Abstract
Mitochondria are a major target in hypoxic/ischemic injury. Mitochondrial impairment increases with age leading to dysregulation of molecular pathways linked to mitochondria. The perturbation of mitochondrial homeostasis and cellular energetics worsens outcome following hypoxic-ischemic insults in elderly individuals. In response to acute injury conditions, cellular machinery relies on rapid adaptations by modulating posttranslational modifications. Therefore, post-translational regulation of molecular mediators such as hypoxia-inducible factor 1α (HIF-1α), peroxisome proliferator-activated receptor γ coactivator α (PGC-1α), c-MYC, SIRT1 and AMPK play a critical role in the control of the glycolytic-mitochondrial energy axis in response to hypoxic-ischemic conditions. The deficiency of oxygen and nutrients leads to decreased energetic reliance on mitochondria, promoting glycolysis. The combination of pseudohypoxia, declining autophagy, and dysregulation of stress responses with aging adds to impaired host response to hypoxic-ischemic injury. Furthermore, intermitochondrial signal propagation and tissue wide oscillations in mitochondrial metabolism in response to oxidative stress are emerging as vital to cellular energetics. Recently reported intercellular transport of mitochondria through tunneling nanotubes also play a role in the response to and treatments for ischemic injury. In this review we attempt to provide an overview of some of the molecular mechanisms and potential therapies involved in the alteration of cellular energetics with aging and injury with a neurobiological perspective.
1 Introduction
The world is experiencing considerable growth in its older population. Estimates from 2010 suggest that there were 524 million people aged 65 or older, this is expected to triple by 2050 (Suzman and Beard, 2011). Given these statistics, significant questions arise about productivity, health, well-being, independence, the need for social constructs to care for them, and factors that can positively impact the health of these individuals. The effect of stress or injury in old age is becoming an increasingly greater challenge. Declining mitochondrial function with aging plays a fundamental role in altered cellular energetics with aging and contributes greatly to outcome following injury (Biala et al., 2015; Gomes et al., 2013; Poulose and Raju, 2014). In this review, we aim to address this specific aspect by discussing the role of mitochondria in hypoxic/ischemic injury and the influence of aging on organ function.
2 The Mitochondrial Theory of Aging
In 1956, Harman suggested that free radicals result in cellular damage that accrues over time and causes aging (Harman, 1956). He later added to his theory in what has now become known as the mitochondrial theory of aging. He suggested that aging is due to accumulated free radical damage to mitochondria, impacting their ability to function (Harman, 1972). However there are mounting evidences that challenge the mitochondrial theory of aging (Lapointe and Hekimi, 2010). Results from rodent models with overexpression of genes important in redox regulation and models with ablation of these genes failed to support this theory. The accumulation of somatic mutations on mitochondrial DNA (mtDNA) has also been suggested to be through random genetic drift (Elson et al., 2001). Further studies found that at least in short-lived animals, this model does not hold (Kowald and Kirkwood, 2013) and based on the relationship between transcription and mtDNA replication, the same authors recently reported that transcription could be the key to the selection advantage of mitochondrial deletion mutants in aging (Kowald and Kirkwood, 2014). Though theories built around mitochondria to address organismal aging remain controversial, the role of mitochondria in health, aging and disease is being increasingly recognized.
2.1 Structure and Function of Mitochondria
Mitochondria are a cellular organelle consisting of a simple outer phospholipid bilayer membrane, an intermembrane space, a complex inner phospholipid bilayer, and a mitochondrial matrix (Palade, 1953). The outer membrane contains VDACs, also known as mitochondrial porin proteins, which make it permeable to small molecules (Palade, 1953; Tomasello et al., 2009). The intermembrane space is between the inner and outer membranes and play critical role in the transport of proteins across mitochondrial membranes, and oxidative phosphorylation (Gellerich, 1992; Herrmann and Riemer, 2010). The inner membrane is freely permeable to oxygen, carbon dioxide, and water (Alberts, 2008). It contains multiple folds called cristae, which significantly increase the total surface area of the inner membrane, allowing it to contain many proteins (Alberts, 2008; Palade, 1953) including transport proteins, all the electron transport chain complexes, and the ATP synthase complex (Alberts, 2008). The inner mitochondrial matrix contains the citric acid cycle reaction enzymes and substrates (Alberts, 2008).
Mitochondria produce energy and their numbers are particularly prominent in cardiac muscle, skeletal muscle, liver, kidney, and neuronal cells as these cells require significant energy for function. Mitochondria use the electron transport chain to produce usable chemical energy from electron donors like reduced nicotinamide adenine dinucleotide (NADH) via a series of oxidation/reduction reactions where molecules transfer electrons and facilitate transmembrane proton transport resulting in an electrochemical gradient that drives adenosine triphosphate (ATP) synthesis (Mitchell, 1961).
Recent reports have suggested that mitochondria undergo rapid and constitutive metabolic oscillations in a coordinated manner in individual cells as well as in supracellular networks in the tissue (Porat-Shliom et al., 2014). Reactive oxygen species (Aon et al., 2004) and gap junctions (Porat-Shliom et al., 2014) control the synchronization of mitochondrial activity, the oscillations were confirmed by intravital microscopy in living animals (Porat-Shliom et al., 2014) These oscillations were found to disappear with interruption of circulation, inhibiting complex I, and by scavenging ROS. However, they persist but are not coordinated when carbenoxolone, a gap junction inhibitor, is administered.
2.2 Mitochondrial Complexes and the Electron Transport Chain
The electron transport chain consists of five enzyme complexes that are comprised of integral inner membrane proteins. They are NADH-CoQ reductase, Succinate-CoQ reductase, CoQ-cytochrome c reductase, cytochrome c oxidase, and ATP synthase (complexes I-V, respectively). Ubiquinone (CoQ) and cytochrome c are two freely diffusible molecules that mediate the transfer of electrons between complexes (Gao et al., 2008). Oxygen is the final acceptor of electrons in this process.
2.2.1 Oxidative Stress
Oxidative stress arises when the system is unable to establish a balance between production and utilization of oxidant molecules. The excess production of ROS during oxidative stress has the potential to cause protein, DNA and lipid damage causing cellular injury, as observed in ischemia/reperfusion models (Bhat et al., 2015; Walters et al., 2016). Reactive oxygen species include hydrogen peroxide, hydroxyl radicals and superoxide anions. Nevertheless, it is known that cellular production of ROS is essential to the normal function of cells as they take part in specific signaling events (Finkel and Holbrook, 2000). ROS are produced inside the mitochondria as well as outside. The extra-mitochondrial sources of ROS include lipoxygenases, peroxisomes and NADPH oxidases. However, mitochondria are the major site of ROS production, inside the mitochondria, complexes I and III are the major sites. Though mitochondrial ROS has been attributed to one of the causes of aging, there are evidences to the contrary (Wang and Hekimi, 2015).
2.2.2 Mitochondrial Complex I
NADH enters the electron transport chain at complex I (NADH-CoQ reductase, or NADH dehydrogenase). NADH binds to the complex and is oxidized, donating 2 electrons. Electrons are transferred through- complex I flavin mononucleotide (FMN) prosthetic group and it is converted to FMNH2 (Hirst, 2010). The electrons pass through a series of nine FeS clusters to Coenzyme Q (CoQ) (Murphy, 2009) and eventually to the primary electron acceptor, ubiquinone.(Sazanov and Hinchliffe, 2006) The fully reduced flavin in complex I also reduces O2 to superoxide (Hirst, 2010). This also results in transfer of four protons from the matrix into the intermembrane space to support the proton motive force that drives ATP synthesis. Both forward and reverse electron flow generate superoxide radicals (Schonfeld et al., 2010). Inhibition of complex I by rotenone increases superoxide production. The superoxide may be converted to hydrogen peroxide (H2O2) by MnSOD within the mitochondria or react with NO to generate peroxynitrite (ONOO-).
