Abstract
Low capacity runner (LCR) and high capacity runner (HCR) rat strains are divergent for running capacity and aerobic fitness. The LCR rats are susceptible to obesity, insulin resistance, and fatty liver while the HCR are protected. We performed studies testing the hypothesis that the divergence in susceptibility for obesity and metabolic dysfunction between HCR/LCR is due to differences in hepatic mitochondrial function that may also drive differences in energy expenditure and substrate utilization.
Keywords: Fitness, aerobic capacity, mitochondria, energy expenditure, obesity, hepatic steatosis, fat oxidation
Summary
High- and low-running capacity rats display differences in mitochondrial metabolism that drive differences in susceptibility for metabolic disease.
Introduction
Aerobic capacity, also termed cardiorespiratory fitness, is the capacity of the body to deliver and utilize oxygen during maximal intensity exercise. Aerobic capacity is commonly measured with direct assessments of oxygen consumption (VO2max) during graded exercise tests to exhaustion in exercise physiology labs and clinics. In addition, exercise tests to failure without direct measures of oxygen consumption and sub-maximal field tests can also provide indirect assessments of maximal aerobic capacity. Thus, aerobic capacity is a physiological outcome measure that can be tested in a variety of settings. Most importantly, aerobic capacity has proven to be critical for health. In fact, it could be argued that aerobic capacity is the most critical health-related outcome measure. Low aerobic capacity increases the risk for the development of multiple disease states and increases the risk for early mortality regardless of disease state (4, 18, 40). In contrast, high aerobic capacity is linked to lower risk for disease and greater overall survivability (4, 18, 40).
Aerobic Capacity, Much is Left To Be Discovered
Factors controlling aerobic capacity are a merging of daily behavior patterns, genetics, and age. Current evidence suggests that aerobic capacity peaks in the second- to third-decade of human life before it begins to slowly decline through the subsequent decades of life (5). Importantly, however, most individuals can embark on an exercise program at any age and see measurable improvements in aerobic capacity in weeks to months. Studies suggest that approximately 70% of fitness is due to genetics and not solely due to daily activities or exercise training (8). Current evidence in both humans and rodents suggests that there is a “lifetime apex” for peak aerobic capacity after which the capacity declines throughout aging despite individuals remaining sedentary or staying highly-active (6). This was recently shown in rats where daily wheel running versus sedentary cage only activity increased VO2peak but did not change the age at which the apex for VO2peak occurred (37). This has led to the concept of achieving a high level of aerobic capacity at a young age, so that the decline that occurs with aging will not reach a critical “frailty” threshold until an older age (6).
Although aerobic capacity is clearly linked to health and mortality it is surprising that so many basic research questions underlying this fundamental physiological readout remain largely unanswered. For example, we are unaware of research that has clearly deciphered whether the possession of low aerobic capacity due to genetics or due to low physical activity differently alters disease and mortality. However, it is more likely that low aerobic capacity due to low physical activity patterns is the primary culprit. A recent report cited that physical inactivity and low aerobic capacity provide a similar increase in mortality rate, and that the greatest protection is provided by staying out of the most “unfit” or most “inactive” groups (7). The concept that fitness due to daily activity patterns and not genetics is most critical for health is supported by a report that individuals who went from being “unfit” to “fit” by increasing physical activity between two doctor visits spread apart by 4.9 years significantly lowered mortality (3). However, evidence in a rat model described later in this review clearly suggests that genetic (intrinsic) fitness can also play an important role.
There are multiple reasons why the mechanistic links between fitness status and disease susceptibility are unknown. First, aerobic capacity status is largely underappreciated by the medical and biomedical science communities (1). Additional issues include that studies in humans would be difficult. Most researchers can only obtain muscle while access to other tissues (heart, lungs, liver, etc.) is difficult or impossible. The primary focus of the biomedical community has been on single gene manipulation within animal models;, approaches that cannot mimic the polygenetic/multi-tissue effects required to study the impact of divergent aerobic capacity levels. A final complication is the difficulty in separating the impact of exercise vs. intrinsic fitness itself. For example, highly fit humans are also usually regular exercisers, and thus it is unknown if the outcomes measured in these individuals is due to fitness or due to the effects of different physical activity or exercise patterns as already mentioned. Because of these limitations, an appropriate model to examine the mechanisms by which high and low aerobic capacity impacts disease was desperately needed.
A Model To Answer “Why” Genetic Aerobic Capacity Impacts Health
To fill the model gap, Drs. Steve Britton and Lauren Koch began breeding high and low capacity running rats (HCR/LCR) over 15 years ago with the goal of creating rats with intrinsically high and low aerobic capacity (15, 41). Outbred NIH:N strain rats were tested for exercise capacity to exhaustion on treadmills, followed by selective breeding for high (top 10%) or low (bottom 10%) running performance over successive generations. The design and breeding of the model is described in detail elsewhere (15). After several generations of breeding, the HCR run dramatically longer than LCR rats during exercise tests, despite the animals receiving no exposure to exercise training and simply being maintained in cages. Therefore, the strains intrinsically possess the phenotype of high or low running capacity due to permanent genetic changes. Importantly, outcomes in the HCR/LCR model mimic the impact of aerobic capacity on human health. LCR rats fed a normal chow diet show multiple cardiovascular/metabolic syndrome risk factors at a young age, while the HCR display no risk factors (41). The LCR show early mortality, living approximately a 6-month shorter lifespan than the HCR rats, studies that were replicated in two different cohorts of rats (17). Also, the HCR displayed 60% higher VO2peak compared to the LCR rats following exercise testing (41). Therefore, by breeding for endurance capacity, Britton and Koch created rats with truly divergent aerobic capacities and phenotypical characterization that largely mimic health outcomes measured in humans. Recent studies continue to match findings in this model to those in humans. LCR rats have now been shown to be more susceptible to a variety of other conditions that are also linked to low aerobic capacity in humans (16, 26, 39).
Why Does Aerobic Capacity Status Impact Susceptibility For Disease?
Our laboratory has primarily been interested in the role of physical activity/exercise and fitness to impact susceptibility for metabolic diseases. The remainder of this review will focus on our studies examining the links between aerobic capacity and metabolic health including a focus on fatty liver disease in the HCR/LCR rat model. The underlying hypothesis of these studies is that intrinsic aerobic fitness impacts hepatic mitochondrial function which impacts susceptibility for metabolic conditions systemically and within the liver.
