Abstract
Background
Diabetes guidelines recommend individualizing glycemic goals (A1C) for older patients. We assess a personalized web-based decision support tool.
Design
We randomized physicians and their patients (≥65 years of age) with type 2 diabetes to support tool or educational pamphlet (75:25 patients). Prior to a visit, intervention patients interacted with the tool, which provided personalized risk predictions and elicited treatment preferences. Main outcomes included 1) patient-doctor communication, 2) decisional conflict, 3) changes in goals, 4) intervention acceptability.
Results
We did not find significant differences in proportions of patients that had an A1C discussion (91% intervention vs. 76% control, p=0.19). Intervention patients had larger declines in the Informed Subscale of Decisional Conflict (-20.0 vs. 0, p=0.04). There were no significant differences in proportions of patients with changes in goals (49% vs. 28%, p=0.08). Most intervention patients reported that the tool was easy to use (91%) and helped them to communicate (84%).
Limitations
Pilot trial at one academic institution.
Conclusions
Web-based decision support tools may be a practical approach to facilitating personalization of goals for chronic conditions.
Trial Registration
ClinicalTrials.govNCT02169999, https://clinicaltrials.gov/show/NCT02169999
Keywords: Type 2 diabetes, aging, personalized medicine, Decision aids, decision support, chronic disease modeling, randomized trial
Introduction
Older diabetes patients are highly heterogeneous in terms of functional status, comorbidities, and life expectancy and these differences may alter the risks and benefits of achieving diabetes care goals. In 2003, the American Geriatrics Society published one of the earliest diabetes care guidelines encouraging providers to consider less intensive glucose control goals (A1C <8%) among frail, older patients with limited life expectancy (<5 years), while continuing to pursue intensive glucose control (A1C <7%) among relatively healthy older patients.1
Despite the dissemination of this guideline, considerable evidence suggests that diabetes care is not individualized in clinical practice.2-4 Lack of individualization may be due to the difficulty of implementing recommendations in busy practices. In a variety of clinical domains, decision support interventions have been found to improve guideline adherence.5-7 We developed a personalized web-based decision support tool (Personal DC) that combines the features of a traditional decision aid with quantitative risk prediction. To generate pilot data for a larger trial, we conducted a randomized trial of the tool in two outpatient clinics.
Methods
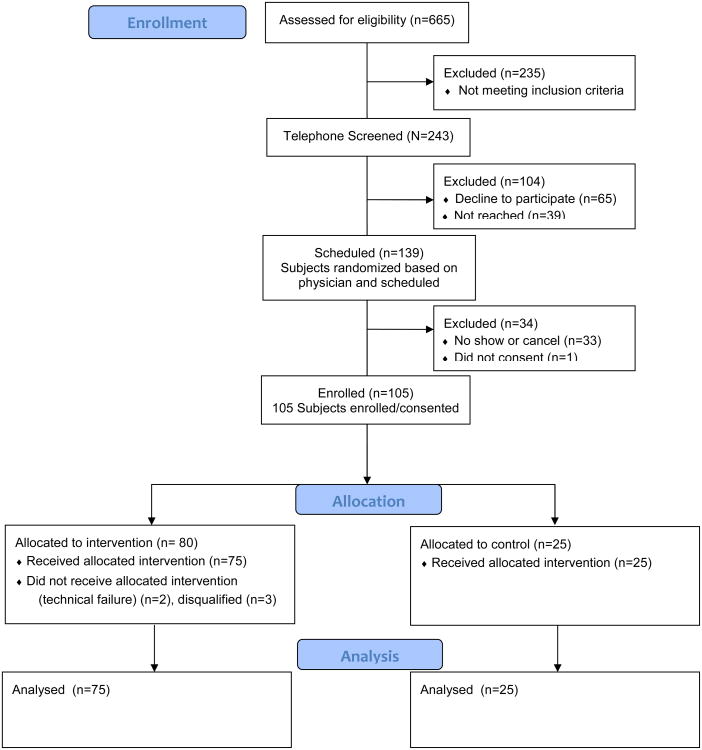
This pilot randomized controlled trial took place in two clinics of the University of Chicago and was approved by the Institutional Review Board. All attending physicians were approached to enroll patients. We enrolled English-speaking patients ≥ 65 years, with type 2 diabetes, no dementia, and who had been seen in 2011. Randomization occurred at the physician level (3:1 intervention to control ratio). We enrolled 20 intervention and 7 control physicians and 75 intervention and 25 control patients (Appendix Figure A). Physicians were not blinded to study objectives or allocation. Patients were blinded to study hypotheses and were unaware of allocation.