2.2.3 Mitochondrial Complex II
Succinate-Q oxidoreductase (complex II) is the only enzyme that is part of both the citric acid cycle and the electron transport chain (Cecchini, 2003). In this reaction, succinate is oxidized to fumarate through a flavin adenine dinucleotide (FAD) cofactor and iron sulfur clusters mediate electron transfer to coenzyme Q. A heme group is also present but is believed to decrease reactive oxygen species production (Yankovskaya et al., 2003). It does not contribute to the proton gradient by transporting protons.
2.2.4 Mitochondrial Complex III
Complex III has been known for a long time to be a significant site of ROS production in the ETC (Murphy, 2009). Q-cytochrome c reductase (complex III) is a dimer with each part containing protein subunits, iron sulfur clusters, and cytochromes (Iwata et al., 1998). In a two step process called the Q cycle, ubiquinol is oxidized and two molecules of cytochrome c are reduced, also resulting in four protons being added to the intermembrane space (Trumpower, 1990). An intermediate in this cycle is a free radical called ubisemiquinone, which can leak electrons directly to oxygen, resulting in superoxide. Superoxide production is further enhanced when complex III is inhibited by antimycin A.
2.2.5 Mitochondrial Complex IV
Cytochrome c oxidase (Complex IV) consists of 13 subunits, two heme groups, and metal cofactors including magnesium, zinc, and three copper ions (Tsukihara et al., 1996). It mediates the transfer of electrons to O2, which is reduced to two H2O molecules. Four reduced cytochrome c molecules serve as electron donors and four protons are consumed from the matrix to make H2O. Four protons are also transferred from the matrix to the intermembrane space, increasing the electrochemical gradient and proton motive force. This complex is highly efficient and releases few partly reduced intermediates.
2.2.6 Mitochondrial Complex V
ATP synthase (complex V) is the final step of oxidative phosphorylation. It uses the proton motive force generated across the inner mitochondrial membrane to drive synthesis of ATP (Boyer, 1997). Three to four protons pass through the complex, driving molecular rotors named Fo and F1, and resulting in one unit of ATP (Fillingame and Steed, 2014; Jastroch et al., 2010).
2.2.7 Oxidative Products and Effects (Complexes 1-III)
Mitochondrial electron transport generates superoxide as an inevitable by-product at complexes I-III (Turrens, 2003). So in the presence of oxygen, reactive oxygen species such as the superoxide radical (O2−) are generated in low levels. Over time, however, small amounts of free radical induced oxidative damage may accumulate, particularly during hypoxic or ischemic episodes (Chan, 2001). Glutathione peroxidase, catalase and superoxide dismutase are effective endogenous antioxidants that can help scavenge free radicals. In the absence of scavengers, ROS can cause deleterious effects on cellular functions. The transcription factors, Nrf-1 and Nrf-2 have been shown to control the expression of antioxidant response genes (Ohtsuji et al., 2008). A decreased expression of several antioxidant response genes have been observed in mice deficient in Nrf-2 (Itoh et al., 1997). Environmental pollutants, therapeutics, and transient hypoxia have all been suggested and confirmed experimentally to be “hits” resulting in injury to mitochondria and specifically mitochondrial DNA (Nohl et al., 1997). Mitochondrial DNA is especially susceptible to free radical induced damage as it is in close proximity to reactive oxygen species production and superoxide is unable to diffuse through the mitochondrial membranes (Gao et al., 2008). Consequently, there is a major role for mitochondria in hypoxic-ischemic injury as well as age dependent decline in metabolic processes and organ function (Correia-Melo and Passos, 2015). Other neurodegenerative diseases such as Alzheimer’s disease (Aliev et al., 2002; Ng et al., 2014; Palacios et al., 2011) and Parkinson’s disease (Snow et al., 2010; Wu et al., 2011) have also shown key roles of age dependent decline of mitochondrial function and increased oxidative stress, although the focus of this review is on hypoxic-ischemic injury.
3 Hypoxic-Ischemic Injury
3.1 Definitions
Hypoxia refers to low levels of oxygen in the body or tissue. Hypoxemia, on the other hand, refers to low levels of oxygen in the blood. Ischemia occurs with a lack of blood flow to tissues. Ischemia always results in tissue hypoxia. However, hypoxia can occur without ischemia as in acute lung injury (decreased oxygen entering blood), anemia (decreased carrying of oxygen by blood), carbon monoxide poisoning (decreased delivery of carried oxygen to tissues), and other mechanisms. Ischemia can be global or focal. Global ischemia can be produced by traumatic hemorrhage, cardiac or pulmonary failure, sepsis and other mechanisms. Focal ischemia can be produced by embolic or thrombotic processes, injury, surgical clamping of a blood vessel, during organ transplantation, and other mechanisms. Whether by hypoxia or ischemia, or both, a dearth of mitochondrial oxygen impairs oxidative phosphorylation and mitochondrial function. As hypoxic and ischemic events are increased in elderly individuals, they are particularly prone to the resulting mitochondrial dysfunction.
3.2 Effect of Hypoxia/Ischemia
Hypoxic/Ischemic insults are important in the generation of reactive oxygen species (ROS). In unstressed healthy individuals, ROS are generated during cellular metabolism. Sources of ROS include xanthine oxidase, nicotinamide adenine dinucleotide reduction, and adenosine triphosphate production via the mitochondrial electron transport chain (Cairns et al., 1997). Normally, cellular antioxidant systems effectively remove ROS. One mechanism by which this occurs is when superoxide dismutase reacts with superoxide radicals produced during cellular respiration to generate hydrogen peroxide, which is subsequently broken down by glutathione peroxidase and catalase (Winterbourn, 2013). However, during hypoxic/ischemic injury, ROS production by the mitochondria overwhelms antioxidant capacities, leading to cellular DNA damage, mitochondrial lipid peroxidation, disruption of Ca2 + homeostasis (Mattson et al., 2000) (Murphy and Steenbergen, 2008), and mitochondrial membrane depolarization (Lemasters et al., 2009). This results in cytochrome c release and cell death by apoptosis (Wu and Bratton, 2013). Thus, mitochondrial function is a crucial determinant of survival during ischemic injury (Brookes et al., 2004).
3.3 Mitochondrial Trafficking
Intracellular trafficking of mitochondria is important in meeting local metabolic demand as well as in renewal and recycling of the organelle.(Chang and Reynolds, 2006). It has been suggested that motility of axonal mitochondria is important in plasticity and reliability of synaptic transmission (Sun et al., 2013b). Intercellular transfer of mitochondria have been demonstrated in both in vitro and in vivo models and are reported to be facilitated by cellular portals formed by tunneling nanotubes (TNTs) (Gerdes et al., 2007; Rogers and Bhattacharya, 2013). TNTs are cellular connections 100–800 nm in diameter and up to 100 μm long that contain a small cytoplasmic bridge and a surrounding phospholipid bilayer (Gerdes et al., 2007). TNTs look like phyllopodia while forming and contain F-actin and motor proteins (Gerdes et al., 2007; Rustom et al., 2004). TNTs have been observed in various cell lines, including but not limited to adrenal cells (Bukoreshtliev et al., 2009), kidney cells (Gurke et al., 2008), and cardiac cells (Koyanagi et al., 2005; Plotnikov et al., 2008).