Initial studies in the HCR/LCR model established a clear metabolic phenotype between the strains. Wisloff et al. (41) reported that skeletal muscle of the HCR rats displayed higher mitochondrial content and oxidative capacity than the LCR, matching the well-established muscle mitochondrial phenotypes described by multiple studies in exercise trained vs. untrained humans (33). Low skeletal muscle-mitochondrial content and fatty acid oxidative (FAO) capacity have been linked to skeletal muscle insulin resistance and obesity for many years, although it remains a controversial topic. Noland et al. (25) utilized a chronic (8 week) high fat diet (HFD; 50% of energy from fat) in male HCR/LCR rats to examine susceptibility for dietary induced obesity and insulin resistance. HFD’s in rodents are a commonly utilized tool to induce insulin resistance and obesity. We expected the LCR to demonstrate greater susceptibility to the HFD than the HCR due to low skeletal muscle mitochondrial content and FAO. As expected, the LCR displayed reduced total FAO in skeletal muscle homogenates and reduced mitochondrial content (citrate synthase enzyme activity) compared to the HCR on a chow diet. Interestingly, both the HCR and LCR increased skeletal muscle FAO on the HFD, resulting in no difference between the strains after the HFD. To compliment this profile, the LCR displayed higher initial body mass, higher fasting glucose and insulin, and higher insulin responses to an oral glucose tolerance test than the HCR on the normal chow diet. After 8 weeks of the HFD, the LCR displayed pronounced weight gain, and significant increases in both glucose and insulin during the oral glucose tolerance test, evidence of worsening insulin resistance. In contrast, the HCR animals showed protection against these effects as the body weight gain and glucose/insulin responses in the HFD fed HCR rats mirrored those of the HCR rats fed normal chow. This was the first evidence that the HCR rats were protected against HFD-induced weight gain and insulin resistance, while the LCR were susceptible. Very similar outcomes were found in female HCR/LCR rats following a HFD in studies from our laboratory (24). At the time of these studies, skeletal muscle mitochondrial oxidative capacity was a prime area of focus linking obesity and insulin resistance. Because of this, and the well-known link between high and low endurance capacity and divergent skeletal muscle mitochondrial content and oxidative capacity, the field largely believed the metabolic differences between the HCR and LCR was tied to divergent skeletal muscle phenotypes (2).
In 2006, Church and colleagues (10) reported that low fit men were at a greater risk for developing non-alcoholic fatty liver disease (NAFLD) compared to moderate and high fit men. NAFLD is a clinical condition (also called hepatic steatosis) in which greater than 5% of liver weight is comprised of fat.. NAFLD commonly occurs with obesity and is linked to elevated hepatic glucose production and an increased risk for the development of type 2 diabetes. The study also reported that controlling for body weight differences did not impact the statistical relationship between fitness and NAFLD. The report by Church et al. (10) led our laboratory to hypothesize that low fit-LCR rats may also have a greater risk for hepatic steatosis. We further questioned if the increased risk for NAFLD in the low fit-LCR would be secondary to reduced hepatic mitochondrial content and/or reduced hepatic FAO.
Hepatic Mitochondria and Susceptibility For Fatty Liver Disease: Impact of Fitness
Mitochondrial FAO (specifically β-oxidation) is the dominant oxidative pathway for the complete disposal of long chain fatty acids in the liver. Total capacity for hepatic FAO is controlled by a variety of conditions including hepatic mitochondrial content or density, enzymatic activity of CPT-1α, the rate limiting step for long chain fatty acid entry into the mitochondrial, and a variety of other factors including substrate supply and energy status. Previous results from our collaborative research team have shown that reduced hepatic mitochondrial FAO (FAO) increased risk for hepatic steatosis. We have shown that hyperphagic, obese OLETF rats display hepatic steatosis in association with reduced hepatic mitochondrial content and lower hepatic mitochondrial complete FAO (oxidation of 14C–palmitate to 14CO2) (28). We have also shown that hepatic steatosis is effectively prevented or treated with exercise effects that track with increased hepatic FAO and other markers of enhanced mitochondrial content and function (27, 28). In addition, we also showed that the OLETF possesses lower hepatic mitochondrial content and FAO in the liver at a young age prior to the development of hepatic steatosis (29).
As expected, we found that the LCR display evidence of lower hepatic mitochondrial content, and lower hepatic mitochondrial FAO compared to high fit-HCR rats (36). The LCR also displayed mild NAFLD at a young age on a normal chow diet, marked by higher hepatic triglycerides, steatosis score, and percent of nuclei associated with lipid droplets in the LCR vs. the HCR. Typically, NAFLD is not witnessed in young rodents unless the animals are obese following a high fat diet, hyperphagic due to altered satiety signals (OLETF and Zucker rats), or with specific genetic alterations that negatively impact lipid metabolism.. The LCR is heavier than the HCR rat, but is not grossly obese like OLETF or Zucker models. Body composition analysis has shown that the 20–30% greater body mass in the LCR over the HCR is partially due to a bigger body size (longer snout to tail), with increases in both lean body mass and slightly higher body fat mass (21, 25). Therefore, young, normal chow fed fit-LCR rats displayed hepatic steatosis without dietary manipulation or excessive obesity. Most importantly, the pre-clinical HCR/LCR data matched the results from Church et al. (10) in human subjects that fitness was an independent risk factor for NAFLD.
Why Would Hepatic Mitochondrial Content and FAO Be Elevated in High vs. Low Fit Rats?
As previously mentioned, the health outcome data first collected in the HCR/LCR rats suggested that skeletal muscle mitochondrial content and function and cardiovascular adaptations were the fundamental reason for differences in susceptibility to metabolic disease in the model (41). This was a logical assumption given that heart and muscle are two highly important tissues for delivering and utilizing oxygen during exercise, respectively. However, the liver also plays a critical, but underappreciated, role in the metabolic demands of endurance exercise capacity. As stated elegantly by Trefts, Williams, and Wasserman et al. (38) “The accelerated demands of working muscle cannot be met without a robust response from the liver. If not for the hepatic response, sustained exercise would be impossible. The liver stores, releases, and recycles potential energy. Exercise would result in hypoglycemia if it were not for the accelerated release of energy as glucose.” The needs for liver glucose production are necessitated by the very limited amounts of circulating glucose (~4 grams) that would be diminished in minutes after the onset of exercise.
High rates of ATP production are required to sustain hepatic gluconeogenic flux during prolonged exercise. The liver generates ATP by increasing mitochondrial oxidation of fatty acids that are being delivered to the liver at a high rate secondary to increased lipolysis of triglycerides from adipose stores. Therefore, the liver acts as a core energetic converter during prolonged exercise.