Intervention protocol
At baseline, intervention physicians underwent one hour of in-person training on principles of geriatric diabetes 1 and use of the decision support tool. Intervention patients met with a research assistant one hour prior to their next scheduled clinic appointment. Patients were given brief instructions on use of the tool and received minor assistance with the computer, if necessary. The website was presented via a touch-screen laptop for easy use if the patient had difficulty manipulating a mouse.
Main components of the decision support tool were 1) interactive diabetes education module, 2) simulation model for calculating life expectancy and risk of developing complications, 3) treatment preference elicitation, 4) geriatric condition screening, and 5) personalized patient printout. Patient responses regarding demographics, biometrics, comorbidities, and functional status were fed into the model. The model integrates the United Kingdom Prospective Diabetes Study (UKPDS) simulation model of diabetes outcomes8 and a geriatric mortality prediction model that accounts for functional status and comorbid illness.9,10 The model calculated life expectancy, lifetime risk of developing a heart attack (at A1C of 7%), and risk of amputation or blindness (at A1C of 7, 8, and 9%). Personalized risks, patient preferences, and geriatric screener results were summarized in the printout (Appendix Figure B). Patients received two copies with instructions to give one copy to their doctor.
Control protocol
Control physicians received no formal training. Control patients met with a research assistant one hour prior to their scheduled appointment and were given an educational brochure regarding the A1C test.11
Outcomes and Follow-up
Sources of data included: 1) patient surveys; 2) physician surveys; 3) electronic medical records. In the pre and post patient surveys, we asked patients questions about their 1) knowledge about the A1C test, 2) decisional conflict related to diabetes management12,13, 3) preferences regarding participation in treatment decisions and relationship with physician14, 4) diabetes and non-diabetes health status questions15,16, and 5) current self-management of diabetes.17 In separate pre and post physician surveys regarding their individual patients, physicians were asked to estimate life expectancy and identify frailty status, A1C goals, patient's knowledge of A1C goal, geriatric syndromes, and patient's preferences. Electronic medical records were abstracted for comorbidities, diabetes-related complications, medications, and current risk factor levels.
The primary outcomes for this study were 1) patient and physician communication about A1C goals, 2) patient decisional conflict, 3) changes in identified goals, and 4) feasibility of intervention.
Statistical Analysis
The targeted sample size (75:25) for the pilot trial was based on the assumption that we would have 80% power to detect a 29% difference (49% intervention and 20% control) in the proportion of patients with guideline adherent goals. Each survey outcome was analyzed with a generalized linear mixed effect model. All models included random effects for physician and patients to account for clustering of patients within a physician and for within-subject correlation between pre- and post-time points. An interaction term between intervention effect and survey time point evaluated the intervention effect on outcomes over time. In sensitivity analyses, we conducted McNemar's tests and two-sample t-tests to compare pre- and post-intervention within each study arm. P values of <0.05 were considered statistically significant. All analyses were performed using SAS 9.3 (SAS Institute, Cary, NC).
Results
Patients in each trial arm were similar in gender, race/ethnicity, age, duration of diabetes, and glycemic control (Table 1). The control group had a significantly larger proportion of patients exclusively using insulin (32%) compared to intervention group (11%) (p=0.02). The control group had a significantly higher proportion of patients with report of hypoglycemia in the last month (56% vs. 28%, p=0.02). Rates of other conditions were similar.