Unidirectional mitochondrial intercellular transfer within nanotubes has been demonstrated (Koyanagi et al., 2005; Plotnikov et al., 2008). Vibration and chemical inhibitors of TNT formation are noted to prevent or decrease mitochondrial intercellular transfer (Gurke et al., 2008; Rustom et al., 2004). Also, overexpression of the mitochondrial GTPase Miro1 has been shown to increase mitochondrial transfer and prevent lung injury by the mitochondrial poison rotenone, while knockdown leads to decreased transfer and loss of efficacy (Ahmad et al., 2014). Among other stressors, oxidative stress leading to p53 activation has been shown to be a stimulant for TNT development in stressed cells to non-stressed cells based on the concentration difference in the S100A4 protein (Wang et al., 2011c). Damaged neurons had decreased S100A4 and produced TNT, while cells with higher levels received the TNT. In another study, lung adenocarcinoma A549 cells without mitochondria due to treatment with ethidium bromide received mitochondria and recovered aerobic respiration from cells with functional mitochondria (Spees et al., 2006). Not only do the cells recover respiration, they are also protected from endotoxin induced acute lung injury associated with the transfer of functioning mitochondria (Islam et al., 2012). Another model using cigarette smoke also demonstrated that mitochondrial transfer from induced pluripotent cells decreased damage to lung epithelium (Li et al., 2014). Given these results, using stem or progenitor cells as a vehicle for healthy mitochondrial transfer to cells with damaged mitochondria as a result of hypoxia or aging, and potentiating this transfer with Miro1 may provide significant therapeutic value but needs more investigation, especially with neuronal cells as few studies have been performed in these cell populations at this time (Las and Shirihai, 2014). Apart from acting as a connecting bridge between cells for mitochondrial transfer, TNTs may also serve important intercellular communication during cellular stress (Wang and Gerdes, 2015).
3.4 Nuclear-Mitochondrial Cross-talk
Mitochondrial structure and function are controlled by the 37 genes encoded in mtDNA as well as a large number of genes in nuclear DNA (Poulose and Raju, 2014). Among the 37 genes on the mtDNA, 13 code for proteins, 22 for tRNAs and 2 for ribosomal RNAs. The transcription and translation of the 13 genes coding for proteins on mtDNA involves a complex process participated by a number of proteins coded on the nuclear genome (Hallberg and Larsson, 2014). Therefore the small number of genes on the mtDNA is not sufficient for the structure and function of mitochondria. The nuclear encoded proteins that are translated are translocated into the mitochondria by a well-orchestrated mechanism. Several molecular and genetic factors have broad impacts on the normal function of mitochondria as well as in the setting of age and ischemia. Therefore, nuclear-mitochondrial talk is an essential mechanism in mitochondrial homeostasis. In order to study nuclear-mitochondria cross talk, our laboratory developed a mitochondrial gene chip with probe sets representing the transcriptome of the genes on the mitochondria and that of the nucleus important for the structure and function of mitochondria, together called the mitoscriptome (Raju et al., 2011). We found significant upregulation of genes involved in glycolytic pathways in cardiomyocytes subjected to hypoxia demonstrating a shift in energy production from mitochondria to glycolysis (Jian et al., 2011b). Recently it has been found that humanin, a mitochondria derived peptide, exhibits strong cytoprotective actions against various stress and disease models and suggested to be part of a retrograde signaling that involves nuclear-mitochondria crosstalk (Gong et al., 2014). This inter-organelle communication is important in the mitochondrial response to hypoxic-ischemic conditions, whether induced by stroke, myocardial-infarction or sepsis. About 1500 distinct proteins are estimated to be involved in the maintenance of mitochondrial structure and function (Calvo and Mootha, 2010), some of the master regulators that control physiological responses are well-studied.
3.3.1 Molecular Mediators of Mitochondrial Function in Hypoxic-Ischemic Injury
One of the hallmarks of hypoxia is the stabilization of HIF-1α and subsequent nuclear translocation with modulation of expression of a number genes important in energy metabolism (Ke and Costa, 2006; Salceda and Caro, 1997; Semenza, 2007). HIF-1 consists of two protein subunits: HIF-1β, which is constitutively expressed, and HIF-1α, which is continuously synthesized then degraded in a prolyl hydroxylase dependent manner (Solaini et al., 2010) by the ubiquitin-proteasome system under normoxic conditions (Salceda and Caro, 1997). During low oxygen tension, proline hydroxylation does not occur and HIF-1α rapidly accumulates, binds with the β subunit and is translocated to the nucleus where it serves as a transcriptional activator of over 100 genes (Semenza, 2007). Many of these genes are involved in glycolytic pathways and lead to a switch in energy production from oxidative phosphorylation to glycolysis (Semenza, 1996). HIF-1 enhances glycolysis by upregulating synthesis. of glycolysis enzymes (Semenza, 1996), increasing glucose transporters, and blocking mitochondrial energy metabolism (Semenza, 2011). HIF-1 facilitates activation of pyruvate dehydrogenase kinase (PdK)1, which phosphorylates and inhibits pyruvate dehydrogenase (PDH), the rate limiting step leading to oxidative phosphorylation, from converting pyruvate to acetyl CoA to fuel the mitochondrial TCA cycle (Papandreou et al., 2006) and blocks assembly of Fe/S clusters required for oxidative phosphorylation (Semenza, 2011). It also concomitantly blocks nuclear-mitochondrial crosstalk inhibiting the expression of mitochondrial encoded subunits in oxidative phosphorylation complexes (Gomes et al., 2013). The Sinclair group (Gomes et al., 2013) suggested the development of a pseudohypoxic state with progressive aging and a role for HIF-1, independent of PGC-1α, in modulating mitochondrial function. HIF-1 has an interesting interplay with a pleotropic transcription factor called c-MYC. c-MYC binding canonical sequence segments are found on several genes related to mitochondrial biogenesis (Kim et al., 2008; Li et al., 2005) and overexpression of c-MYC was shown to prevent loss of mtDNA and cellular ATP levels with upstream inhibition (Gomes et al., 2013). c-MYC appears to act via increasing transcription factor A mitochondrial (TFAM) and is critically important in hypoxia/ischemia. In a mitochondrial gene expression profiling of a rat model of hemorrhagic shock in our laboratory, c-MYC exhibited most change following the injury (Jian et al., 2011b).
The c-MYC -HIF-1 interrelationship and their role in cellular homeostasis remain unsettled. Though there are contradicting results, HIF-1 modulation of c-MYC is evident from many studies. c-MYC increases both glycolysis and mitochondrial oxidative phosphorylation. Both c-MYC and HIF-1α are upregulated with hypoxia, yet HIF-1 directs c-MYC repression and inhibition of oxidative phosphorylation (Kim et al., 2006), a result similar to Warburg reprogramming (Christofk et al., 2008; Warburg, 1956). HIF-1 also impacts neovascularization, angiogenesis and cell survival. It has also been shown to be a critical determinant of lifespan in C. elegans, a nematode frequently used in aging research (Leiser and Kaeberlein, 2010). While Warburg postulated aerobic glycolysis in cancer, notwithstanding the anaerobic environment in the core of solid tumors, injury conditions such as ischemia or hemorrhage reflect an anaerobic status with mitochondrial functional deficiency and promotion of glycolysis. This is clearly evident in models like hemorrhagic shock where increased plasma lactate and decreased tissue ATP levels are consistently observed (Jian et al., 2012; Pearce et al., 1985). These relationships and others are depicted in Figure 1. Further investigations on the fine balance of function between HIF-1 and c-MYC in hypoxic/ischemic insult with the aging phenotype is likely to yield more information on the dysregulation of mitochondrial homeostasis.
