto-fusion. Furthermore the actin nucleation factor complex Arp2/3, a potential target for Rac1 and Cdc42, accumulates transiently around the pillar and is also required for auto-fusion.
Another fundamental aspect of this auto-fusion event is the involvement of E-Cadherin in the establishment of the auto-junction before fusion. Fibroblasts, which lack E-cadherin, are able to establish self-contact but do not form adherens junctions and do not fuse. Removing E-cadherin from MDCK cells decreases the rate of auto-fusion, but expression of E-cadherin is not sufficient to promote auto-fusion in fibroblasts [91]. Inhibitors of Rac1 or Cdc42 do not prevent the establishment of auto-junctions [51], but do prevent fusion, suggesting an increase of Arp2/3 activity might be involved in E-cadherin junction dissipation.
The auto-fusion described here is interesting because it shows how a cell can easily auto-fuse under appropriate conditions. It is tempting to speculate that such auto-fusion may also occur in vivo. Furthermore, it provides a cell culture system to study the mechanisms of auto-fusion, and may permit identification of relevant mammalian fusogen(s).
4.6. Similarities and differences between auto-fusion and macropinocytic mechanisms of seamless tube formation
Another proposed mechanism for forming seamless tubes involves macropinocytosis. Endothelial cells grown in three-dimensional collagen gels develop large intracellular vacuoles that can be labeled with an exogenously applied dextran-fluorescein tracer, indicating fluid phase uptake [30, 92]. These vacuoles fuse, enlarge and eventually connect to similar vacuoles in adjacent cells through exocytosis or anastomosis. Some imaging studies in zebrafish have supported this mechanism [31, 32].
Are macropinocytosis and auto-fusion really distinct mechanisms, or could they be related? In both cases, exoplasmic membrane merging converts basal plasma membrane into an internal apical membrane domain. Furthermore, like auto-fusion, macropinocytosis can involve a transient cadherin-positive self-contact [93]. However, macropinocytosis involves fission to generate a topologically isolated vacuole compartment within the cytosol (Fig. 1A), which later connects to other apical domains via a separate fusion step. In contrast, wrapping and auto-fusion are supposed to accomplish the same result in a single step (Fig. 1C).
Many questions remain about the molecular mechanisms involved in each process. In mammalian cells, some of the same molecular players, such as Rac and Cdc42, have been implicated in both processes, but there are also reported differences in the requirements for Rho and ROCK, which inhibit pinocytic lumen formation but promote auto-fusion [51, 91, 94]. Curiously, cytoskeletal regulators have not yet been implicated in either auto-fusion or cell-cell fusion in C. elegans. Finally, auto-fusion in C. elegans clearly requires the fusogens EFF-1 or AFF-1, whereas no fusogens have been identified for either auto-fusion or macropinocytosis in the other systems. Further studies are needed to understand the core similarities and differences among these various types of exoplasmic membrane merging events.
5. The importance of auto-fusion
As the above discussion has shown, auto-fusion and other exoplasmic membrane merging events shape individual neuronal, epithelial and endothelial cells in multiple animal systems. The importance of some of these shaping events is clear. Precise dendrite branching patterns and axon integrity determine synaptic connections essential for complex behaviors [1]. Indeed, the dendrite pruning defects of eff-1 mutants are associated with defects in sensing or responding to touch stimulation [4].
But what is the advantage of a seamless tube compared to a seamed tube? Small unicellular capillaries maximize surface area and allow relatively thin walls for efficient nutrient exchange with surrounding tissues. One likely advantage of seamlessness is that limiting junctions also limits paracellular leakage so that such exchange can be highly regulated, which is especially important in some capillary beds such as the blood-brain barrier [95]. A related advantage might be one of tube strength – a seamless structure does not have a weak seam along which it could potentially rupture. Another possibility is that seamlessness facilitates complex shaping of the cells and lumens. Indeed, some seamless tubes adopt highly branched shapes, as exemplified by Drosophila tracheal terminal cells and the C. elegans excretory canal cell [33]. The excretory duct begins as a simple toroid with a donut-like shape, but later adopts a more complicated shape, with a narrow extension linking it to the pore cell and an elongated lumen that takes a looping path inside the cytoplasm (Fig. 3G). The pharyngeal pm8 cell is conical and its lumen has a tri-radiate shape rather than a simple oval shape when viewed in cross-section [5] (Fig. 3B). Potentially, removal of the autocellular junctions could facilitate subsequent cytoskeletal and trafficking mechanisms involved in generating such shapes, or continued rounds of auto-fusion could sculpt the membranes more directly.