Table 1. Baseline Patient Characteristics.
| Patient Characteristics, %a | Intervention (n=75) | Control (n=25) | P value |
|---|---|---|---|
| Female, % | 77 | 80 | .78 |
| Age, mean (SD), y | 74.5 (6.4) | 72.4 (5.6) | .14 |
| 60-70, % | 31 | 44 | |
| 71-80 | 49 | 40 | |
| > 80 | 20 | 16 | |
| Race/ethnicity,% | 0.99 | ||
| Caucasian | 8 | 8 | |
| Black | 89 | 92 | |
| Hispanic/Latino | 1 | 0 | |
| Other | 1 | 0 | |
| A1C value, mean (SD), % | 7.6 (0.9) | 7.4 (0.6) | .23 |
| ≤ 7.0, % | 31 | 28 | |
| 7.1-8.0 | 45 | 60 | |
| 8.1-9.0 | 15 | 12 | |
| > 9.0 | 9 | 0 | |
| Body mass index, mean (SD), kg/m2 | 31.7 (6.7) | 29.8 (5.3) | .20 |
| Weight status, % | .46 | ||
| Underweight | 0 | 4 | |
| Normal weight | 11 | 12 | |
| Overweight | 36 | 36 | |
| Obese | 53 | 48 | |
| No. of years w/diabetes, mean (SD) | 15.6 (9.6) | 17.1 (12.1) | .53 |
| 0-10, % | 36 | 40 | |
| 11-20 | 41 | 28 | |
| 21-30 | 17 | 20 | |
| > 30 | 5 | 12 | |
| No. of medications currently taking, mean (SD) | 6.7 (3.4) | 7.6 (3.6) | .25 |
| 0-5, % | 40 | 32 | |
| 6-10 | 48 | 52 | |
| > 10 | 12 | 16 | |
| Type of oral antidiabetic (OAD) medication use, % | |||
| No OAD medication | 9 | 8 | 0.99 |
| Single oral OAD | 32 | 16 | .20 |
| Multiple oral OAD | 32 | 24 | .18 |
| Insulin only (≥1 insulin type) | 11 | 32 | .02 |
| Insulin and OAD(s) | 16 | 20 | .64 |
| No. of years seeing current doctor, % | .18 | ||
| < 1 | 9 | 0 | |
| 1-10 | 64 | 80 | |
| > 10 | 27 | 20 | |
| Patient self-reported history, % | |||
| Heart disease | 25 | 28 | .69 |
| Lung disease | 8 | 12 | .69 |
| Cancer | 24 | 28 | .69 |
| Body pains (within past 2 weeks) | 75 | 68 | .52 |
| Hypoglycemic episode in the last month b | 28 | 56 | .02 |
| Patient who have taken diabetes education class, % | 38 | 52 | .21 |
Values reported are percentages of patients, unless otherwise stated in the table.
One control patient and 3 intervention patients self-reported requiring an ambulance or hospitalization due to hypoglycemic episode(s).
Patient and Physician Communication about A1C Goals
Intervention patients (77% vs. 64%, p=0.24) and their physicians (91% vs. 76%, p=0.19) did not report significantly different rates of A1C discussions compared to controls (Table 2). Additionally, the proportion of intervention patients with a physician reporting patient knowledge of their A1C goal rose from 32% to 81%, but this was not significantly different from the experience of the control group (52% to 60%, p=0.09).
Table 2. Patient and Physician Communication about A1C Goals and Insulin Use.
| Intervention (n=75) | Control (n=25) | P valueb | |||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Patient and Physician Communication, %a | Pre | Post | P value | Pre | Post | P value | |
| Patients who stated they knew their A1C goalc | 35 | 65 | <0.001 | 36 | 60 | .09 | 0.64 |
| Patients with physician reporting that patient knows his/her A1C goal | 32 | 81 | .004 | 52 | 60 | 0.89 | 0.09 |
| Patients reporting an A1C discussion at visit | -- | 77 | -- | -- | 64 | -- | 0.24 |
| Patients with a physician reporting an A1C discussion at visit | -- | 91 | -- | -- | 76 | -- | 0.19 |
| Patients stated they knew their A1C goal when the physician also stated their patient knew their A1C goal | 17 | 59 | <0.001 | 20 | 36 | 0.17 | 0.14 |
| Physicians who said insulin would be considered for patient in the future | 77 | 71 | 0.28 | 96 | 88 | 0.30 | 0.52 |
Values reported are the percentage of patients, unless otherwise stated in the table.