Importantly, the graded exercise test to failure used to select the HCR/LCR rats was designed differently than what is commonly utilized in humans. In human subjects, the goal is to reach maximal effort and thus VO2max between ~8 to ~15 minutes of duration. However, the goal of the breeding program designed by Britton and Koch was to selectively breed for endurance running capacity, therefore, they designed a graded exercise test (increased treadmill speed every 2 minutes) that was longer in distance and duration and truly tested “endurance” capacity (15). In the founder population the rats ran 355 meters for ~30 minutes before reaching exhaustion. By generation 6 the HCR rats were running for an average of 839 meters for 42 (15). The HCR rats have continued to increase running distance and duration over several generations of breeding and are now running an average distance of ~2,000 meters at generation 28. In converse, the LCR are running only an average of 200 meters at generation 28 (30).
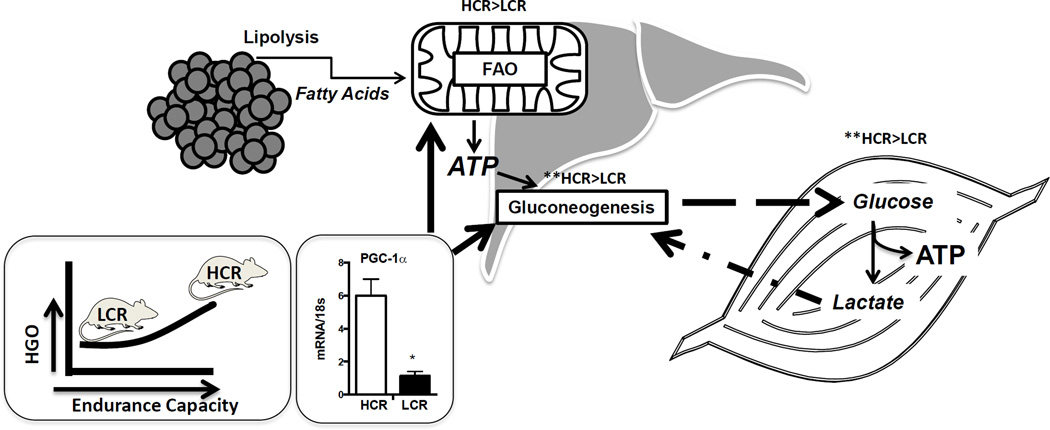
Pronounced differences in hepatic mitochondrial content and function between the HCR and LCR are one of the primary traits acquired as a result of selectively breeding for high and low endurance exercise capacity . We posit that the higher hepatic mitochondrial content and function in the HCR is necessary for fueling the elevated rates of gluconeogenesis that would be needed for prolonged endurance capacity.. In contrast, we posit that selectively breeding for reduced endurance capacity led to decreased hepatic mitochondrial content and function. PGC-1α is a ubiquitously expressed transcriptional co-activator that functions as a master regulator of mitochondrial biogenesis, oxidative phosphorylation, and fatty acid oxidation (11) and is also a major regulator of gluconeogenesis in the liver (42). As expected the HCR chronically display 4 fold higher expression of hepatic PGC-1α compared to the LCR (Figure 1). PGC-1α is known to be increased by exercise in muscle and liver (11), but differences in hepatic PGC-1α between the HCR and LCR would also indicate that expression patterns are inherited, a factor that is worthy of future exploration. It should be noted that we do not know if hepatic PGC-1a levels between the HCR and LCR rats have continued to become more divergent over several generations of selective breeding. Figure 1 depicts a diagram showing the relationship between endurance running capacity, hepatic PGC-1α, hepatic FAO, and hepatic glucose output capacity. Although we have not proven that HCR have greater hepatic glucose output, we do have unpublished data showing that the LCR have an impaired ability to maintain circulating glucose during prolonged fasting (unpublished), data that supports reduced hepatic glucose output in the LCR vs. HCR.
Figure 1.
Theoretical mechanism by which aerobic capacity differences in the HCR/LCR rats impacts hepatic metabolism through PGC-1α. Elevated endurance exercise capacity would be dependent upon the ability to maintain euglycemia through sustained gluconeogenesis. The lipolysis of free fatty acids during prolonged exercise provides necessary substrate for hepatic mitochondrial fatty acid oxidation which generates ATP to fuel gluconeogenesis. Hepatic mitochondrial content, fat oxidation, and gluconeogenesis are transcriptionally regulated by the transcriptional co-activator peroxisome gamma co-activator 1 alpha (PGC1α) that we have found to be expressed consistently higher in the liver of HCR vs. LCR rats.
As previously mentioned, skeletal muscle of the HCR/LCR rats also display differences in mitochondrial content and FAO (25), an effect that we have found to be less pronounced in females (24). Interestingly, other tissues have also shown similar differences in mitochondrial content, function, or proteins controlling mitochondrial biogenesis between the HCR and LCR including white (35) and brown adipose tissue (20, 39). How selective breeding for endurance exercise capacity would necessitate differences in mitochondrial phenotypes in adipose are beyond the scope of this review, but deserve attention and also likely play an important role for disease susceptibility between the HCR and LCR rats.
Testing Susceptibility for Dietary Induced Steatosis in High vs. Low Fit Rats: Integrative Responses
We next sought to determine if the divergence in hepatic mitochondrial content and FAO between the HCR and LCR rats would alter susceptibility for fatty liver disease induced by a high fat diet (HFD). Chronic HFD’s (45 to 60% of energy from fat) are often utilized to induce hepatic steatosis, but other work had shown that acute 3 day HFD could be used to induce hepatic steatosis. We favored the acute HFD approach in order to examine the acute responses to the diet rather than examine responses after a chronic HFD when it is less likely to decipher what is cause or effect. Weight gain in humans can occur incrementally as a result of acute bouts of overconsumption that occur on holidays or weekends, periods of time that would match a 3 day HFD approach. Our hypothesis was that the high fit-HCR rats would be protected against acute HFD induced weight gain and hepatic steatosis due to their elevated hepatic mitochondrial content and FAO. In contrast, the LCR would be very susceptible to the insult. However, we also suspected that other peripheral factors including metabolic flexibility and energy balancer were implicated.
Mechanism(s) for excessive fat storage in the liver are: reduced FAO, increased or excessive fatty acid uptake, reduced triacylglycerol secretion, or increased de novo lipogenesis. Alterations in all of these factors may be involved to one degree or another in the development of hepatic steatosis depending on the model or condition that is being studied. In addition, factors related to energy balance are also likely at play. Conditions in which energy intake is significantly greater than energy expenditure leads to weight gain and expansion of lipid stores in not only adipocytes but also in ectopic storage into muscle and liver. The best evidence for this is that over 70% of obese, and 100% of extremely obese individuals reportedly have fatty liver (9). Therefore, although we believe the hepatic mitochondrial phenotype impacts susceptibility for hepatic steatosis there is no doubt that peripheral metabolic factors also play a role. Given that the HCR/LCR already had known differences in peripheral metabolism, we designed 3-day HFD studies to carefully examine integrative metabolism from the whole body to isolated hepatic mitochondria.