Tube networks containing seamed vs. seamless tubes may also differ in their propensity for dynamic rearrangements. In C. elegans, seamed unicellular tubes such as the excretory pore cell can delaminate and/or divide to give rise to other cell types, without disrupting network continuity [96, 97]. In contrast, seamless tubes are invariably post-mitotic. On the other hand, in zebrafish, the assembly and dis-assembly of blood vessels involves dynamic behaviors of both seamed and seamless tubes [50, 86, 87, 98].
6. Open questions and future directions
Future studies of auto-fusion must focus on its biological importance in shaping neurons and tubes, as discussed above, but also on its mechanism and regulation. How does a cell decide which membranes to fuse, and whether to execute auto-fusion or cell-cell fusion?
In C. elegans, the current model is that both types of cell fusion require a homotypic interaction between the same FF fusogen on both fusing membranes, so that close membrane apposition and fusogen expression and localization are the major determining factors. If two epithelial cells are in tight contact via an adherens junction, and if they both expose the same fusogen on their plasma membranes, then the two cells will fuse. If a single cell makes an autocellular adherens junction with itself and also exposes a fusogen on its plasma membranes, then it will auto-fuse. Similarly, in the case of neuronal dendrites or axons, self-contact between different parts of the same cell may be mediated by other adhesive factors and allow auto-fusion as long as the same fusogen is exposed on both plasma membranes. However, this simple model seems insufficient to explain fusion regulation in more complex organisms. In particular, this model cannot explain the exquisite specificity of MDCK cell auto-fusion [51, 91], where all cells in the culture should be genetically identical. The MDCK cell results suggest that other factors, such as the degree of membrane curvature at the contact point, must help distinguish self from non-self and influence the fusion decision.
In C. elegans, knowledge of the relevant fusogens, EFF-1 and AFF-1, will facilitate future studies of fusion regulation. Elegant studies in the hypodermis have already identified RAB-5 and dynamin-dependent endocytosis of EFF-1 as a key mechanism in restricting cell-cell fusion [49], and it will be interesting to test if this mechanism also regulates when and where neuronal auto-fusion occurs. Other potential regulatory mechanisms might include post-translational modifications or binding partners that regulate fusogen oligomerization or function. Finally, there is evidence for alternative splice isoforms of EFF-1 [18], which could contribute to specific functions.
In vertebrates, the relevant fusogens need to be found in order to understand auto-fusion mechanisms and regulation. So far, Syncytins are the only cell fusogens identified in vertebrates, but it is not known if Syncytins can mediate auto-fusion or are relevant to the auto-fusion events observed in the vascular system or in MDCK cells. The MDCK system should be particularly useful for identifying the relevant fusogen(s) and elucidating their functions.
Acknowledgments
We are grateful to Jennifer Cohen and Hasreet Gill, for assistance with preparation of this manuscript. This work was supported by NIH grant GM58540 to M.S.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lefebvre JL, Sanes JR, Kay JN. Development of dendritic form and function. Annu Rev Cell Dev Biol. 2015;31:741–77. doi: 10.1146/annurev-cellbio-100913-013020. [DOI] [PubMed] [Google Scholar]