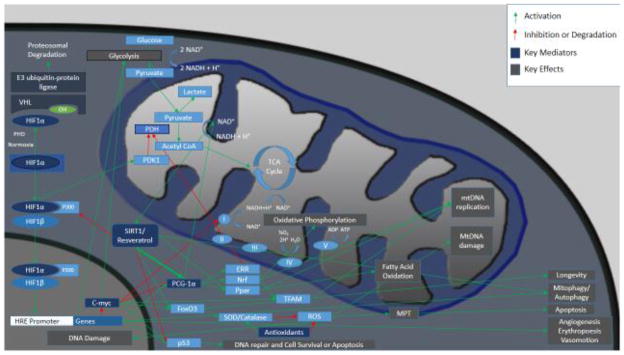
Figure 1. Critical molecular mediators in hypoxia/ischemia.
HIF-α is a critical modulator of hypoxic response. SIRT1 deacetylates Lys674 of HIF-1α, which inhibits P300 binding and decreases HIF-1α signaling. During hypoxia, SIRT1 is suppressed consequent to decreased NAD+, and facilitates HIF-1 signaling (Lim et al., 2010). HIF-1α induces gene transcription leading to activation of many mediators including glycolytic enzymes, and also activates pyruvate dehydrogenase kinase 1 (PDK1) which inhibits pyruvate dehydrogenase (PDH) leading to inhibition of mitochondrial oxidation. PGC-1α activation leads to NRF, ERR, and PPAR activation, which increase mitochondrial function and biogenesis.
One of the critical mediators of mitochondrial function is PGC-1α, the expression and activity of which have been demonstrated with several age-associated neurodegenerative diseases (Wenz, 2011). PGC-1α is a cofactor to peroxisome proliferator-activated receptor (PPAR) family transcription factors leading to increased mitochondrial fatty acid oxidation gene expression (Finck and Kelly, 2007). It also binds to and activates nuclear respiratory factor 1 (NRF1), a transcription factor that increases oxidative phosphorylation and also stimulates the synthesis of transcription factor A mitochondrial (TFAM), leading to mtDNA transcription, maintenance, and replication (Kang and Hamasaki, 2005). Additionally it targets and activates estrogen receptor related receptor (ERR) family proteins. ERRα increases nuclear respiratory factor 2 (NRF2), which regulates the cell cycle, cell differentiation, and mitochondrial biogenesis (Scarpulla, 2008). PGC-1α has also been noted to have effects on energy production and defense systems against ROS (Jian et al., 2011b). PGC-1α activity is modulated by nutrient availability, calcium, ROS, insulin, thyroid hormone, estrogens, hypoxia, ATP demand, cytokines, and c-MYC (Puigserver and Spiegelman, 2003). It has also been noted to decrease with age (Anderson and Prolla, 2009). A SIRT1 pathway that regulates mitochondria independently of PGC-1α was recently postulated (Gomes et al., 2013). These researchers were able to revert key biochemical parameters in 22-month old mice to that of 6-month old mice by treating these mice with NAD+, a substrate and therefore a modulator of SIRT1 activity, indicating that regulation of mitochondrial function can alter aging trajectory.
3.4.1 Age Related Effect of Hypoxia/Ischemia on Cells and Mitochondria
Aging, as discussed by Harman, leads to mitochondrial dysfunction (Harman, 1972). Mitochondrial dysfunction causes energy failure and is associated with multi-organ dysfunction in severely injured patients (Cairns et al., 1997). Consequently, mitochondria are a common denominator in the increased mortality, injury, and organ dysfunction associated with aging during hypoxia and ischemia. Mitochondria are increasingly implicated as central players in the development of ischemic cell death both through impairment in generating ATP for cell function and as key mediators in cell death pathways (Sims and Muyderman, 2010). This has been attributed to multiple mechanisms.
During hypoxia or ischemia, oxidative phosphorylation via the electron transport chain is inhibited or reduced, resulting in accumulation of superoxide radicals along with decreased ATP (Solaini et al., 2010). Superoxide at low levels does little cellular damage by itself; however, at higher levels, pathologic free radicals such as hydro-peroxyl radicals, hydroxyl radicals, and peroxynitrite (Stowe and Camara, 2009) result in oxidative stress that can damage DNA, lipids, and proteins, (Wheaton and Chandel, 2011). This oxidative stress, in conjunction with calcium increases due to glutamate toxicity (in the brain) and/or ischemia induced loss of efflux associated with less Na+/K+ ATPase and Na+/Ca+2+ exchanger activity, causes the inner mitochondrial membrane to become more permeable with resultant loss of the mitochondrial membrane potential (Green and Kroemer, 2004). This leads to mitochondrial swelling with release of cytochrome c and apoptotic protease activating factors (Murphy et al., 1999) into the cytoplasm. Together, they activate caspases and induce apoptosis (Zou et al., 1997). This process is amplified when aged cells are exposed to injury, particularly hypoxic/ischemic injury as progressive loss of mitochondrial function potentiates further injury. Less efficient mitochondria in aged cells have increased free radical generation and decreased energy production during stress (Nohl et al., 1997; Sasaki et al., 2008b). Consequently, not only is the damage greater in aged cells for a given degree of injury, the healing response is also diminished (Gupta et al., 2004). There are also age-related decreases in the activities of mitochondrial carrier proteins—such as for phosphate, ATP/ADP (Paradies and Ruggiero, 1991; Tummino and Gafni, 1991) and Ca 2+ (Hansford and Castro, 1982), adenine nucleotide (Kim et al., 1988; Nohl and Kramer, 1980; Tummino and Gafni, 1991), and pyruvate (Paradies and Ruggiero, 1990). Humanin, a mitochondria-derived small peptide has been shown to exhibit strong cytoprotective actions against pathological stress (Lee et al., 2013a). Interestingly this 24-amino acid peptide is derived from a 75bp open reading frame in the 16S ribosomal RNA sequence on the mtDNA. One recent study found that a humanin-derivative, AGA(C8R)-HNG17, enhanced the activity of ATP synthase complex (Cohen et al., 2015). Humanin is also implicated to play a role in lifespan extension (Gong et al., 2014). As such mitochondria are believed to be a key modulator of neuronal viability during hypoxic/ischemic stress.
3.4.2 Mitochondrial dynamics and role in cell death
Hypoxic-ischemic insults are associated with increased apoptosis (programmed cell death). Mitochondrial control of apoptosis has been extensively studied and a number of players included in this mechanism are known. During ischemia, increased levels of ROS and influx of calcium leads to membrane permeability transition, and release of cytochrome c from mitochondrial intermembrane space leading to the activation of the apoptosome and caspase mediated apoptosis (Mattson et al., 2000) (Bonanni et al., 2006). Studies showed an increased release of cytochrome c with aging (Marzetti et al., 2013). Calcium mediated activation of mitochondrial calpains 1 and 10 results in the cleavage of AIF from inner membrane and translocation to nucleus facilitating caspase independent programmed cell death (Polster, 2013). A growing number of known proteins are known to be involved in initiating and executing mitochondria-mediated apoptosis or inhibiting the process, and these include Bax, Bad, Bid, Apaf-1, Bcl-2, and Bcl-xL.