All reported P values are from generalized linear mixed models that account for clustering by physician. For the analysis of the intervention effect over time, P values were estimated for the interaction between groups and assessment time.
Patients stated whether they knew their A1C goal or not (binary). All of these individuals were also asked to state a numeric A1C goal value (those results are not shown).
Decisional conflict
Decisional conflict scores regarding A1C goal selection declined for both intervention and control patients but the decline was not significantly different (Table 3). The overall decisional conflict score declined from 52.7 to 24.5 for intervention patients compared to 51.2 to 36.6 for control patients (p= 0.07). Among subscale scores, the largest differences were for the informed subscale score, where intervention patients had a significant decline from 60.9 to 40.9 while control patients' scores remained unchanged from pre to post results (p = 0.04).
Table 3. Decisional Conflict among Intervention and Control Patients.
| Intervention (n=75) | Control (n=25) | ||||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Patients Answering Yes, %a | Pre | Post | P value | Pre | Post | P value | P value c |
| Overall DC Score, mean (SD)b | 52.7 (33.0) | 24.5 (26.7) | <0.001 | 51.2 (35.5) | 36.6 (33.8) | 0.04 | 0.07 |
| Informed subscale DC Score, mean (SD) | 60.9 (36.8) | 40.9 (39.2) | <0.001 | 57.3 (39.1) | 57.3 (42.5) | 1.00 | 0.04 |
| Do you know what A1c goals are available to you? | 19 | 44 | 0.002 | 28 | 36 | 0.53 | |
| Do you know the benefits to each A1c goal? | 31 | 56 | 0.002 | 36 | 36 | 1.00 | |
| Do you know the risks and side effects of each A1c goal? | 31 | 61 | <0.001 | 32 | 36 | 0.74 | |
| Values clarity subscale DC Score, mean (SD) | 54.0 (40.1) | 18.3 (33.7) | <0.001 | 56.0 (38.4) | 31.0 (41.0) | <0.001 | 0.28 |
| Are you clear about which benefits matter most? | 31 | 79 | <0.001 | 28 | 60 | 0.03 | |
| Are you clear on which risks/side effects matter most to you? | 39 | 77 | <0.001 | 32 | 68 | 0.02 | |
| Support subscale DC Score, mean (SD) | 40.9 (36.0) | 13.8 (23.0) | <0.001 | 40.0 (37.6) | 25.3 (33.7) | 0.12 | 0.16 |
| Do you have enough support to make a choice? | 61 | 89 | <0.001 | 60 | 64 | 0.77 | |
| Are you choosing your goal without pressure? | 49 | 85 | <0.001 | 48 | 88 | 0.01 | |
| Do you have enough advice to make a choice? | 40 | 79 | <0.001 | 52 | 60 | 0.57 | |
| Uncertainty subscale DC Score, mean (SD) | 56.7 (40.8) | 22.0 (35.6) | <0.001 | 54.0 (44.3) | 28.0 (39.7) | 0.01 | 0.38 |
| Are you clear about the best choice for you? | 31 | 79 | <0.001 | 36 | 60 | 0.08 | |
| Do you feel sure about what to choose? | 27 | 68 | <0.001 | 44 | 68 | 0.08 | |
Values reported represent the percentage of patients who answered “yes” to the question, unless otherwise specified in the table.
All scores reported range from 0 (no decisional conflict [DC]) to 100 (extremely high decisional conflict). The response was given 0 points if answered “yes”, 4 points if answered “no”, 2 points if answered “unsure”. Points were then entered into an equation to determine final decisional conflict score for the overall score and subscale scores.