- 2.Andrew DJ, Ewald AJ. Morphogenesis of epithelial tubes: Insights into tube formation, elongation, and elaboration. Dev Biol. 2010;341(1):34–55. doi: 10.1016/j.ydbio.2009.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ghosh-Roy A, Wu Z, Goncharov A, Jin Y, Chisholm AD. Calcium and cyclic AMP promote axonal regeneration in Caenorhabditis elegans and require DLK-1 kinase. J Neurosci. 2010;30(9):3175–83. doi: 10.1523/JNEUROSCI.5464-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oren-Suissa M, Hall DH, Treinin M, Shemer G, Podbilewicz B. The fusogen EFF-1 controls sculpting of mechanosensory dendrites. Science. 2010;328(5983):1285–8. doi: 10.1126/science.1189095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rasmussen JP, English K, Tenlen JR, Priess JR. Notch signaling and morphogenesis of single-cell tubes in the C. elegans digestive tract. Dev Cell. 2008;14(4):559–69. doi: 10.1016/j.devcel.2008.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stone CE, Hall DH, Sundaram MV. Lipocalin signaling controls unicellular tube development in the Caenorhabditis elegans excretory system. Dev Biol. 2009;329(2):201–11. doi: 10.1016/j.ydbio.2009.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stegmann T, Doms RW, Helenius A. Protein-mediated membrane fusion. Annu Rev Biophys Biophys Chem. 1989;18:187–211. doi: 10.1146/annurev.bb.18.060189.001155. [DOI] [PubMed] [Google Scholar]
- 8.Podbilewicz B. Virus and cell fusion mechanisms. Annu Rev Cell Dev Biol. 2014;30:111–39. doi: 10.1146/annurev-cellbio-101512-122422. [DOI] [PubMed] [Google Scholar]
- 9.Thorn P, Zorec R, Rettig J, Keating DJ. Exocytosis in non-neuronal cells. J Neurochem. 2016 doi: 10.1111/jnc.13602. [DOI] [PubMed] [Google Scholar]
- 10.Lo Cicero A, Stahl PD, Raposo G. Extracellular vesicles shuffling intercellular messages: for good or for bad. Curr Opin Cell Biol. 2015;35:69–77. doi: 10.1016/j.ceb.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 11.Hu J, Shibata Y, Zhu PP, Voss C, Rismanchi N, Prinz WA, Rapoport TA, Blackstone C. A class of dynamin-like GTPases involved in the generation of the tubular ER network. Cell. 2009;138(3):549–61. doi: 10.1016/j.cell.2009.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Orso G, Pendin D, Liu S, Tosetto J, Moss TJ, Faust JE, Micaroni M, Egorova A, Martinuzzi A, McNew JA, Daga A. Homotypic fusion of ER membranes requires the dynamin-like GTPase atlastin. Nature. 2009;460(7258):978–83. doi: 10.1038/nature08280. [DOI] [PubMed] [Google Scholar]
- 13.Cipolat S, Martins de Brito O, Dal Zilio B, Scorrano L. OPA1 requires mitofusin 1 to promote mitochondrial fusion. Proc Natl Acad Sci U S A. 2004;101(45):15927–32. doi: 10.1073/pnas.0407043101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hales KG, Fuller MT. Developmentally regulated mitochondrial fusion mediated by a conserved, novel, predicted GTPase. Cell. 1997;90(1):121–9. doi: 10.1016/s0092-8674(00)80319-0. [DOI] [PubMed] [Google Scholar]
- 15.Santel A, Fuller MT. Control of mitochondrial morphology by a human mitofusin. J Cell Sci. 2001;114(Pt 5):867–74. doi: 10.1242/jcs.114.5.867. [DOI] [PubMed] [Google Scholar]
- 16.Blond JL, Lavillette D, Cheynet V, Bouton O, Oriol G, Chapel-Fernandes S, Mandrand B, Mallet F, Cosset FL. An envelope glycoprotein of the human endogenous retrovirus HERV-W is expressed in the human placenta and fuses cells expressing the type D mammalian retrovirus receptor. J Virol. 2000;74(7):3321–9. doi: 10.1128/jvi.74.7.3321-3329.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mi S, Lee X, Li X, Veldman GM, Finnerty H, Racie L, LaVallie E, Tang XY, Edouard P, Howes S, Keith JC, Jr., McCoy JM. Syncytin is a captive retroviral envelope protein involved in human placental morphogenesis. Nature. 2000;403(6771):785–9. doi: 10.1038/35001608. [DOI] [PubMed] [Google Scholar]
- 18.Mohler WA, Shemer G, del Campo JJ, Valansi C, Opoku-Serebuoh E, Scranton V, Assaf N, White JG, Podbilewicz B. The type I membrane protein EFF-1 is essential for developmental cell fusion. Dev Cell. 2002;2(3):355–62. doi: 10.1016/s1534-5807(02)00129-6. [DOI] [PubMed] [Google Scholar]
- 19.Sapir A, Choi J, Leikina E, Avinoam O, Valansi C, Chernomordik LV, Newman AP, Podbilewicz B. AFF-1, a FOS-1-regulated fusogen, mediates fusion of the anchor cell in C. elegans. Dev Cell. 2007;12(5):683–98. doi: 10.1016/j.devcel.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gong R, Peng X, Kang S, Feng H, Huang J, Zhang W, Lin D, Tien P, Xiao G. Structural characterization of the fusion core in syncytin, envelope protein of human endogenous retrovirus family W. Biochem Biophys Res Commun. 2005;331(4):1193–200. doi: 10.1016/j.bbrc.2005.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Renard M, Varela PF, Letzelter C, Duquerroy S, Rey FA, Heidmann T. Crystal structure of a pivotal domain of human syncytin-2, a 40 million years old endogenous retrovirus fusogenic envelope gene captured by primates. J Mol Biol. 2005;352(5):1029–34. doi: 10.1016/j.jmb.2005.07.058. [DOI] [PubMed] [Google Scholar]