Subheading 1: Energy Balance and Weigh Gain
Indirect calorimetry experiments were performed with the HCR/LCR rats prior to and during the transition to the 3-day HFD so that precise measurements of energy expenditure, energy intake, and energy balance could be made (21). Total energy expenditure was not different between the HCR/LCR rats on either diet condition. However, given that the LCR displayed 30% higher body mass, it was obvious that energy expenditure per unit of mass was not the same. Therefore, we adjusted for body mass differences by covariate analysis, which revealed that the HCR displayed higher basal energy expenditure than the LCR. As was reported previously (36), the HCR showed higher spontaneous activity in their cages than the LCR as measured by X and Y beam breaks. However, the increased spontaneous activity was not the sole reason for differences in total energy expenditure. To eliminate the effects of the differences in spontaneous activity, we approximated “resting energy expenditure” from periods of time when the rats had the least amount of movement and displayed a prolonged period of reduced oxygen consumption. Resting energy expenditure, when adjusted for body mass by covariate analysis, was still higher in the HCR than the LCR. Importantly, resting energy expenditure makes up more than 70% of total energy expenditure in most cages rodents and thus is a critical driver of total energy expenditure in rodents.
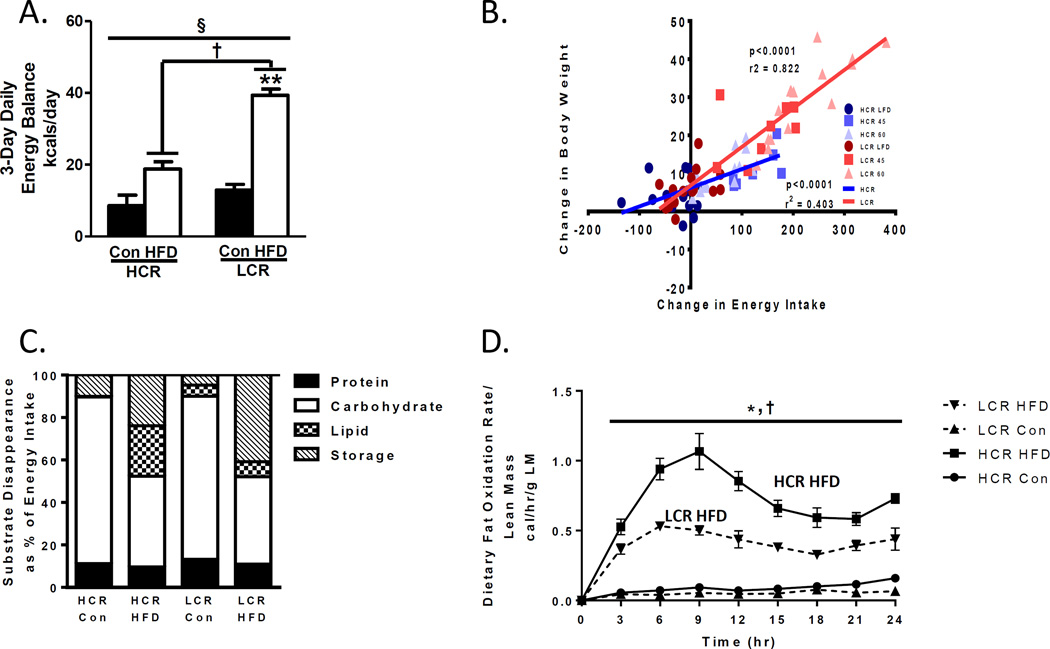
Food and energy intake values were also different between strains after the transition to the HFD (21). Although both groups significantly increased the energy intake of the calorically rich HFD, the increase was two-fold higher in the LCR than the HCR. As a result, both groups displayed increases in positive energy balance with the 3 day HFD. However, the HFD-induced positive energy balance was 50% higher in the LCR than the HCR (Figure 2A).
Figure 2.
Energy balance and substrate utilization differences in HCR/LCR after transition to a 3-day HFD. A) LCR displays a significantly higher increase in energy balance then HCR after transition to a 3 day HFD. B) Energy intake during a 1 week HFD (45 or 60% kcals) correlate with weight gain more strongly in the LCR than the HCR suggesting that energy intake plays an important role in HFD induced weight gain differences between strains (previously published in. C) HCR display a greater capacity to increase lipid utilization as a % of total energy intake after transitioning to an acute HFD compared to the LCR. D) HCR rats display a greater increase in dietary fatty acid oxidation upon transition to a HFD than LCR. Figure 2 A), C), and D) (Reprinted from (21). Copyright © 2014 The American Physiological Society. Used with permission.) Figure 2B) (Reprinted from (20). Copyright © 2016 John Wiley & Sons. Used with permission.)
The explanation for the increased energy intake in the LCR over the HCR rats upon transition to the HFD is unknown. However, we have completed additional studies (unpublished) and found the HFD induces elevated energy intake in the LCR over the HCR for up to 3 weeks. We have also found this energy intake to be predictive of weight gain during a 1 week HFD (Figure 2B) (20). Existing data from two separate groups may explain these results, and interestingly, it could be related to hepatic mitochondrial FAO and energy state differences. The Friedman group showed that chemical inhibition of hepatic FAO lowered hepatic ATP and acutely increased (up to 4 hours) food intake in rats (12). They further showed that this acute increase in food intake was signaled to the brain through vagal afferent signaling (13). The only other polygenetic rat model to show a protection and susceptibility to HFD induced obesity is the obese prone and obesity resistant rat model developed by Dr. Barry Levin. Sprague Dawley rats were bred over several generations to be prone or a resistant to HFD induced weight gain (31). A primary driver of the phenotype for weight gain in the obese prone is greater energy intake upon transition to the HFD. Interestingly, Friedman et al. found the obese prone rats to also show lower hepatic FAO and ATP levels, which he hypothesized to be a primary driver of their energy intake(14). Clearly more studies are needed determine the role of fitness on hepatic mitochondrial FAO and links to energy intake. Of note, obese humans have been reported repeatedly to display lower hepatic ATP than lean counterparts (23), suggesting that this same phenomenon could occur in humans. However, we are unaware of studies that have tested this hypothesis, or have even examined if sedentary low fitness humans display different ATP levels than high fit humans.
The acute HFD caused both groups to gain total body mass and fat mass, but again, the LCR displayed a greater weight gain and a larger increase in total body fat following the HFD. As expected by the whole body fat mass data, the HFD induced changes in fat pads weights were altered differently between the HCR and LCR rats. The percent increase in fat pad mass in the HFD vs. low fat control diet groups was double in the LCR what it was in the HCR. This increase occurred in the omental, retroperitoneal, mesenteric, and epididymal fat pads providing evidence that the LCR display higher risk for visceral fat pad expansion on a short term HFD.
