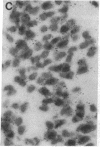
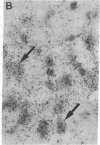
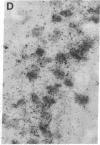
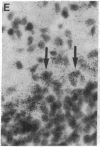
Abstract
Non-N-methyl-D-aspartate glutamate receptors (GluRs) are encoded by a gene family, known members of which are designated GluR-1, -2, -3, -4, and -5. The present study examined the developmental pattern of GluR-1, -2, and -3 gene expression in rat brain. In situ hybridization revealed different spatial patterns throughout the brain for the cognate mRNAs at all ages examined, as well as different temporal patterns during development. In the adult all three mRNAs were expressed prominently in the pyramidal and granule layers of the hippocampus and in the Purkinje cell layer of the cerebellum, where detailed differences were apparent at the cellular level. In neocortex, GluR-2 mRNA exhibited prominent lamination and regional differences, which were less marked for GluR-1 and -3 mRNAs. In caudate-putamen GluR-2 mRNA was at high levels, but GluR-1 and -3 mRNAs were not. At early ages transcripts were transiently elevated relative to adult levels. GluR-1 mRNA reached peak expression in cortex at postnatal day 14 (P14) (225% of adult), in striatum at P4 (255% of adult), in hippocampus at P14 (195% of adult), and in cerebellum at P21 (150% of adult). GluR-3 exhibited more modest peaks in neocortex and hippocampus. In contrast, GluR-2 mRNA was at near adult levels throughout the first days of postnatal life and exhibited a peak only in cerebellum at P14 (168% of adult). The finding of differential developmental regulation of the GluR-1, -2, and -3 genes indicates that the receptors they encode may have different influences on synaptic plasticity, neuronal survival, and susceptibility to excitatory amino acid toxicity.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boulter J., Hollmann M., O'Shea-Greenfield A., Hartley M., Deneris E., Maron C., Heinemann S. Molecular cloning and functional expression of glutamate receptor subunit genes. Science. 1990 Aug 31;249(4972):1033–1037. doi: 10.1126/science.2168579. [DOI] [PubMed] [Google Scholar]
- Cambray-Deakin M. A., Foster A. C., Burgoyne R. D. The expression of excitatory amino acid binding sites during neuritogenesis in the developing rat cerebellum. Brain Res Dev Brain Res. 1990 Jul 1;54(2):265–271. doi: 10.1016/0165-3806(90)90149-s. [DOI] [PubMed] [Google Scholar]
- Campochiaro P., Coyle J. T. Ontogenetic development of kainate neurotoxicity: correlates with glutamatergic innervation. Proc Natl Acad Sci U S A. 1978 Apr;75(4):2025–2029. doi: 10.1073/pnas.75.4.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle J. T. Neurotoxic action of kainic acid. J Neurochem. 1983 Jul;41(1):1–11. doi: 10.1111/j.1471-4159.1983.tb11808.x. [DOI] [PubMed] [Google Scholar]
- Gall C., Sumikawa K., Lynch G. Levels of mRNA for a putative kainate receptor are affected by seizures. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7643–7647. doi: 10.1073/pnas.87.19.7643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garthwaite G., Garthwaite J. In vitro neurotoxicity of excitatory acid analogues during cerebellar development. Neuroscience. 1986 Mar;17(3):755–767. doi: 10.1016/0306-4522(86)90043-6. [DOI] [PubMed] [Google Scholar]
- Greenamyre T., Penney J. B., Young A. B., Hudson C., Silverstein F. S., Johnston M. V. Evidence for transient perinatal glutamatergic innervation of globus pallidus. J Neurosci. 1987 Apr;7(4):1022–1030. doi: 10.1523/JNEUROSCI.07-04-01022.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollmann M., O'Shea-Greenfield A., Rogers S. W., Heinemann S. Cloning by functional expression of a member of the glutamate receptor family. Nature. 1989 Dec 7;342(6250):643–648. doi: 10.1038/342643a0. [DOI] [PubMed] [Google Scholar]
- Insel T. R., Miller L. P., Gelhard R. E. The ontogeny of excitatory amino acid receptors in rat forebrain--I. N-methyl-D-aspartate and quisqualate receptors. Neuroscience. 1990;35(1):31–43. doi: 10.1016/0306-4522(90)90117-m. [DOI] [PubMed] [Google Scholar]