- 22.Perez-Vargas J, Krey T, Valansi C, Avinoam O, Haouz A, Jamin M, Raveh-Barak H, Podbilewicz B, Rey FA. Structural basis of eukaryotic cell-cell fusion. Cell. 2014;157(2):407–19. doi: 10.1016/j.cell.2014.02.020. [DOI] [PubMed] [Google Scholar]
- 23.Zeev-Ben-Mordehai T, Vasishtan D, Siebert CA, Grunewald K. The full-length cell-cell fusogen EFF-1 is monomeric and upright on the membrane. Nat Commun. 2014;5:3912. doi: 10.1038/ncomms4912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cole ES, Cassidy-Hanley D, Fricke Pinello J, Zeng H, Hsueh M, Kolbin D, Ozzello C, Giddings T, Jr., Winey M, Clark TG. Function of the male-gamete-specific fusion protein HAP2 in a seven-sexed ciliate. Curr Biol. 2014;24(18):2168–73. doi: 10.1016/j.cub.2014.07.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson MA, von Besser K, Zhou Q, Smith E, Aux G, Patton D, Levin JZ, Preuss D. Arabidopsis hapless mutations define essential gametophytic functions. Genetics. 2004;168(2):971–82. doi: 10.1534/genetics.104.029447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wong JL, Johnson MA. Is HAP2-GCS1 an ancestral gamete fusogen? Trends Cell Biol. 2010;20(3):134–41. doi: 10.1016/j.tcb.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 27.Doherty GJ, McMahon HT. Mechanisms of endocytosis. Annu Rev Biochem. 2009;78:857–902. doi: 10.1146/annurev.biochem.78.081307.110540. [DOI] [PubMed] [Google Scholar]
- 28.Kabayama H, Nakamura T, Takeuchi M, Iwasaki H, Taniguchi M, Tokushige N, Mikoshiba K. Ca2+ induces macropinocytosis via F-actin depolymerization during growth cone collapse. Mol Cell Neurosci. 2009;40(1):27–38. doi: 10.1016/j.mcn.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 29.Kabayama H, Takeuchi M, Taniguchi M, Tokushige N, Kozaki S, Mizutani A, Nakamura T, Mikoshiba K. Syntaxin 1B suppresses macropinocytosis and semaphorin 3A-induced growth cone collapse. J Neurosci. 2011;31(20):7357–64. doi: 10.1523/JNEUROSCI.2718-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Davis GE, Camarillo CW. An alpha 2 beta 1 integrin-dependent pinocytic mechanism involving intracellular vacuole formation and coalescence regulates capillary lumen and tube formation in three-dimensional collagen matrix. Exp Cell Res. 1996;224(1):39–51. doi: 10.1006/excr.1996.0109. [DOI] [PubMed] [Google Scholar]
- 31.Yu JA, Castranova D, Pham VN, Weinstein BM. Single-cell analysis of endothelial morphogenesis in vivo. Development. 2015;142(17):2951–61. doi: 10.1242/dev.123174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kamei M, Saunders WB, Bayless KJ, Dye L, Davis GE, Weinstein BM. Endothelial tubes assemble from intracellular vacuoles in vivo. Nature. 2006;442(7101):453–6. doi: 10.1038/nature04923. [DOI] [PubMed] [Google Scholar]
- 33.Cohen J, Sundaram MV. Time to make the doughnuts: Building and shaping seamless tubes. Seminars in Cell & Developmental Biology Submitted. 2016 doi: 10.1016/j.semcdb.2016.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oren-Suissa M, Podbilewicz B. Evolution of programmed cell fusion: common mechanisms and distinct functions. Dev Dyn. 2010;239(5):1515–28. doi: 10.1002/dvdy.22284. [DOI] [PubMed] [Google Scholar]