Subheading 2: Metabolic Flexibility and Dietary Fat Trafficking
We also used two separate approaches to examine metabolic flexibility and whole body FAO between the LCR and HCR rats after transition to the 3 day HFD. Metabolic flexibility is the ability to switch between a reliance on fat or carbohydrate depending on nutrient conditions. Metabolic flexibility is commonly measured by respiratory quotient using indirect calorimetry whereby a RQ of 0.7 is 100% fat utilization while a RQ of 1.0 is 100% carbohydrate utilization according to stoichiometry. Metabolic flexibility can be assessed in transition from a low to a HFD where the RQ should be reduced quickly after the onset of the HFD. The HCR and LCR displayed clear differences in metabolic flexibility according to RQ results (21). The lowering of RQ induced by the HFD was significantly greater in the HCR than the LCR. Using the RQ data it is possible to calculate the % of energy utilization from the substrates of carbohydrate, fat, or protein. Examining the data in this manner revealed clear differences in substrate utilization between the HCR and LCR. On the control diet there was no differences between strains, but upon transition to the 3 day HFD, the HCR were able to increase fat utilization by ~20 fold while the LCR could only increase it by 1.35 fold (Figure 2B). Therefore, intrinsic fitness dramatically impacts the ability to switch between fuel sources acutely.
As a second method for analyzing fat utilization, we also measured whole body dietary FAO. Both the low fat control diet and the HFD were labeled with radiolabeled lipids (14C labeled oleate and palmitate) (21) allowing us to measure whole body FAO by measuring 14C tracer in expired CO2. Both groups displayed increased dietary FAO on the HFD, but the HCR response was 46% greater than the LCR (Figure 2C). Interestingly, the HCR also displayed 43% greater dietary FAO on the low fat control diet. In conclusion, the HCR displayed significantly more robust responses in changing whole body fat utilization in response to the HFD compared to the LCR. Interestingly, these dramatic differences occurred even though the LCR consumed a greater quantity of the HFD containing more tracer than the LCR.
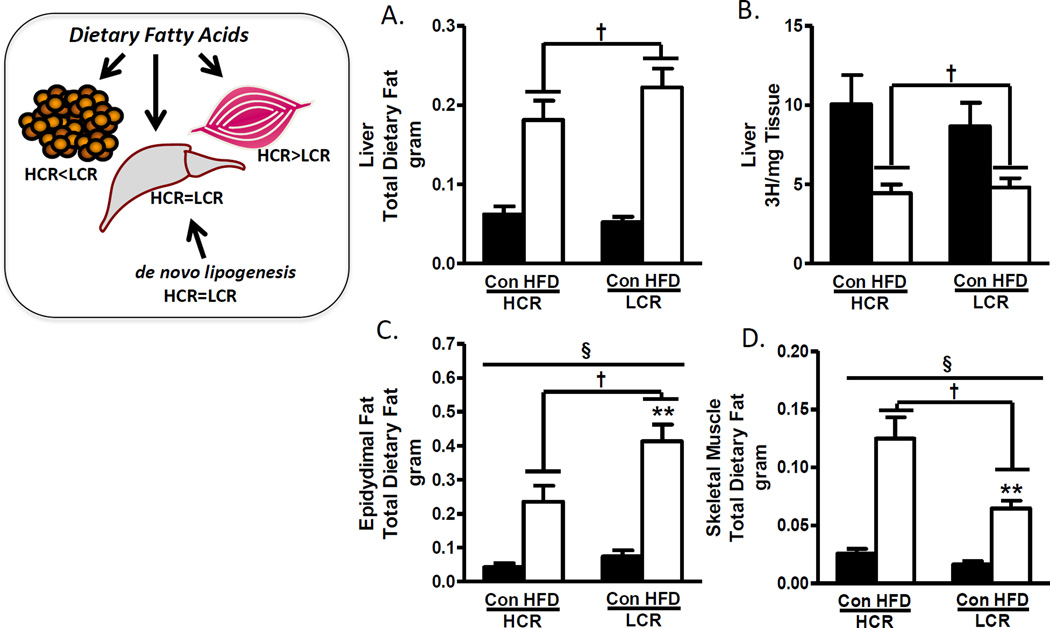
The radiolabeled dietary lipids also allowed us to quantify the trafficking of dietary lipids into liver, adipose, and skeletal muscle. The HFD resulted in a robust 3-fold increase in dietary lipids trafficked to the liver in both groups suggesting that the greater steatosis found in the LCR rats is not simply due to greater trafficking of dietary lipids to liver. However, both skeletal muscle and adipose displayed differential lipid trafficking between the strains on a HFD. The HCR increased dietary lipid trafficking into skeletal muscle 2-fold higher than the LCR. While the HFD increased dietary lipid trafficking to epididymal fat in both groups, the LCR had a 75% greater net retention of dietary fat than the HCR. Similar patterns of lipid retention between the HCR and LCR were observed in other fat pads (retroperitoneal, mesenteric, omental, and inguinal).
In addition to the robust differences in whole body dietary FAO, the differential trafficking of dietary fat into skeletal muscle or adipose provides additional insight. Figure 3 depicts the dietary lipid trafficking differences between the HCR and LCR rats. The high fit-HCR primarily traffics lipids to muscle in which they will eventually be oxidized and cannot be re-released into circulation, while in contrast, the LCR primarily traffic dietary lipids to adipose pads where the lipids will be stored for a period of time, but will then be mobilized and subsequently oxidized in other metabolic tissues (liver, muscle, heart). Therefore, the HCR appear to have an advantage in trafficking lipids to muscle over liver, but the role of this trafficking on the steatosis outcomes and overall differences in susceptibility or protection to obesity is associative and further mechanistic studies are required.
Figure 3.
HCR/LCR partition dietary lipids differently after transition to a 3-day HFD. A. HCR and LCR show similar increases in dietary lipid deposition into the liver following 3-day HFD. B. Both groups show suppressed de novo lipogenesis after 3-day HFD. B&C. Transition to 3-day high fat diet increases dietary lipid deposition into adipose and skeletal muscle of both strains, however, the LCR primarily increase deposition in adipose, while the HCR primarily increase deposition into skeletal muscle. Figure 3A–D (Reprinted from (20). Copyright © 2016 John Wiley & Sons. Used with permission.)
The central mechanisms driving the differences in whole body metabolic flexibility and substrate trafficking between the HCR/LCR are unknown, but we have previously reported evidence that the differences in hepatic-PGC-1α and –mitochondrial oxidative capacity may play a role (22). We overexpressed hepatic PGC-1α in obesity prone rats prior to a 3-day high fat diet. The 3-day HFD lowed hepatic mitochondrial respiratory capacity and reduced metabolic flexibility in rats receiving a control virus, however, rats with hepatic PGC-1α overexpression maintained both hepatic respiratory capacity and whole body metabolic flexibility despite the HFD. IN addition, we found that dietary lipid trafficking to skeletal muscle was significantly increased in the rats with PGC-1α overexpression (unpublished). All told, these findings suggest that hepatic mitochondrial content and function cannot only impact hepatic metabolism, but also impact energy intake and substrate utilization at the whole body level.