All reported P values are from generalized linear mixed models that account for clustering by physician. For the analysis of the intervention effect over time, P values were estimated for the interaction between groups and assessment time.
Changes in Physician Identified Goals
Nearly half of intervention patients (49%) had their physician report a change in A1C goal following the intervention in comparison to 28% of control patients; this was not statistically different (p=0.08) (Table 4). More detailed analysis of specific patient subgroups (e.g., life expectancy groups) and goal selection did not reveal statistically significant differences between intervention and control groups (Appendix Table A).
Table 4. Changes in Physician-Stated A1C Goals among the Total Population and Stratified by Model Predicted Life Expectancy.
| Change in A1C Goal | Intervention (n=75) | Control (n=25) | P valueb | |
|---|---|---|---|---|
|
|
||||
| Total population, %a | Goal stayed the same | 51 | 72 | |
| Goal changedc | 49 | 28 | 0.08 | |
| Lower goal | 59 | 29 | ||
| Higher goal | 41 | 71 | ||
|
| ||||
| Model predicted life expectancy d | ||||
|
| ||||
| Life expectancy ≤5 y | Sample size | 22 | 9 | |
| Goal stayed the same | 55 | 78 | ||
| Goal changed | 45 | 22 | 0.36 | |
| Lower goal | 60 | 0 | ||
| Higher goal | 40 | 100 | ||
|
| ||||
| Life expectancy >5 y | Sample size | 53 | 16 | |
|
|
||||
| Goal stayed the same | 49 | 69 | ||
| Goal changed | 51 | 31 | 0.18 | |
| Lower goal | 59 | 40 | ||
| Higher goal | 41 | 60 | ||
Values reported are the percentage of patients, unless otherwise stated in the table.
All reported P values are from generalized linear mixed models that account for clustering by physician.
A change in goal was defined as a 0.5% increase or decrease from pre to post survey responses. When a range was specified, the upper A1c goal was used to assess change. The percentage of patients whose goal changed to either a higher or lower goal is reported as italicized values.
Model predicted life expectancy was taken from the life expectancy predicted by the embedded prediction model in the Personal DC tool. Control patients were also run through the model to generate model predicted life expectancy for result comparison.
Feasibility
Most intervention patients reported that the website was easy to use and understand (91%) and that the site helped them to talk with their doctor about their diabetes care (84%). Average time on the site was 7 minutes. Most physicians reported that the experience utilizing the decision aid with the patient was acceptable (53%).
Discussion
Multiple clinical guidelines are encouraging active personalization of diabetes care goals.18-22 Our web-based decision support intervention differs from the interventions of prior studies because we focus on 1) glucose goal setting, 2) geriatric populations, and 3) personalization of risk estimates. Previous diabetes decision aids have focused on therapeutic decisions such as statin use and choice of glucose lowering drugs, and have enhanced decision making and sometimes improved medication adherence.23-28 Our decision support tool significantly reduced patients' Informed Subscale of Decisional Conflict scores. Other findings were not statistically significant, but promising. Based on point estimates, intervention patients had more communication about A1C goals during clinic visits, more awareness of their personal A1C goal, and more changes in goal selection by their physicians. The intervention was also acceptable to patients and required very little time prior to visits.
Our study has limitations. Our simulation model was based on the original UKPDS model.8 We used this model because of its widespread use, public availability, and prior external validation.29 Future versions of this intervention will need to incorporate updates to the UKPDS model and more recent clinical trial findings.30,31 Our pilot study was underpowered and was primarily designed to address feasibility issues and to gather data in preparation for a larger trial. Due to resource limitations, the study design was purposely imbalanced to maximize experience with the intervention. By chance, our control patients were more likely to be insulin users and had higher rates of hypoglycemia. Our trial also took place at the clinics of a single urban academic institution and findings may differ in other settings. Our data collection did not include direct observations of clinical encounters which would have helped us understand intervention effects on the quality of discussions.