- 35.Blaise S, de Parseval N, Benit L, Heidmann T. Genomewide screening for fusogenic human endogenous retrovirus envelopes identifies syncytin 2, a gene conserved on primate evolution. Proc Natl Acad Sci U S A. 2003;100(22):13013–8. doi: 10.1073/pnas.2132646100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dupressoir A, Vernochet C, Bawa O, Harper F, Pierron G, Opolon P, Heidmann T. Syncytin-A knockout mice demonstrate the critical role in placentation of a fusogenic, endogenous retrovirus-derived, envelope gene. Proc Natl Acad Sci U S A. 2009;106(29):12127–32. doi: 10.1073/pnas.0902925106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shemer G, Podbilewicz B. Fusomorphogenesis: cell fusion in organ formation. Dev Dyn. 2000;218(1):30–51. doi: 10.1002/(SICI)1097-0177(200005)218:1<30::AID-DVDY4>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 38.Avinoam O, Fridman K, Valansi C, Abutbul I, Zeev-Ben-Mordehai T, Maurer UE, Sapir A, Danino D, Grunewald K, White JM, Podbilewicz B. Conserved eukaryotic fusogens can fuse viral envelopes to cells. Science. 2011;332(6029):589–92. doi: 10.1126/science.1202333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Podbilewicz B, Leikina E, Sapir A, Valansi C, Suissa M, Shemer G, Chernomordik LV. The C. elegans developmental fusogen EFF-1 mediates homotypic fusion in heterologous cells and in vivo. Dev Cell. 2006;11(4):471–81. doi: 10.1016/j.devcel.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 40.Shemer G, Suissa M, Kolotuev I, Nguyen KC, Hall DH, Podbilewicz B. EFF-1 is sufficient to initiate and execute tissue-specific cell fusion in C. elegans. Curr Biol. 2004;14(17):1587–91. doi: 10.1016/j.cub.2004.07.059. [DOI] [PubMed] [Google Scholar]
- 41.del Campo JJ, Opoku-Serebuoh E, Isaacson AB, Scranton VL, Tucker M, Han M, Mohler WA. Fusogenic activity of EFF-1 is regulated via dynamic localization in fusing somatic cells of C. elegans. Curr Biol. 2005;15(5):413–23. doi: 10.1016/j.cub.2005.01.054. [DOI] [PubMed] [Google Scholar]
- 42.Brabin C, Appleford PJ, Woollard A. The Caenorhabditis elegans GATA factor ELT-1 works through the cell proliferation regulator BRO-1 and the Fusogen EFF-1 to maintain the seam stem-like fate. PLoS Genet. 2011;7(8):e1002200. doi: 10.1371/journal.pgen.1002200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cassata G, Shemer G, Morandi P, Donhauser R, Podbilewicz B, Baumeister R. ceh-16/engrailed patterns the embryonic epidermis of Caenorhabditis elegans. Development. 2005;132(4):739–49. doi: 10.1242/dev.01638. [DOI] [PubMed] [Google Scholar]
- 44.Pellegrino MW, Farooqui S, Frohli E, Rehrauer H, Kaeser-Pebernard S, Muller F, Gasser RB, Hajnal A. LIN-39 and the EGFR/RAS/MAPK pathway regulate C. elegans vulval morphogenesis via the VAB-23 zinc finger protein. Development. 2011;138(21):4649–60. doi: 10.1242/dev.071951. [DOI] [PubMed] [Google Scholar]
- 45.Shemer G, Podbilewicz B. LIN-39/Hox triggers cell division and represses EFF-1/fusogen-dependent vulval cell fusion. Genes Dev. 2002;16(24):3136–41. doi: 10.1101/gad.251202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mason DA, Rabinowitz JS, Portman DS. dmd-3, a doublesex-related gene regulated by tra-1, governs sex-specific morphogenesis in C. elegans. Development. 2008;135(14):2373–82. doi: 10.1242/dev.017046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Choi J, Richards KL, Cinar HN, Newman AP. N-ethylmaleimide sensitive factor is required for fusion of the C. elegans uterine anchor cell. Dev Biol. 2006;297(1):87–102. doi: 10.1016/j.ydbio.2006.04.471. [DOI] [PubMed] [Google Scholar]
- 48.Kontani K, Moskowitz IP, Rothman JH. Repression of cell-cell fusion by components of the C. elegans vacuolar ATPase complex. Dev Cell. 2005;8(5):787–94. doi: 10.1016/j.devcel.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 49.Smurova K, Podbilewicz B. RAB-5- and DYNAMIN-1-Mediated Endocytosis of EFF-1 Fusogen Controls Cell-Cell Fusion. Cell Rep. 2016;14(6):1517–27. doi: 10.1016/j.celrep.2016.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lenard A, Daetwyler S, Betz C, Ellertsdottir E, Belting HG, Huisken J, Affolter M. Endothelial cell self-fusion during vascular pruning. PLoS Biol. 2015;13(4):e1002126. doi: 10.1371/journal.pbio.1002126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sumida GM, Yamada S. Rho GTPases and the downstream effectors actin-related protein 2/3 (Arp2/3) complex and myosin II induce membrane fusion at self-contacts. J Biol Chem. 2015;290(6):3238–47. doi: 10.1074/jbc.M114.612168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Halevi S, McKay J, Palfreyman M, Yassin L, Eshel M, Jorgensen E, Treinin M. The C. elegans ric-3 gene is required for maturation of nicotinic acetylcholine receptors. EMBO J. 2002;21(5):1012–20. doi: 10.1093/emboj/21.5.