Sub-heading 3: Mitochondrial-Respiratory Capacity and Fat Oxidation
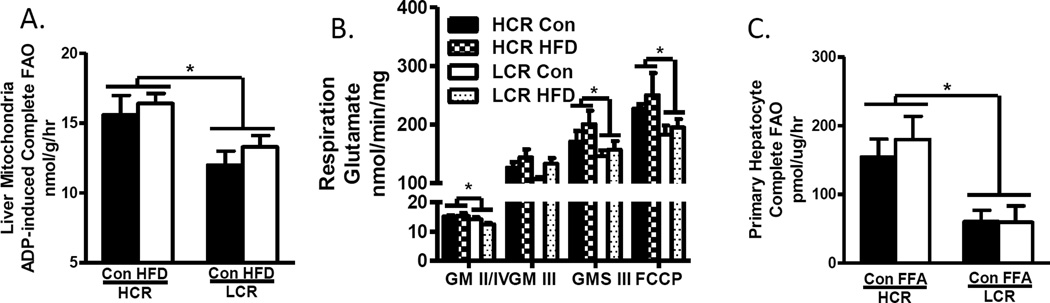
As had been reported previously, we found the HCR to have higher complete FAO to CO2 in both isolated hepatic mitochondria (Figure 4A) and liver homogenates on the control diet (21). However, the LCR displayed higher incomplete FAO (14C to acid soluble metabolites) in liver homogenate on control diet, suggesting that they likely divert more acetyl-CoA to ketogenesis following β-oxidation of long chain fatty acids. In response to the 3 day HFD, the HCR retained a higher complete FAO but were also able to increase incomplete oxidation (21). Hepatic mitochondrial respiration showed similar results as the HCR displayed higher rates of respiratory capacity than the LCR under basal, ADP-stimulated, and uncoupled conditions using glutamate as a substrate (Figure 4B). Similar results were found with lipid (palmitoylcarnitine) as a substrate (unpublished). Assays performed in the presence of maximal concentrations of ADP and substrates assess the maximal capacity of the OXPHOS system. In-vivo however, FAO and mitochondrial respiration are driven by the energy turnover rate (i.e., energy demand). Mitochondrial density and function typically adapt to and reflect the local chronic level of energy turnover with a cell or tissue. Given that the HCR displays greater energy expenditure it is logical that their mitochondria are characterized by higher maximal functional capacity. The important question is how does selective breeding for running capacity lead to differences in mitochondrial functional capacity and susceptibility for hepatic steatosis? Are the livers of the HCRs protected simply by virtue of the greater energy expenditure, or does the selective breeding for endurance capacity imprint changes to the hepatocytes? Evidence of an imprinted or stable phenotype comes from studies on primary hepatocytes isolated from the HCR and LCR, which retain the difference in phenotype for FAO (21). This suggests that differences in peripheral factors are not driving the liver phenotypes between the HCRs and LCRs but rather it is a permanent intrinsic imprinting to hepatocyte mitochondria. Further work including examination of mitochondrial heteroplasmy and the nuclear genome is underway to understand the potential imprinting of running capacity on hepatic mitochondria between the HCR and LCR.
Figure 4.
Hepatic mitochondrial fat oxidation and respiratory capacity. A. HCR display elevated (A.) hepatic mitochondrial FAO and (B.) respiration than LCR. C. Primary hepatocytes isolated from the HCR/LCR also display differences in FAO capacity. Figures 4A, 4B, and 4C (Reprinted from (20). Copyright © 2016 John Wiley & Sons. Used with permission.)
In addition, these data suggest that the higher starting point for hepatic FAO and respiratory capacity in the HCRs may play a role in protection against hepatic steatosis, and that a 3-day HFD induces very little change in these mitochondrial measures despite the dramatic change in dietary lipid influx and energy balance experienced in both strains. Chronic HFD studies have now been conducted in the HCR/LCR rats, which do alter FAO and respiratory function (unpublished), but those studies still suggest that a higher FAO and respiratory capacity starting point is critical for protection against dietary induced steatosis. Further work is needed to determine if a higher level of FAO and mitochondrial respiration is associated with protection against hepatic steatosis, or plays a direct mechanistic role in the phenotype.
The elevated energy expenditure in the HCR vs. LCR indicates differences in efficiency. The LCR are efficient (shown by greater weight gain and lipid storage) while the HCR are inefficient. Clearly an inefficient phenotype is advantageous for warding off weight gain during periods of energy excess. The differences in efficiency between the strains are driven by differences in energy demand and uncoupling of mitochondria effects that are again indicative of imprinting due to breeding for running capacity. Importantly, previous work in mice demonstrated that higher vs. lower metabolic rate (whole body oxygen consumption) is associated with greater mitochondrial uncoupling and greater longevity in mice (34). This has been determined the “uncoupling to survive hypothesis”. Therefore, there are other lines of evidence suggesting that inefficient phenotype found in the HCR is beneficial and may play a role in there greater longevity than the LCR (17).
Conclusion
The results discussed here demonstrate several metabolic differences between the HCR and LCR rats that occur at the level of the whole body, tissue, and hepatic mitochondria. Of interest is the potential role that differences in hepatic mitochondrial content and function may play on impacting not only hepatic lipid disposal, but also whole body regulation of energy intake, metabolic flexibility, and substrate utilization. Importantly, these results begin to shed light on the mechanism(s) by which a divergence for aerobic capacity impacts susceptibility for obesity and metabolic disease. Evidence continues to accumulate showing that high or low fitness impacts susceptibility for metabolic disease. Previously mentioned cross-sectional studies examining fitness in those who do or do not develop metabolic diseases provided the first line of evidence. Monozygotic twins with discordant physical activity patterns and thus disparate aerobic fitness levels have also revealed large differences in body composition, central adiposity, liver fat content and glucose homeostasis (19, 32). Therefore, convincing evidence in humans with the same genes and early life environment also links high and low fitness to susceptibility for obesity and metabolic pathologies. Despite the potential mechanisms highlighted in this review, future work is needed to examine molecular mechanisms in multiple tissues including adipose, skeletal muscle, liver, and brain. It will also be important to determine if the metabolic effects of fitness on each of these tissues works in isolation or if there is hormonal, neural, or metabolic communication between these tissues, which integrate metabolic health. In addition, it should be highlighted that exercise research, and, by extension work examining aerobic capacity, has historically ignored tissues other than the lungs, heart, and skeletal muscle. While other tissues may not impact aerobic capacity directly, aerobic capacity may certainly impact other tissue structure and function. This is most evidence by the findings that adipose tissue mitochondrial phenotypes are different. Future work needs to determine if exercise training can induce similar metabolic changes in rodents or humans that were reported here or if the differences in the HCR/LCR rat are due to selective breeding for fitness over several generations. These questions could also largely be tested in human subjects who are matched for age and body weight but have dramatic differences in aerobic capacity. In conclusion, the HCR/LCR model is beginning to provide important insight into the mechanism(s) by which aerobic capacity impacts aerobic capacity. Moreover, the HCR/LCR rat model is further proof to emerging human data that aerobic capacity is important for long-term metabolic health.