Cellular machinery is a robust biological system with meticulous built-in mechanisms to maintain homeostasis. Autophagy is one such mechanism that works together with the ubiquitin-proteosome system to recycle damaged organelles and act as a survival mechanism in nutrient poor conditions. Activation of autophagy has been observed following cerebral ischemia (Polster, 2013) and declining autophagy is noted with age (Wang et al., 2011a). Inhibition of mTOR by rapamycin was found to augment autophagy and consistent with this observation, rapamycin prolonged longevity in mice (Ehninger et al., 2014; Harrison et al., 2009). These results demonstrate a key role for autophagy in the restoration of cellular homeostasis and aging phenotype. An autophagy process called mitophagy is utilized by cells to clear damaged mitochondria. This is a critical process for healthy mitochondrial biogenesis considering the dynamic nature of this organelle, which undergoes constant fission and fusion process. Mitophagy involves a selective recruitment of mitochondria into isolation membranes and fusion with lysosomes resulting in the elimination of entrapped mitochondria (Kane and Youle, 2010). However, it is not clear how soon this process is effected in the case of sudden injuries such as myocardial infarction, stroke or hemorrhagic shock. Nevertheless, it is known that one hour after induction of stress by mitochondrial uncouplers, Parkin can translocate from cytosol to mitochondria (Kane and Youle, 2010). PINK1 is required for the Parkin recruitment to mitochondria (Sebastian et al., 2012). In healthy cells, PINK1 is rapidly degraded by proteolysis and maintains low levels, where as PINK1 hydrolysis is inhibited in damaged mitochondria. Parkin inhibits mitochondrial fusion by ubiquitinylating mitofusins (Gomes et al., 2011). Dynamin-related protein 1 (Drp1) and Fission 1 (Fis1) are fission-promoting proteins, whereas Mitofusin 1 and -2 are mainly responsible for outer membrane fusion, and Opa1 mediates inner membrane fusion. Mitofusin 2 is also reported to tether endoplasmic reticulum to mitochondria (Ding and Yin, 2012). It has been shown that starvation resulted in elongated mitochondria, resulting in resistance to autophagic degradation. This is postulated to be due to increased cAMP and activation of protein kinase A resulting in phosphorylation of Drp1 (Gomes et al., 2011; Rambold et al., 2011). It may be noted that a major pathway implicated in caloric restriction mediated longevity is increase in the expression and/or activity of SIRT1, and SIRT1 is known to promote mitochondrial biogenesis. In lower organisms it has been shown that, though controversial, resveratrol can mimic the beneficial effects of caloric restriction. In addition, Bnip3 and Nix are highly induced during hypoxia and are important in mitochondrial autophagy. Mitochondrial fission and fusion control quality of the mitochondria, and together with mitophagy prevent accumulation of damaged mitochondria and maintain mitochondrial homeostasis.
3.5 Age Related Effects of Hypoxia/Ischemia on Organ Function
3.5.1 Neurologic
The CNS is highly susceptible to mitochondrial impairment because of its aerobic requirements. It is reported that blood flow to an average brain is 15% of the cardiac output (Udomphorn et al., 2008). While the human brain comprises only 5% of the body, it is responsible for 20% of respiration and is dependent on glucose and oxygen for energy. More injury occurs with smaller insults compared to other tissues (Wang and Michaelis, 2010). It has been shown that young individuals have increased neuronal aerobic glycolysis compared to aged individuals (Goyal et al., 2014). Aerobic glycolysis, also known as Warburg reprogramming and first described in cancer cells, is defined as glucose utilization in excess of that used for mitochondrial respiration despite sufficient oxygen to completely oxidize glucose. Positron emission tomographgy based studies suggest that aerobic glycolysis commonly occurs in healthy adult brains (Fox and Raichle, 1986; Fox et al., 1988; Vaishnavi et al., 2010). Changes in brain energetics, neuronal phosphate, and ATP metabolism occur with age (Forester et al., 2010), and elderly individuals have virtually absent global neuronal aerobic glycolysis (Goyal et al., 2014), which creates a pseudohypoxic state. This is particularly relevant in the face of hypoxic injury, as aged individuals have less oxygen dependent cellular metabolism needed for post injury repair.
The unique structure of the neuron necessitates mitochondrial transport to facilitate local energy demand. Mitochondria are trasported to synaptic terminals along microtubules to meet the energy demand due to synaptic transmission (Ma et al., 2009). About 20–30% of axonal mitochondria are reported to be motile in mature neurons (Lin and Sheng, 2015), though the localized non-motile mitochondria serve local energy needs the motile ones may have complementary functions in energy needs. Furthermore it has been suggested that there is a correlation between mitochondrial membrane potential and the direction of mitochondrial transport (Miller and Sheetz, 2004). The speed of mobilization is important in critical events and influence of age and hypoxic/ischemic injury in mitochondrial trafficking may be an important factor in determining neuronal survival following injury.
Stroke is a major cause of death in the United States, with one person dying from stroke every four minutes (Mozaffarian et al., 2015). The incidence of stroke onset seems to be higher in men at middle age than older age, whereas women show fewer stroke incidences during middle age that increases significantly at older age. Several preclinical studies show that estrogen treatment is beneficial after stroke, but its effects have been found to be age and sex dependent and in particular estrogen therapy might be detrimental in aged women (Kim and Vemuganti, 2015).
Stroke may be associated with local or global ischemia depending on the nature of the initiating event. For example, embolic occlusion of the middle cerebral artery can result in focal ischemia while heart failure can cause a low flow state resulting in global ischemia. During stroke, in the penumbral region, large decreases are seen in ATP (Folbergrova et al., 1995). Some of the ADP generated from ATP hydrolysis is further metabolised to AMP and ATP. The AMP in ischemic tissue is then converted to inosine and hypoxanthine resulting in overall depletion of the adenine nucleotide pool (Onodera et al., 1986). Ischemic preconditioning has been suggested to modulate miRNAs that are upstream of neuroprotective signaling (Vemuganti, 2010).
Another frequent cause of neuronal injury is traumatic brain injury (TBI). Adults aged 75 and older have the highest rates of traumatic brain injury related hospitalization and death (Thompson et al., 2006). Repeated stress prior to TBI increase expression of cerebral mitochondrial electron transport chain complex proteins (Xing et al., 2013). Traumatic brain injury frequently results in intracranial hemorrhage leading to increased intracranial pressure and reduced cerebral perfusion pressure, which causes ischemia. According to a Congressional Research Service report of 2014, over 300,000 Defense service personnel of the United States suffered traumatic brain injury during the period 2000–2014 which include wars in Iraq and Afghanistan (Fischer, 2014). Neurologic outcomes are worse for individuals at the extremes of age following ischemia. In one experiment, juvenile, young adult, middle-aged, and aged (18–19 months) mice had transient middle cerebral artery occlusion. On postoperative day seven, aged mice had significantly increased infarct volume. In addition, aged mice had prolonged disability and less functional recovery after stroke. The aged animals had decreased mitochondrial function and less antioxidant detoxification following ischemia, thereby inducing oxidative damage (Li et al., 2011b). While the study by Li and coworkers implied the neurological deficits were associated with increased infarct volumes sustained with age, another study found that tissue loss failed to explain the increased functional deficits seen in aged animals and suggested that prolonged edema, increased opening of the blood-brain barrier and increased neurodegeneration were responsible for age-related differences in outcome (Onyszchuk et al., 2008). Some groups have suggested that altered apoptotic proteins with age play a role. There is age related increased expression of apoptosis proteins (heat shock protein 27, hippocalcin, and LANP [acidic nuclear phosphoprotein]) after injury (Sun et al., 2013a). Less cellular proliferation and differentiation into neurons after injury in aged animals has been noted (Sun et al., 2005). Electrolyte and neurotransmitter excess may also contribute to age related neurotoxicity. Higher levels of K+ and glutamate with aging disrupt ionic and neurotransmitter homeostasis in neuronal cells through injury induced activation of AMPA (non NMDA) and NMDA receptors leading to calcium influx (Yi and Hazell, 2006) and apoptosis. This glutamate mediated excitotoxicity is known to cause expansion of the injury; however, glutamate receptor antagonists have been ineffective (Yi and Hazell, 2006). Others have suggested metabolic differences contribute, noting that brain pH and high energy phosphate levels remain lower for longer in older gerbil brains during transient ischemia (Funahashi et al., 1994). The authors suggested that brain mitochondria from older animals are less capable of responding to oxidative stress brought on by ischemia and that oxidative damage accumulates as the pH stays lower. Increased oxidative damage due to amplified levels of free radicals with age is another mechanism by which neurologic outcomes are worse after cerebral ischemia (Buga et al., 2008; Itoh et al., 2013). Superoxide levels are greater in the brains of aged mammals and birds during oxidative stress (Sasaki et al., 2008b). This is likely due to not only increased superoxide generation (Sasaki et al., 2008b), but also reduced antioxidant levels following ischemic stroke (Flamm et al., 1978). Importantly, a recent study identified that cardiolipin oxidation generates mitochondrial death signals and the global lipodomics-based study in an experimental traumatic brain injury model demonstrated significant oxidation of polyunsaturated cardiolipin following the injury (Ji et al., 2012).