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tsalik EL, Niacaris T, Wenick AS, Pau K, Avery L, Hobert O. LIM homeobox gene-dependent expression of biogenic amine receptors in restricted regions of the C. elegans nervous system. Dev Biol. 2003;263(1):81–102. doi: 10.1016/s0012-1606(03)00447-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liang X, Dong X, Moerman DG, Shen K, Wang X. Sarcomeres Pattern Proprioceptive Sensory Dendritic Endings through UNC-52/Perlecan in C. elegans. Dev Cell. 2015;33(4):388–400. doi: 10.1016/j.devcel.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dong X, Liu OW, Howell AS, Shen K. An extracellular adhesion molecule complex patterns dendritic branching and morphogenesis. Cell. 2013;155(2):296–307. doi: 10.1016/j.cell.2013.08.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Salzberg Y, Diaz-Balzac CA, Ramirez-Suarez NJ, Attreed M, Tecle E, Desbois M, Kaprielian Z, Bulow HE. Skin-derived cues control arborization of sensory dendrites in Caenorhabditis elegans. Cell. 2013;155(2):308–20. doi: 10.1016/j.cell.2013.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu OW, Shen K. The transmembrane LRR protein DMA-1 promotes dendrite branching and growth in C. elegans. Nat Neurosci. 2012;15(1):57–63. doi: 10.1038/nn.2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Salzberg Y, Ramirez-Suarez NJ, Bulow HE. The proprotein convertase KPC-1/furin controls branching and self-avoidance of sensory dendrites in Caenorhabditis elegans. PLoS Genet. 2014;10(9):e1004657. doi: 10.1371/journal.pgen.1004657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yip ZC, Heiman MG. Duplication of a Single Neuron in C. elegans Reveals a Pathway for Dendrite Tiling by Mutual Repulsion. Cell Rep. 2016;15(10):2109–17. doi: 10.1016/j.celrep.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 60.Smith CJ, Watson JD, VanHoven MK, Colon-Ramos DA, Miller DM., 3rd Netrin (UNC-6) mediates dendritic self-avoidance. Nat Neurosci. 2012;15(5):731–7. doi: 10.1038/nn.3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Smith CJ, O'Brien T, Chatzigeorgiou M, Spencer WC, Feingold-Link E, Husson SJ, Hori S, Mitani S, Gottschalk A, Schafer WR, Miller DM., 3rd Sensory neuron fates are distinguished by a transcriptional switch that regulates dendrite branch stabilization. Neuron. 2013;79(2):266–80. doi: 10.1016/j.neuron.2013.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zipursky SL, Grueber WB. The molecular basis of self-avoidance. Annu Rev Neurosci. 2013;36:547–68. doi: 10.1146/annurev-neuro-062111-150414. [DOI] [PubMed] [Google Scholar]
- 63.Schreiner D, Weiner JA. Combinatorial homophilic interaction between gamma-protocadherin multimers greatly expands the molecular diversity of cell adhesion. Proc Natl Acad Sci U S A. 2010;107(33):14893–8. doi: 10.1073/pnas.1004526107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wojtowicz WM, Flanagan JJ, Millard SS, Zipursky SL, Clemens JC. Alternative splicing of Drosophila Dscam generates axon guidance receptors that exhibit isoform-specific homophilic binding. Cell. 2004;118(5):619–33. doi: 10.1016/j.cell.2004.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hoy RR, Bittner GD, Kennedy D. Regeneration in crustacean motoneurons: evidence for axonal fusion. Science. 1967;156(3772):251–2. doi: 10.1126/science.156.3772.251. [DOI] [PubMed] [Google Scholar]
- 66.Birse SC, Bittner GD. Regeneration of giant axons in earthworms. Brain Res. 1976;113(3):575–81. doi: 10.1016/0006-8993(76)90058-5. [DOI] [PubMed] [Google Scholar]
- 67.Deriemer SA, Elliott EJ, Macagno ER, Muller KJ. Morphological evidence that regenerating axons can fuse with severed axon segments. Brain Res. 1983;272(1):157–61. doi: 10.1016/0006-8993(83)90373-6. [DOI] [PubMed] [Google Scholar]