Despite its limitations, our study indicates that web-based personalized decision support can be feasibly introduced into clinical practice. A much larger clinical trial is needed to determine how longitudinal use of decision support influences goal selection and treatments over time and if structured personalization in diabetes care will influence health outcomes such as hypoglycemia and quality of life.
Supplementary Material
Acknowledgments
Contributors: The authors thank the participating physicians for their dedication to this project, and the patients for their contributions to this work. We also thank Don Saner and Tim Holper of the University of Chicago Center for Research Informatics for their guidance in developing the Personal DC.
Funding: Financial support for this study was provided by the Retirement Research Foundation (2007-250), the American Diabetes Association (1-08-CR-25), and the National Institute of Diabetes and Digestive and Kidney Diseases (P30 DK092949, K24 DK071933, K24 DK105340). None of these funders played any role in the design and conduct of the study; collection, management, analysis, and interpretation of data; and preparation, review, or approval of the manuscript. The funding agreements ensured the authors' independence in designing the study, interpreting the data, writing, and publishing the report.
Appendix Table A. Movement in the Intensity of Physician-Stated A1C Goals among the Total Population and Stratified by Model Predicted Life Expectancy.
| Intervention (n=75) | Control (n=25) | P valueb | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Upper A1c Goalc | Pre | Post | P value | Pre | Post | P value | ||
|
|
||||||||
| Total population, %a | ≤7.0 | 49 | 56 | 0.34 | 68 | 60 | 0.51 | 0.30 |
| ≥7.1 | 51 | 44 | 32 | 40 | ||||
|
| ||||||||
| Model predicted life expectancyd | ||||||||
|
| ||||||||
| Sample size | 22 | 9 | ||||||
|
|
||||||||
| LE ≤5 | ≤7.0 | 41 | 41 | 1.00 | 67 | 56 | 0.57 | 0.65 |
| ≥7.1 | 59 | 59 | 33 | 44 | ||||
|
| ||||||||
| Sample size | 53 | 16 | ||||||
|
|
||||||||
| LE >5 | ≤7.0 | 53 | 62 | 0.27 | 69 | 63 | 0.68 | 0.37 |
| ≥7.1 | 47 | 38 | 31 | 38 | ||||
Values reported are the percentage of patients, unless otherwise stated in the table.
All reported P values are from generalized linear mixed models that account for clustering by physician.
An intensive goal was defined as an A1C goal ≤7.0. A moderate goal was defined as an A1C goal ≥7.1.
Model predicted life expectancy was taken from the life expectancy predicted by the embedded prediction model in the Personal DC tool. Control patients were also run through the model to generate model predicted life expectancy for result comparison.
Appendix Figure A. Consort Flow Diagram.
Footnotes
Presentations: This paper was presented in part at the 2013 Society for Medical Decision Making Meeting in Baltimore, MD and at the 2014 Mount Hood Diabetes Challenge in Palo Alto, California.
Conflicts of Interest: None of the authors had any relevant financial interests, activities, relationships, and affiliations that might be construed as conflicts of interest. Dr. Col serves as an advisor for Epi-Q, Emmi Solutions, and Jansen Scientific Affairs and has been involved in developing other decision aids targeting patients with diabetes.