The changes to neuronal mitochondria during ischemic stress in aged individuals have been studied by multiple groups. A study of age related changes in the blood brain barrier revealed altered function that was associated with a decrease in the number of mitochondria in endothelial cells of brain capillaries in monkeys (Mooradian, 1988). Not only are there less mitochondria, but the remaining neuronal mitochondria have associated functional decline (Xu et al., 2008). The etiology of the functional decline is likely multifactorial. The age related mutations of mitochondrial DNA lead to less effective production of various proteins needed for respiratory chain function (Bowling et al., 1993). Over time various studies have reported a decline in mitochondrial function in aged rats associated with impairment of complexes in the respiratory chain (Davis et al., 1997; Tatarkova et al., 2011).
3.5.2 Cardiac
Aging increases propensity for cardiac ischemia injury. Aged individuals have demonstrated higher mortality after myocardial infarction despite similar infarct size to younger patients (Corsini et al., 2006; Day et al., 1987). It has been reported that ROS generated from mitochondria is significantly elevated in the heart with progression of age (Judge et al., 2005). One study examined the cardiac effects of ischemia in aged versus adult rat hearts and found that age results in greater tissue damage (creatine kinase measurements 3.4 times greater) and limits hemodynamic recovery (pressure 31% vs 57% of preischemic baseline) (Lesnefsky et al., 1994). Consequently, aged hearts are more susceptible than the adult hearts to ischemia-reperfusion injury. There are likely multiple mechanisms by which this occurs; ionotropic, metabolic, genetic, oxidative stress, and hormonal pathways have all been suggested. Many of these mechanisms involve the mitochondria.
A component of the increased cardiac injury seen with aging may be ionic imbalance (Tani et al., 1997). Aged individuals start with higher intracellular sodium levels and have greater sodium increase with injury associated with a decline in myocardial function. The lack of cellular ATP due to hypoxia and mitochondrial dysfunction with aging may limit the function of the sodium-potassium ATPase, resulting in higher intracellular sodium levels.
Changes in metabolism due to altered mitochondrial gene expression in aged individuals following hypoxia/ischemia may also explain the increased cardiac injury. A number of genes related to cellular energetics are altered with age following trauma-hemorrhage based on an analysis with a custom-made rodent mitochondrial gene chip (RoMitochip) in 6 and 22 month old rats (Jian et al., 2011b). Aged rats had a decline in the number and amplitude of expression of mitochondria-related genes compared to the younger rats following trauma-hemorrhage.
Aged animals appear to have less ability to withstand significant oxidative stress and have more oxidative injury following cardiac ischemia. One study found that aging selectively decreases the oxidative capacity of rat heart interfibrillar mitochondria. They found increased oxidative damage occurs in aged hearts, noted via increased oxidation of cardiolipin in the inner mitochondrial membrane following ischemia (Lesnefsky et al., 2009).
Hormonal changes may also account for the increased cardiac injury with aging seen following hypoxic/ischemic injury. Based on proteomic screens of mitochondria, there appears to be a highly selective response to estradiol (E2) deficiency in aged versus adult rat hearts (Korzick and Lancaster, 2013) after ischemic injury. They suggested that estradiol deficiency induced perturbations of electron transport chain (ETC) proteins may upset the stoichiometry of the ETC and contribute to increased ROS production and cardiac injury with aging.
There is an association between higher levels of mitochondrial DNA damage and coronary atherosclerotic heart disease (Ballinger, 2005; Corral-Debrinski et al., 1992). Corral and coworkers specifically noted that in subjects with coronary artery disease certain mitochondrial DNA deletions were increased 7,220-fold over age-matched controls (Corral-Debrinski et al., 1992). So not only are there increased errors in mitochondrial DNA, there are also decreased absolute amounts. In a model of coronary artery disease where the left anterior descending artery was ligated for 4 weeks, the mitochondrial DNA copy number was noted to decrease significantly. Interestingly, associated decreased complex I, complex III, and complex IV levels and activities were also observed (Ide et al., 2001). Therefore, worse outcomes in aged individuals with cardiac ischemia are associated with mitochondrial DNA damage leading to decreased mitochondrial function. Consequently, modulation of mitochondrial respiration and function during and immediately following an episode of ischemia can attenuate the extent of myocardial injury (Chen et al., 2007) and may maintain cardiac function in the context of injury and aging (Judge and Leeuwenburgh, 2007).
3.5.3 Interconnectedness of Mitochondria in Different Tissues, Mitokines
Interestingly, a recent study showed that mitochondrial perturbations in one tissue may trigger mitochondrial stress response in distal tissues (Durieux et al., 2011). This provides a mechanism by which multiorgan dysfunction and survival can be affected in aged individuals exposed to ischemic insults in one tissue. Retrograde signaling by mitochondrial unfolded protein response (UPR) to the nucleus is suggested to be a likely mechanism of mitochondrial control of metabolic events following stress. Extension of such signaling events through hypothesized “mitokine” messengers to the distal parts of the body needs further investigation to substantiate (Riera and Dillin, 2015). One study that may support this hypothesis is the demonstration that neuronal ROS signaling through SKN/NRF2 may be sufficient to extend lifespan extension in C. elegans (Schmeisser et al., 2013). If such a scenario exists, as a result of multi-tissue extension of mitochondria dysfunction, increased age significantly increases predisposition to critical illness, complications, and poor healing and is a strong independent risk factor for mortality after focal ischemia.
3.5.4. Age Related Effects of Global Hypoxia/Ischemia on Survival
While focal ischemia as described frequently has organ specific effects, global ischemia can cause multiple organ system failure. Traumatic hemorrhage is one of the models used to investigate the effects of global hypoxia and ischemia. In fact, almost half of deaths due to trauma are due to hemorrhage or its resultant hypoxic/ischemic injury (Curry et al., 2011). Following hemorrhagic shock, aging has been shown to increase tissue damage (Mees et al., 2008) and particularly amplified the effects of hypoxia/ischemia after trauma (Gong et al., 2004; Gong et al., 2005; Thompson et al., 2006). Shock index (heart rate/systolic blood pressure) has been found to be an indicator of mortality in trauma patients (King et al., 1996) and age multiplied by shock index is an even better predictor of early mortality in elderly patients (Zarzaur et al., 2008). Consequently, increased age is associated with worse prognosis in these scenarios.