Key Points.
Aerobic capacity is tremendously important for human health, but mechanisms are largely unknown
We have utilized a rat model in which rats are selectively bred for high or low endurance exercise capacity resulting in high and low running rat strains with dramatic differences in aerobic capacity.
Rats with high aerobic capacity have metabolic adaptations to high fat diets that make them protected against obesity, fatty liver, and insulin resistance. These adaptations are not found in the LCR.
Differences in hepatic mitochondrial content and function may play a central role in mediating the metabolic differences between the high and low aerobic capacity rats.
Acknowledgments
Funding: The work described was supported in part by R01DK088940 from the NIDDK (JPT), Merit Review Award # 1I01BX002567-01 from the Biomedical Laboratory Research and Development of the U.S. Department of Veterans Affairs (JPT) and AHA Postdoctoral Grant Award from the American Heart Association (EMM).
Footnotes
There is nothing to disclose.
References
- 1.Baber U, Boffetta P. Improving fitness to achieve health: shifting the focus from theory to practice. J Am Coll Cardiol. 2015;65(19):2101–2103. doi: 10.1016/j.jacc.2015.03.542. [DOI] [PubMed] [Google Scholar]
- 2.Bernal-Mizrachi C, Semenkovich CF. Fast predators or fast food, the fit still survive. Nat Med. 2006;12(1):46–47. doi: 10.1038/nm0106-46. discussion 7. [DOI] [PubMed] [Google Scholar]
- 3.Blair SN, Kohl HW, 3rd, Barlow CE, Paffenbarger RS, Jr, Gibbons LW, Macera CA. Changes in physical fitness and all-cause mortality. A prospective study of healthy and unhealthy men. Jama. 1995;273(14):1093–1098. [PubMed] [Google Scholar]
- 4.Blair SN, Kohl HW, 3rd, Paffenbarger RS, 3rd, Clark DG, Cooper KH, Gibbons LW. Physical fitness all-cause mortality A prospective study of healthy men and women. JAMA : the journal of the American Medical Association. 1989;262(17):2395–2401. doi: 10.1001/jama.262.17.2395. [DOI] [PubMed] [Google Scholar]
- 5.Booth FW, Laye MJ, Roberts MD. Lifetime sedentary living accelerates some aspects of secondary aging. J Appl Physiol. 2011;111(5):1497–1504. doi: 10.1152/japplphysiol.00420.2011. [DOI] [PubMed] [Google Scholar]
- 6.Booth FW, Roberts CK, Laye MJ. Lack of exercise is a major cause of chronic diseases. Compr Physiol. 2012;2(2):1143–1211. doi: 10.1002/cphy.c110025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bouchard C, Blair SN, Katzmarzyk PT. Less Sitting, More Physical Activity, or Higher Fitness? Mayo Clin Proc. 2015;90(11):1533–1540. doi: 10.1016/j.mayocp.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 8.Bouchard C, Rankinen T, Timmons JA. Genomics and genetics in the biology of adaptation to exercise. Compr Physiol. 2011;1(3):1603–1648. doi: 10.1002/cphy.c100059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Browning JD, Szczepaniak LS, Dobbins R, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40(6):1387–1395. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 10.Church TS, Kuk JL, Ross R, Priest EL, Biltoft E, Blair SN. Association of cardiorespiratory fitness, body mass index, and waist circumference to nonalcoholic fatty liver disease. Gastroenterology. 2006;130(7):2023–2030. doi: 10.1053/j.gastro.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 11.Fernandez-Marcos PJ, Auwerx J. Regulation of PGC-1alpha, a nodal regulator of mitochondrial biogenesis. Am J Clin Nutr. 2011;93(4):884S–890S. doi: 10.3945/ajcn.110.001917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Horn CC, Ji H, Friedman MI. Etomoxir, a fatty acid oxidation inhibitor, increases food intake and reduces hepatic energy status in rats. Physiol Behav. 2004;81(1):157–162. doi: 10.1016/j.physbeh.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 13.Horn CC, Tordoff MG, Friedman MI. Role of vagal afferent innervation in feeding and brain Fos expression produced by metabolic inhibitors. Brain Res. 2001;919(2):198–206. doi: 10.1016/s0006-8993(01)02963-8. [DOI] [PubMed] [Google Scholar]
- 14.Ji H, Friedman MI. Reduced capacity for fatty acid oxidation in rats with inherited susceptibility to diet-induced obesity. Metabolism. 2007;56(8):1124–1130. doi: 10.1016/j.metabol.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koch LG, Britton SL. Artificial selection for intrinsic aerobic endurance running capacity in rats. Physiol Genomics. 2001;5(1):45–52. doi: 10.1152/physiolgenomics.2001.5.1.45. [DOI] [PubMed] [Google Scholar]
- 16.Koch LG, Britton SL, Wisloff U. A rat model system to study complex disease risks, fitness, aging, and longevity. Trends Cardiovasc Med. 2012;22(2):29–34. doi: 10.1016/j.tcm.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koch LG, Kemi OJ, Qi N, et al. Intrinsic aerobic capacity sets a divide for aging and longevity. Circ Res. 2011;109(10):1162–1172. doi: 10.1161/CIRCRESAHA.111.253807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kokkinos P, Myers J, Kokkinos JP, et al. Exercise capacity and mortality in black and white men. Circulation. 2008;117(5):614–622. doi: 10.1161/CIRCULATIONAHA.107.734764. [DOI] [PubMed] [Google Scholar]
- 19.Leskinen T, Sipila S, Alen M, et al. Leisure-time physical activity and high-risk fat: a longitudinal population-based twin study. Int J Obes (Lond) 2009;33(11):1211–1218. doi: 10.1038/ijo.2009.170. [DOI] [PubMed] [Google Scholar]