References
- 1.Brown AF, Mangione CM, Saliba D, Sarkisian CA California Healthcare Foundation/American Geriatrics Society Panel on Improving Care for Elders with D. Guidelines for improving the care of the older person with diabetes mellitus. J Am Geriatr Soc. 2003;51:S265–80. doi: 10.1046/j.1532-5415.51.5s.1.x. [DOI] [PubMed] [Google Scholar]
- 2.Lipska KJ, Ross JS, Miao Y, Shah ND, Lee SJ, Steinman MA. Potential Overtreatment of Diabetes Mellitus in Older Adults With Tight Glycemic Control. JAMA Intern Med. 2015 doi: 10.1001/jamainternmed.2014.7345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tseng CL, Soroka O, Maney M, Aron DC, Pogach LM. Assessing potential glycemic overtreatment in persons at hypoglycemic risk. JAMA Intern Med. 2014;174:259–68. doi: 10.1001/jamainternmed.2013.12963. [DOI] [PubMed] [Google Scholar]
- 4.Thorpe CT, Gellad WF, Good CB, et al. Tight Glycemic Control and Use of Hypoglycemic Medications in Older Veterans With Type 2 Diabetes and Comorbid Dementia. Diabetes Care. 2015 doi: 10.2337/dc14-0599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ennis J, Gillen D, Rubenstein A, et al. Clinical decision support improves physician guideline adherence for laboratory monitoring of chronic kidney disease: a matched cohort study. BMC Nephrol. 2015;16:163. doi: 10.1186/s12882-015-0159-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gonzales R, Anderer T, McCulloch CE, et al. A cluster randomized trial of decision support strategies for reducing antibiotic use in acute bronchitis. JAMA Intern Med. 2013;173:267–73. doi: 10.1001/jamainternmed.2013.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gupta A, Ip IK, Raja AS, Andruchow JE, Sodickson A, Khorasani R. Effect of clinical decision support on documented guideline adherence for head CT in emergency department patients with mild traumatic brain injury. Journal of the American Medical Informatics Association : JAMIA. 2014;21:e347–51. doi: 10.1136/amiajnl-2013-002536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clarke PM, Gray AM, Briggs A, et al. A model to estimate the lifetime health outcomes of patients with type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS) Outcomes Model (UKPDS no. 68) Diabetologia. 2004;47:1747–59. doi: 10.1007/s00125-004-1527-z. [DOI] [PubMed] [Google Scholar]
- 9.Lee SJ, Lindquist K, Segal MR, Covinsky KE. Development and validation of a prognostic index for 4-year mortality in older adults. JAMA. 2006;295:801–8. doi: 10.1001/jama.295.7.801. [DOI] [PubMed] [Google Scholar]
- 10.Huang ES, Zhang Q, Gandra N, Chin MH, Meltzer DO. The effect of comorbid illness and functional status on the expected benefits of intensive glucose control in older patients with type 2 diabetes: a decision analysis. Ann Intern Med. 2008;149:11–9. doi: 10.7326/0003-4819-149-1-200807010-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.The A1C Test and Diabetes. Accessed at http://www.niddk.nih.gov/health-information/health-topics/diagnostic-tests/a1c-test-diabetes/Pages/index.aspx.
- 12.O'Connor AM. Validation of a decisional conflict scale. Med Decis Making. 1995;15:25–30. doi: 10.1177/0272989X9501500105. [DOI] [PubMed] [Google Scholar]
- 13.Linder SK, Swank PR, Vernon SW, Mullen PD, Morgan RO, Volk RJ. Validity of a low literacy version of the Decisional Conflict Scale. Patient Educ Couns. 2011;85:521–4. doi: 10.1016/j.pec.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krupat E, Rosenkranz SL, Yeager CM, Barnard K, Putnam SM, Inui TS. The practice orientations of physicians and patients: the effect of doctor-patient congruence on satisfaction. Patient Educ Couns. 2000;39:49–59. doi: 10.1016/s0738-3991(99)00090-7. [DOI] [PubMed] [Google Scholar]
- 15.Ware JEJ, Kosinski M, Keller SD. SF-12: How to score the SF-12 physical and mental health summary scales. 2nd. Boston, MA: The Health Institute, New England Medical Center; 1995. [Google Scholar]