4 Pharmacologic Therapies
While time dependent thrombolytic agents like tissue plasminogen activator (tPA) exist to restore blood flow, few treatments improve outcomes once ischemic injury has occurred or after the three hour time window, especially in aged individuals. Several agents that are shown to have profound effect on mitochondrial function and have therapeutic potential for hypoxic/ischemic injury are being investigated in multiple laboratories.
4.1 Agents with Multiple Mechanisms
4.1.1 Sirtuins and Resveratrol
4.1.1.1 Overview
In response to acute injury conditions, cellular machinery can rapidly mobilize molecular pathways through posttranslational modifications, rather than relying solely on transcriptional control. Therefore processes such as phosphorylation-dephosphorylation and acetylation-deacetylation become important in restoration of cellular homeostasis underlining the significance of regulatory mediators like sirtuins, AMPK and PGC-1 in the control of mitochondrial function. Sirtuins are a class of NAD+ dependent histone deacetylases (Imai et al., 2000; Landry et al., 2000; Smith et al., 2000) that may also have mono-ADP ribosyl transferase (Frye, 1999; Haigis et al., 2006), demalonylation (Du et al., 2011), and desuccinylation activities (Du et al., 2011). They were first discovered in yeast as a transcriptional repressor called silent information regulator 2 protein (Sir2) (Klar et al., 1979; Rine and Herskowitz, 1987). All eukaryotes encode sirtuins in their genomes as sirtuins have important functions in regulating cellular stress responses (Brunet et al., 2004; Dai et al., 2014; Maksin-Matveev et al., 2015), mitochondrial biogenesis (Brenmoehl and Hoeflich, 2013), apoptosis (Alcendor et al., 2004; Motta et al., 2004), metabolism (Purushotham et al., 2009), fatty acid oxidation (Hirschey et al., 2010), and inflammation (Cao et al., 2013; Liu and McCall, 2013).
4.1.1.2 Types of Mammalian Sirtuins
Mammals have seven sirtuins (SIRT 1–7) distributed in different subcellular compartments. SIRT 1 and 2 are located in the nucleus and cytoplasm, SIRT 3, 4, and 5 are located in the mitochondria, and SIRT 6 and 7 are located in the nucleus (Haigis and Guarente, 2006; Michishita et al., 2005). SIRT 1–3 primarily function by deacetylation, and deacetylation of histones leads to DNA coiling and gene silencing (Braunstein et al., 1993). SIRT 4–7 have weaker deacetylase activity (Du et al., 2011; Haigis et al., 2006; Michishita et al., 2008). SIRT 4 has ADP ribosyl transferase activity (Haigis et al., 2006), and SIRT 5 also has demalonylation and desuccinylation activity (Du et al., 2011). SIRT1 is the most well studied among all sirtuins.
4.1.1.3 Regulators and Targets
SIRT1 is the mammalian ortholog of yeast Sir2 and has numerous regulators and targets. SIRT1 expression and activity have been shown to increase after exercise (Ferrara et al., 2008) and with ischemic preconditioning (Hsu et al., 2010). SIRT1’s activity is NAD+ dependent (Imai et al., 2000). Nicotinamide phosphoribosyltransferase (Nampt) catalyzes the production of nicotinamide mononucleotide (NMN) and is the rate limiting enzyme in NAD+ production (Imai, 2011). Nampt levels are reduced with aging and stress (Hsu et al., 2009), resulting in decreased NAD+ and decreased SIRT1 activity (Imai, 2011). Accordingly, overexpression of Nampt in the heart (Hsu et al., 2009) and brain (Erfani et al., 2015) has been shown to reduce infarct size and apoptosis in response to ischemia and reperfusion. In a negative feedback loop, Nampt is also regulated by SIRT1 mediated deacetylation and inactivation of its transcription factors (Ramsey et al., 2009).
Many other regulators have also been shown to influence SIRT1 activity. Hu antigen R (HuR) is one that dissociates from SIRT1 mRNA during oxidative stress thereby destabilizing it (Abdelmohsen et al., 2007). Other regulators include but are not limited to C-Jun N-terminal kinase 1 (JNK1) (Nasrin et al., 2009), cyclin B/cyclin-dependent kinase 1 (Cdk1) (Sasaki et al., 2008a), small ubiquitin-related modifier 1 (SUMO1)/sentrin specific peptidase 1 (SENP1) (Yang et al., 2007), caspases (Ohsawa and Miura, 2006), MicroRNAs, (miR-34a (Yamakuchi et al., 2008), miR-134 (Gao et al., 2010), miR-199a (Rane et al., 2009), and miR-217 (Menghini et al., 2009)), insulin-like growth factor 1 (Vinciguerra et al., 2010), hypermethylated in cancer (repressor) (Chen et al., 2005b) and deleted in breast cancer 1 (repressor) (Zhao et al., 2008)
Histones are a significant target of SIRT1. When activated, SIRT1 deacetylates histones, such as the lysine 26 residue of histone 1 (H1K26), lysine 9 residue of histone 3 (H3K9), and lysine 16 residue of histone 4 (H4K16). This results in tight DNA coiling and transcriptional silencing (Vaquero et al., 2004; Vaquero et al., 2007). SIRT1 is known to have two nuclear localization signals and two nuclear export signals have been noted (Tanno et al., 2007). During ischemic stress, SIRT 1 is shuttled out of the nucleus and into the cytoplasm leading to decreased deacetylation and decreased SIRT1 function in aged hearts compared to young hearts (Tong et al., 2013). Preferentially overexpressing nuclear SIRT1 has been shown to suppress apoptosis in cells exposed to oxidative stress (Tanno et al., 2007). SIRT1 also has multiple non-histone targets. It binds to and deacetylates hypoxia inducible factor 1α (HIF-1 α) at Lys674, which inactivates it leading to repression of HIF-1 target genes (Lim et al., 2010). SIRT1 also catalyzes PGC-1α deacetylation which impacts cellular metabolism and mitochondrial biogenesis (Nemoto et al., 2005). PGC-1α and SIRT1 appear to be part of a pathway also involving adenosine monophosphate activated protein kinase (AMPK), a master switch sensing and regulating cellular energy processes (Canto et al., 2009). AMPK activation increases NAD+ levels, leading to SIRT1 activation and deacetylation of targets such as PCG-1α and forkhead box proteins (FOXO1 and FOXO3a) (Canto et al., 2009), transcription factors for genes that modulate metabolism, upregulate manganese superoxide dismutase, and apoptosis (Hsu et al., 2010). SIRT1 also stimulates AMPK by deacetylating serine/threonine kinase 11 (LKB1), an upstream kinase of AMPK (Wang et al., 2011b). SIRT1 represses genes controlled by the beta oxidation regulator peroxisome proliferator-activated receptor-gamma (PPAR-γ) by docking with its cofactors nuclear receptor co-repressor and silencing mediator of retinoid and thyroid hormone receptors leading to attenuated adipogenesis, lipolysis, and increased lifespan (Picard et al., 2004).
SIRT1 inactivates p53 by deacetylating the C-terminal Lys382 residue and inhibits stress induced p53 transcription (Cheng et al., 2003; Vaziri et al., 2001). Active Regulator of SIRT1 (AROS) facilitates this process (Kim et al., 2007b). This leads to increased anti-apoptotic molecules like thioredoxin-1 and Bcl-xL, and decreased pro-apoptotic molecules like Bax and cleaved caspase-3 (Hsu et al., 2010). Additionally, the cell-cycle and apoptosis regulator E2F1 induces SIRT1 expression while SIRT1 inhibits E2F1 activity (Wang et al., 2006).