- 68.Neumann B, Nguyen KC, Hall DH, Ben-Yakar A, Hilliard MA. Axonal regeneration proceeds through specific axonal fusion in transected C. elegans neurons. Dev Dyn. 2011;240(6):1365–72. doi: 10.1002/dvdy.22606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Neumann B, Coakley S, Giordano-Santini R, Linton C, Lee ES, Nakagawa A, Xue D, Hilliard MA. EFF-1-mediated regenerative axonal fusion requires components of the apoptotic pathway. Nature. 2015;517(7533):219–22. doi: 10.1038/nature14102. [DOI] [PubMed] [Google Scholar]
- 70.Pinan-Lucarre B, Gabel CV, Reina CP, Hulme SE, Shevkoplyas SS, Slone RD, Xue J, Qiao Y, Weisberg S, Roodhouse K, Sun L, Whitesides GM, Samuel A, Driscoll M. The core apoptotic executioner proteins CED-3 and CED-4 promote initiation of neuronal regeneration in Caenorhabditis elegans. PLoS Biol. 2012;10(5):e1001331. doi: 10.1371/journal.pbio.1001331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hammarlund M, Nix P, Hauth L, Jorgensen EM, Bastiani M. Axon regeneration requires a conserved MAP kinase pathway. Science. 2009;323(5915):802–6. doi: 10.1126/science.1165527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yan D, Wu Z, Chisholm AD, Jin Y. The DLK-1 kinase promotes mRNA stability and local translation in C. elegans synapses and axon regeneration. Cell. 2009;138(5):1005–18. doi: 10.1016/j.cell.2009.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sexton KW, Rodriguez-Feo CL, Boyer RB, Del Corral GA, Riley DC, Pollins AC, Cardwell NL, Shack RB, Nanney LB, Thayer WP. Axonal fusion via conduit-based delivery of hydrophilic polymers. Hand (N Y) 2015;10(4):688–94. doi: 10.1007/s11552-015-9780-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bittner GD, Sengelaub DR, Trevino RC, Peduzzi JD, Mikesh M, Ghergherehchi CL, Schallert T, Thayer WP. The curious ability of polyethylene glycol fusion technologies to restore lost behaviors after nerve severance. J Neurosci Res. 2016;94(3):207–30. doi: 10.1002/jnr.23685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bar T, Guldner FH, Wolff JR. “Seamless” endothelial cells of blood capillaries. Cell Tissue Res. 1984;235(1):99–106. doi: 10.1007/BF00213729. [DOI] [PubMed] [Google Scholar]
- 76.Wolff JR, Bar T. ‘Seamless’ endothelia in brain capillaries during development of the rat's cerebral cortex. Brain Res. 1972;41(1):17–24. doi: 10.1016/0006-8993(72)90613-0. [DOI] [PubMed] [Google Scholar]
- 77.Sundaram MV, Cohen JD. Time to make the doughnuts: Building and shaping seamless tubes. Semin Cell Dev Biol. 2016 doi: 10.1016/j.semcdb.2016.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sundaram MV, Buechner M. The Caenorhabditis elegans Excretory System: A Model for Tubulogenesis, Cell Fate Specification, and Plasticity. Genetics. 2016;203(1):35–63. doi: 10.1534/genetics.116.189357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sharma-Kishore R, White JG, Southgate E, Podbilewicz B. Formation of the vulva in Caenorhabditis elegans: a paradigm for organogenesis. Development. 1999;126(4):691–9. doi: 10.1242/dev.126.4.691. [DOI] [PubMed] [Google Scholar]
- 80.Shemer G, Kishore R, Podbilewicz B. Ring formation drives invagination of the vulva in Caenorhabditis elegans: Ras, cell fusion, and cell migration determine structural fates. Dev Biol. 2000;221(1):233–48. doi: 10.1006/dbio.2000.9657. [DOI] [PubMed] [Google Scholar]
- 81.Rasmussen JP, Feldman JL, Reddy SS, Priess JR. Cell interactions and patterned intercalations shape and link epithelial tubes in C. elegans. PLoS Genet. 2013;9(9):e1003772. doi: 10.1371/journal.pgen.1003772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nelson FK, Albert PS, Riddle DL. Fine structure of the Caenorhabditis elegans secretory-excretory system. J Ultrastruct Res. 1983;82(2):156–71. doi: 10.1016/s0022-5320(83)90050-3. [DOI] [PubMed] [Google Scholar]
- 83.Nelson FK, Riddle DL. Functional study of the Caenorhabditis elegans secretory-excretory system using laser microsurgery. J Exp Zool. 1984;231(1):45–56. doi: 10.1002/jez.1402310107. [DOI] [PubMed] [Google Scholar]
- 84.Berry KL, Bulow HE, Hall DH, Hobert O. A C. elegans CLIC-like protein required for intracellular tube formation and maintenance. Science. 2003;302(5653):2134–7. doi: 10.1126/science.1087667. [DOI] [PubMed] [Google Scholar]