- 16.Welch GW, Jacobson AM, Polonsky WH. The Problem Areas in Diabetes Scale. An evaluation of its clinical utility. Diabetes Care. 1997;20:760–6. doi: 10.2337/diacare.20.5.760. [DOI] [PubMed] [Google Scholar]
- 17.Toobert DJ, Hampson SE, Glasgow RE. The summary of diabetes self-care activities measure: results from 7 studies and a revised scale. Diabetes Care. 2000;23:943–50. doi: 10.2337/diacare.23.7.943. [DOI] [PubMed] [Google Scholar]
- 18.Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38:140–9. doi: 10.2337/dc14-2441. [DOI] [PubMed] [Google Scholar]
- 19.Ismail-Beigi F, Moghissi E, Tiktin M, Hirsch IB, Inzucchi SE, Genuth S. Individualizing Glycemic Targets in Type 2 Diabetes Mellitus: Implications of Recent Clinical Trials. Ann Intern Med. 2011;154:554–9. doi: 10.7326/0003-4819-154-8-201104190-00007. [DOI] [PubMed] [Google Scholar]
- 20.Association AD. Executive summary: Standards of medical care in diabetes--2012. Diabetes care. 2012;35(1):S4–S10. doi: 10.2337/dc12-s004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Standards of Medical Care in Diabetes-2016: Summary of Revisions. Diabetes Care. 2016;39(1):S4–5. doi: 10.2337/dc16-S003. [DOI] [PubMed] [Google Scholar]
- 22.Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive Type 2 Diabetes Management Algorithm - 2016 Executive Summary. Endocrine practice : official journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists. 2016;22:84–113. doi: 10.4158/EP151126.CS. [DOI] [PubMed] [Google Scholar]
- 23.Branda ME, LeBlanc A, Shah ND, et al. Shared decision making for patients with type 2 diabetes: a randomized trial in primary care. BMC health services research. 2013;13:301. doi: 10.1186/1472-6963-13-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weymiller AJ, Montori VM, Jones LA, et al. Helping patients with type 2 diabetes mellitus make treatment decisions: statin choice randomized trial. Arch Intern Med. 2007;167:1076–82. doi: 10.1001/archinte.167.10.1076. [DOI] [PubMed] [Google Scholar]
- 25.Mathers N, Ng CJ, Campbell MJ, Colwell B, Brown I, Bradley A. Clinical effectiveness of a patient decision aid to improve decision quality and glycaemic control in people with diabetes making treatment choices: a cluster randomised controlled trial (PANDAs) in general practice. BMJ Open. 2012;2 doi: 10.1136/bmjopen-2012-001469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mann DM, Ponieman D, Montori VM, Arciniega J, McGinn T. The Statin Choice decision aid in primary care: a randomized trial. Patient Educ Couns. 2010;80:138–40. doi: 10.1016/j.pec.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 27.Mullan RJ, Montori VM, Shah ND, et al. The diabetes mellitus medication choice decision aid: a randomized trial. Arch Intern Med. 2009;169:1560–8. doi: 10.1001/archinternmed.2009.293. [DOI] [PubMed] [Google Scholar]
- 28.Perestelo-Perez L, Rivero-Santana A, Boronat M, et al. Effect of the statin choice encounter decision aid in Spanish patients with type 2 diabetes: A randomized trial. Patient Educ Couns. 2016;99:295–9. doi: 10.1016/j.pec.2015.08.032. [DOI] [PubMed] [Google Scholar]
- 29.The Mount Hood 4 Modeling Group. Computer modeling of diabetes and its complications. Diabetes Care. 2007;30:1638–46. doi: 10.2337/dc07-9919. [DOI] [PubMed] [Google Scholar]
- 30.Hayes AJ, Leal J, Gray AM, Holman RR, Clarke PM. UKPDS outcomes model 2: a new version of a model to simulate lifetime health outcomes of patients with type 2 diabetes mellitus using data from the 30 year United Kingdom Prospective Diabetes Study: UKPDS 82. Diabetologia. 2013;56:1925–33. doi: 10.1007/s00125-013-2940-y. [DOI] [PubMed] [Google Scholar]
- 31.Montori VM, Fernandez-Balsells M. Glycemic control in type 2 diabetes: time for an evidence-based about-face? Ann Intern Med. 2009;150:803–8. doi: 10.7326/0003-4819-150-11-200906020-00008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.