- Kano M., Kato M. Quisqualate receptors are specifically involved in cerebellar synaptic plasticity. Nature. 1987 Jan 15;325(6101):276–279. doi: 10.1038/325276a0. [DOI] [PubMed] [Google Scholar]
- Keinänen K., Wisden W., Sommer B., Werner P., Herb A., Verdoorn T. A., Sakmann B., Seeburg P. H. A family of AMPA-selective glutamate receptors. Science. 1990 Aug 3;249(4968):556–560. doi: 10.1126/science.2166337. [DOI] [PubMed] [Google Scholar]
- Kennedy M. B. Regulation of synaptic transmission in the central nervous system: long-term potentiation. Cell. 1989 Dec 1;59(5):777–787. doi: 10.1016/0092-8674(89)90601-6. [DOI] [PubMed] [Google Scholar]
- Levitan E. S., Schofield P. R., Burt D. R., Rhee L. M., Wisden W., Köhler M., Fujita N., Rodriguez H. F., Stephenson A., Darlison M. G. Structural and functional basis for GABAA receptor heterogeneity. Nature. 1988 Sep 1;335(6185):76–79. doi: 10.1038/335076a0. [DOI] [PubMed] [Google Scholar]
- Mattson M. P., Dou P., Kater S. B. Outgrowth-regulating actions of glutamate in isolated hippocampal pyramidal neurons. J Neurosci. 1988 Jun;8(6):2087–2100. doi: 10.1523/JNEUROSCI.08-06-02087.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson M. P., Kater S. B. Excitatory and inhibitory neurotransmitters in the generation and degeneration of hippocampal neuroarchitecture. Brain Res. 1989 Jan 30;478(2):337–348. doi: 10.1016/0006-8993(89)91514-x. [DOI] [PubMed] [Google Scholar]
- Mattson M. P., Lee R. E., Adams M. E., Guthrie P. B., Kater S. B. Interactions between entorhinal axons and target hippocampal neurons: a role for glutamate in the development of hippocampal circuitry. Neuron. 1988 Nov;1(9):865–876. doi: 10.1016/0896-6273(88)90134-1. [DOI] [PubMed] [Google Scholar]
- Mattson M. P. Neurotransmitters in the regulation of neuronal cytoarchitecture. Brain Res. 1988 Apr-Jun;472(2):179–212. doi: 10.1016/0165-0173(88)90020-3. [DOI] [PubMed] [Google Scholar]
- McDonald J. W., Johnston M. V. Physiological and pathophysiological roles of excitatory amino acids during central nervous system development. Brain Res Brain Res Rev. 1990 Jan-Apr;15(1):41–70. doi: 10.1016/0165-0173(90)90011-c. [DOI] [PubMed] [Google Scholar]
- McDonald J. W., Silverstein F. S., Johnston M. V. Neurotoxicity of N-methyl-D-aspartate is markedly enhanced in developing rat central nervous system. Brain Res. 1988 Aug 30;459(1):200–203. doi: 10.1016/0006-8993(88)90306-x. [DOI] [PubMed] [Google Scholar]
- Miller L. P., Johnson A. E., Gelhard R. E., Insel T. R. The ontogeny of excitatory amino acid receptors in the rat forebrain--II. Kainic acid receptors. Neuroscience. 1990;35(1):45–51. doi: 10.1016/0306-4522(90)90118-n. [DOI] [PubMed] [Google Scholar]
- Mishina M., Takai T., Imoto K., Noda M., Takahashi T., Numa S., Methfessel C., Sakmann B. Molecular distinction between fetal and adult forms of muscle acetylcholine receptor. Nature. 1986 May 22;321(6068):406–411. doi: 10.1038/321406a0. [DOI] [PubMed] [Google Scholar]
- Silverstein F. S., Chen R., Johnston M. V. The glutamate analogue quisqualic acid is neurotoxic in striatum and hippocampus of immature rat brain. Neurosci Lett. 1986 Oct 30;71(1):13–18. doi: 10.1016/0304-3940(86)90249-1. [DOI] [PubMed] [Google Scholar]
- Sommer B., Keinänen K., Verdoorn T. A., Wisden W., Burnashev N., Herb A., Köhler M., Takagi T., Sakmann B., Seeburg P. H. Flip and flop: a cell-specific functional switch in glutamate-operated channels of the CNS. Science. 1990 Sep 28;249(4976):1580–1585. doi: 10.1126/science.1699275. [DOI] [PubMed] [Google Scholar]
- Witzemann V., Barg B., Criado M., Stein E., Sakmann B. Developmental regulation of five subunit specific mRNAs encoding acetylcholine receptor subtypes in rat muscle. FEBS Lett. 1989 Jan 2;242(2):419–424. doi: 10.1016/0014-5793(89)80514-9. [DOI] [PubMed] [Google Scholar]
- Wolf G., Keilhoff G. Kainate and glutamate neurotoxicity in dependence on the postnatal development with special reference to hippocampal neurons. Brain Res. 1984 May;316(1):15–21. doi: 10.1016/0165-3806(84)90004-x. [DOI] [PubMed] [Google Scholar]