- 85.Abdus-Saboor I, Mancuso VP, Murray JI, Palozola K, Norris C, Hall DH, Howell K, Huang K, Sundaram MV. Notch and Ras promote sequential steps of excretory tube development in C. elegans. Development. 2011;138(16):3545–55. doi: 10.1242/dev.068148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Betz C, Lenard A, Belting HG, Affolter M. Cell behaviors and dynamics during angiogenesis. Development. 2016;143(13):2249–60. doi: 10.1242/dev.135616. [DOI] [PubMed] [Google Scholar]
- 87.Kochhan E, Lenard A, Ellertsdottir E, Herwig L, Affolter M, Belting HG, Siekmann AF. Blood flow changes coincide with cellular rearrangements during blood vessel pruning in zebrafish embryos. PLoS One. 2013;8(10):e75060. doi: 10.1371/journal.pone.0075060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lobov IB, Cheung E, Wudali R, Cao J, Halasz G, Wei Y, Economides A, Lin HC, Papadopoulos N, Yancopoulos GD, Wiegand SJ. The Dll4/Notch pathway controls postangiogenic blood vessel remodeling and regression by modulating vasoconstriction and blood flow. Blood. 2011;117(24):6728–37. doi: 10.1182/blood-2010-08-302067. [DOI] [PubMed] [Google Scholar]
- 89.Phng LK, Potente M, Leslie JD, Babbage J, Nyqvist D, Lobov I, Ondr JK, Rao S, Lang RA, Thurston G, Gerhardt H. Nrarp coordinates endothelial Notch and Wnt signaling to control vessel density in angiogenesis. Dev Cell. 2009;16(1):70–82. doi: 10.1016/j.devcel.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lee GS, Filipovic N, Lin M, Gibney BC, Simpson DC, Konerding MA, Tsuda A, Mentzer SJ. Intravascular pillars and pruning in the extraembryonic vessels of chick embryos. Dev Dyn. 2011;240(6):1335–43. doi: 10.1002/dvdy.22618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sumida GM, Yamada S. Self-contact elimination by membrane fusion. Proc Natl Acad Sci U S A. 2013;110(47):18958–63. doi: 10.1073/pnas.1311135110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sacharidou A, Stratman AN, Davis GE. Molecular mechanisms controlling vascular lumen formation in three-dimensional extracellular matrices. Cells Tissues Organs. 2012;195(1-2):122–43. doi: 10.1159/000331410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sabatini PJ, Zhang M, Silverman-Gavrila RV, Bendeck MP. Cadherins at cell-autonomous membrane contacts control macropinocytosis. J Cell Sci. 2011;124(Pt 12):2013–20. doi: 10.1242/jcs.076901. [DOI] [PubMed] [Google Scholar]
- 94.Bayless KJ, Davis GE. The Cdc42 and Rac1 GTPases are required for capillary lumen formation in three-dimensional extracellular matrices. J Cell Sci. 2002;115(Pt 6):1123–36. doi: 10.1242/jcs.115.6.1123. [DOI] [PubMed] [Google Scholar]
- 95.Zhao Z, Nelson AR, Betsholtz C, Zlokovic BV. Establishment and Dysfunction of the Blood-Brain Barrier. Cell. 2015;163(5):1064–78. doi: 10.1016/j.cell.2015.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Parry JM, Sundaram MV. A non-cell-autonomous role for Ras signaling in C. elegans neuroblast delamination. Development. 2014;141(22):4279–84. doi: 10.1242/dev.112045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sulston JE, Horvitz HR. Post-embryonic cell lineages of the nematode, Caenorhabditis elegans. Dev Biol. 1977;56(1):110–56. doi: 10.1016/0012-1606(77)90158-0. [DOI] [PubMed] [Google Scholar]
- 98.Lenard A, Ellertsdottir E, Herwig L, Krudewig A, Sauteur L, Belting HG, Affolter M. In vivo analysis reveals a highly stereotypic morphogenetic pathway of vascular anastomosis. Dev Cell. 2013;25(5):492–506. doi: 10.1016/j.devcel.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 99.Koppen M, Simske JS, Sims PA, Firestein BL, Hall DH, Radice AD, Rongo C, Hardin JD. Cooperative regulation of AJM-1 controls junctional integrity in Caenorhabditis elegans epithelia. Nat Cell Biol. 2001;3(11):983–91. doi: 10.1038/ncb1101-983. [DOI] [PubMed] [Google Scholar]
- 100.Gill HK, Cohen JD, Ayala-Figueroa J, Forman-Rubinsky R, Poggioli C, Bickard K, Parry JM, Pu P, Hall DH, Sundaram MV. Integrity of narrow epithelial tubes in the C. elegans excretory system requires a transient luminal matrix. PLOS Genetics. 2016 doi: 10.1371/journal.pgen.1006205. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]