- 20.Morris EMGME, Koch LG, Britton SL, MacLean PS, Thyfault JP. Increased Aerobic Capacity Reduces Susceptibility to Acute High-fat Diet-induced Weight Gain. Obesity. 2016 doi: 10.1002/oby.21564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morris EM, Jackman MR, Johnson GC, et al. Intrinsic aerobic capacity impacts susceptibility to acute high-fat diet-induced hepatic steatosis. American journal of physiology. Endocrinology and metabolism. 2014;307(4):E355–E364. doi: 10.1152/ajpendo.00093.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morris EM, Jackman MR, Meers GM, et al. Reduced hepatic mitochondrial respiration following acute high-fat diet is prevented by PGC-1alpha overexpression. American journal of physiology. Gastrointestinal and liver physiology. 2013;305(11):G868–G880. doi: 10.1152/ajpgi.00179.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nair S, PC V, Arnold C, Diehl AM. Hepatic ATP reserve and efficiency of replenishing: comparison between obese and nonobese normal individuals. Am J Gastroenterol. 2003;98(2):466–470. doi: 10.1111/j.1572-0241.2003.07221.x. [DOI] [PubMed] [Google Scholar]
- 24.Naples SP, Borengasser SJ, Rector RS, et al. Skeletal muscle mitochondrial and metabolic responses to a high-fat diet in female rats bred for high and low aerobic capacity. Applied physiology, nutrition, and metabolism = Physiologie appliquee, nutrition et metabolisme. 2010;35(2):151–162. doi: 10.1139/h09-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Noland RC, Thyfault JP, Henes ST, et al. Artificial selection for high-capacity endurance running is protective against high-fat diet-induced insulin resistance. Am J Physiol Endocrinol Metab. 2007;293(1):E31–E41. doi: 10.1152/ajpendo.00500.2006. [DOI] [PubMed] [Google Scholar]
- 26.Park YM, Rector RS, Thyfault JP, et al. Effects of ovariectomy and intrinsic aerobic capacity on tissue-specific insulin sensitivity. Am J Physiol Endocrinol Metab. 2015;2015 doi: 10.1152/ajpendo.00434.2015. ajpendo 00434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rector RS, Thyfault JP. Does physical inactivity cause nonalcoholic fatty liver disease? J Appl Physiol. 2011;111(6):1828–1835. doi: 10.1152/japplphysiol.00384.2011. [DOI] [PubMed] [Google Scholar]
- 28.Rector RS, Thyfault JP, Morris RT, et al. Daily exercise increases hepatic fatty acid oxidation and prevents steatosis in Otsuka Long-Evans Tokushima Fatty rats. Am J Physiol Gastrointest Liver Physiol. 2008;294(3):G619–G626. doi: 10.1152/ajpgi.00428.2007. [DOI] [PubMed] [Google Scholar]
- 29.Rector RS, Thyfault JP, Uptergrove GM, et al. Mitochondrial dysfunction precedes insulin resistance and hepatic steatosis and contributes to the natural history of non-alcoholic fatty liver disease in an obese rodent model. J Hepatol. 2010;52(5):727–736. doi: 10.1016/j.jhep.2009.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ren YY, Overmyer KA, Qi NR, et al. Genetic analysis of a rat model of aerobic capacity and metabolic fitness. PLoS One. 2013;8(10):e77588. doi: 10.1371/journal.pone.0077588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ricci MR, Levin BE. Ontogeny of diet-induced obesity in selectively bred Sprague-Dawley rats. Am J Physiol Regul Integr Comp Physiol. 2003;285(3):R610–R618. doi: 10.1152/ajpregu.00235.2003. [DOI] [PubMed] [Google Scholar]
- 32.Rottensteiner M, Leskinen T, Niskanen E, et al. Physical activity, fitness, glucose homeostasis, and brain morphology in twins. Medicine and science in sports and exercise. 2015;47(3):509–518. doi: 10.1249/MSS.0000000000000437. [DOI] [PubMed] [Google Scholar]
- 33.Saltin B, Nazar K, Costill DL, et al. The nature of the training response; peripheral and central adaptations of one-legged exercise. Acta Physiol Scand. 1976;96(3):289–305. doi: 10.1111/j.1748-1716.1976.tb10200.x. [DOI] [PubMed] [Google Scholar]
- 34.Speakman JR, Talbot DA, Selman C, et al. Uncoupled and surviving: individual mice with high metabolism have greater mitochondrial uncoupling and live longer. Aging Cell. 2004;3(3):87–95. doi: 10.1111/j.1474-9728.2004.00097.x. [DOI] [PubMed] [Google Scholar]
- 35.Stephenson EJ, Lessard SJ, Rivas DA, et al. Exercise training enhances white adipose tissue metabolism in rats selectively bred for low- or high-endurance running capacity. Am J Physiol Endocrinol Metab. 2013;305(3):E429–E438. doi: 10.1152/ajpendo.00544.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thyfault JP, Rector RS, Uptergrove GM, et al. Rats selectively bred for low aerobic capacity have reduced hepatic mitochondrial oxidative capacity and susceptibility to hepatic steatosis and injury. J Physiol. 2009;587(Pt 8):1805–1816. doi: 10.1113/jphysiol.2009.169060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Toedebusch RG, Ruegsegger GN, Braselton JF, et al. AMPK-agonist AICAR delays the initial decline in lifetime-apex VO2peak while voluntary wheel running fails to delay its initial decline in female rats. Physiol Genomics. 2015;2015 doi: 10.1152/physiolgenomics.00078.2015. physiolgenomics 00078. [DOI] [PubMed] [Google Scholar]
- 38.Trefts E, Williams AS, Wasserman DH. Exercise and the Regulation of Hepatic Metabolism. Prog Mol Biol Transl Sci. 2015;135:203–225. doi: 10.1016/bs.pmbts.2015.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vieira-Potter VJ, Padilla J, Park YM, et al. Female rats selectively bred for high intrinsic aerobic fitness are protected from ovariectomy-associated metabolic dysfunction. Am J Physiol Regul Integr Comp Physiol. 2015;308(6):R530–R542. doi: 10.1152/ajpregu.00401.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Willis BL, Gao A, Leonard D, Defina LF, Berry JD. Midlife fitness and the development of chronic conditions in later life. Archives of internal medicine. 2012;172(17):1333–1340. doi: 10.1001/archinternmed.2012.3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wisloff U, Najjar SM, Ellingsen O, et al. Cardiovascular risk factors emerge after artificial selection for low aerobic capacity. Science. 2005;307(5708):418–420. doi: 10.1126/science.1108177. [DOI] [PubMed] [Google Scholar]
- 42.Yoon JC, Puigserver P, Chen G, et al. Control of hepatic gluconeogenesis through the transcriptional coactivator PGC-1. Nature. 2001;413(6852):131–138. doi: 10.1038/35093050. [DOI] [PubMed] [Google Scholar]