SIRT1 has a complex feedback loop with the inflammatory cytokine NF-κB. SIRT1 directly inhibits NF-κB signaling via deacetylating its p65 subunit and indirectly by increasing NF-κB inhibitors such as AMPK, PPARα and PGC-1α (Kauppinen et al., 2013). NF-κB diminishes SIRT1 activity through miR-34a, IFN-γ, and ROS (Kauppinen et al., 2013). Nitric oxide, another molecule implicated in inflammatory processes, also diminishes SIRT1 activity by nitrosylation (Shinozaki et al., 2014).
4.1.1.4 Sirtuin Activators – Resveratrol
One of the most studied sirtuin activators is resveratrol. Resveratrol is a naturally occurring polyphenol compound synthesized in many plants, such as peanuts, blueberries, pine nuts, and grapes, which protects them against fungal infection and ultraviolet irradiation (Bavaresco, 2003). It is a significant component of red wine and has been suggested to explain the “French paradox” in which the southwestern population of France has a low occurrence of coronary heart diseases and cardiovascular diseases despite a high saturated fat diet (Renaud and de Lorgeril, 1992). It has anti-cancer (Udenigwe et al., 2008), anti-inflammatory (Chen et al., 2005a; Udenigwe et al., 2008), antiapoptotic (Baarine et al., 2011; Nicolini et al., 2001), antioxidant (Chang et al., 2012; Spanier et al., 2009), antidiabetic (Chang et al., 2012), and antiviral (Clouser et al., 2012) properties. It is a potent activator of SIRT1 by lowering SIRT1’s Michaelis constant for its acetylated substrate and NAD+ (Howitz et al., 2003). Consequently, it is often used to increase sirtuin activity as well as expression in in vitro as well as in vivo models. While this effect is strongly supported (Alcain and Villalba, 2009; Pervaiz, 2003; Raval et al., 2008; Sun et al., 2010), the underlying mechanisms are not fully understood (Zhang et al., 2011). Recently it has been suggested that the effect of resveratrol on SIRT1 is a secondary effect of its cognate inhibitory interaction with phosphodiesterase (PDE) (Chung et al., 2012; Park et al., 2012) as shown in Figure 2. The investigators demonstrated a rolipram mimicking effect for resveratrol, resulting in increased cAMP, activation of AMPK and augmented levels of NAD+. AMPK phosphorylates the mitochondrial biogenesis factor PGC-1α and augmented NAD+ levels lead to enhanced SIRT1 activity and deacetylation of PGC-1α. Phosphorylation and deacetylation are the necessary and sufficient condition for the activation of PGC-1α. However later studies by Sinclair and colleagues demonstrated a dose dependent target specificity for resveratrol, with SIRT1 being the primary target at low concentrations and PDE at higher concentrations (Price et al., 2012). Further studies are necessary to determine whether additional targets exists for resveratrol and the contribution of SIRT1 activation in in vivo studies using resveratrol. A recent study published from our laboratory demonstrates that salutary effect of resveratrol in prolonging life after severe hemorrhage could be at least partially mimicked by the STAC SRT1720 (Ayub et al., 2015).
Figure 2. SIRT1-AMPK axis in resveratrol mediated metabolic regulation.

Resveratrol has been demonstrated to be an allosteric activator of SIRT1. (Price et al, 2013). Resveratrol may also act through phosphodiesterase (PDE) leading to AMPK activation, increased NAD+ and phosphorylation of Pgc-1α (Park et al, 2012). SIRT1 deacetylates Pgc-1α. Phosphorylated and deacetylated Pgc-1α augments mitochondrial biogenesis and function. The pathway of action of resveratrol has been suggested to be dose-dependent (Price et al, 2013). Among other functions of SIRT1 include deacetylation and inactivation of Nfkb and p53. P= phosphate group, Ac= acetyl group.
In one study it was found that oral administration of resveratrol is associated with 70% absorption, but a bioavailability of 1% (Walle, 2011). It has a half-life of approximately 9 h and its phenol groups are rapidly conjugated in the liver with sulfate and glucuronic acid (Walle et al., 2004). Its metabolites remain in the plasma significantly longer (Pervaiz and Holme, 2009). It is distributed to the liver, kidney, heart, and brain, among others (Andres-Lacueva et al., 2012; Clark et al., 2012; Walle, 2011). Greater than 50% of resveratrol and its metabolites are protein bound in plasma (Burkon and Somoza, 2008; Jannin et al., 2004). It is predominately eliminated by the kidneys (Boocock et al., 2007). In summary, resveratrol is frequently used experimentally to increase sirtuin activity and also has pleotropic downstream effects. Many other SIRT1-activating compounds (STACs) have been synthesized and analyzed (Lavu et al., 2008). Many appear to stimulate SIRT1’s enzymatic activity allosterically (Dai et al., 2010). Clinical trials on diabetes, aging, obesity, cardiovascular disease and neurologic disease are in progress to evaluate the efficacies of resveratrol formulations and STACs such as SRT501, SRT2104, and SRT2379 (Camins et al., 2010).
4.1.1.5 Role of SIRT1 and Resveratrol in Hypoxia, Ischemia, and Reperfusion
Sirtuins play a key role in the cellular response to injury by influencing metabolic pathways, cell death, and repair (Poulose and Raju, 2015). After the publication of mechanistic studies linking SIRT1 and resveratrol to longevity, a large number of laboratories began investigating them in a number of models in health and disease, both in vitro and in vivo. However human and non-human primate studies have lagged behind. SIRT1 and AMPK, irrespective of whether direct or indirect target of resveratrol, are stress dependent metabolic sensors that respectively deacetylate and phosphorylate PGC-1α to affect mitochondrial function and biogenesis (Canto and Auwerx, 2009). After ischemia and reperfusion, studies have noted a reduction in SIRT1 mRNA and protein expression (Hsu et al., 2010). The effect on and modulation of SIRT1 levels with ischemia/reperfusion has been studied in multiple tissues.
4.1.1.5.1 Neurologic
SIRT1’s neuroprotective effects have been demonstrated in multiple studies of ischemic stroke, traumatic brain injury, and other neurodegenerative diseases. A direct role for SIRT1 in ischemic stroke is suggested by the fact that SIRT1 knockout mice have increased infarct size when subjected to middle cerebral artery occlusion (Hernandez-Jimenez et al., 2013; Liu et al., 2013a). SIRT1 activators decrease infarct size (Gao et al., 2006; Huang et al., 2001; Li et al., 2012; Lu et al., 2006; Tsai et al., 2007; Yousuf et al., 2009) while SIRT1 inhibitors had an adverse effect (Hernandez-Jimenez et al., 2013). Administration of the SIRT1 activator resveratrol remarkably enhances glucose and ATP levels and decreases lactate levels in ischemic brain (Li et al., 2011a). It also improves blood brain barrier function via PPAR mediated regulation of MMP-9 and TIMP-1 (Cheng et al., 2009), increases functional outcomes, and lessens brain edema (Yousuf et al., 2009). Studies have also demonstrated increased survival (Wang et al., 2014). The neuroprotective effects of SIRT1 activators appear to be mediated by mitochondrial anti-oxidative, anti-apoptotic, and anti-inflammatory pathways (Shin et al., 2012).
Rodents treated with resveratrol demonstrate decreased mitochondrial swelling, improved mitochondrial membrane potential, and less mitochondria in autophagosomes after cerebral ischemia (Wang et al., 2014). The activity of mitochondrial respiratory complexes I, II and III in the hippocampus of resveratrol treated rodents exposed to middle cerebral artery cerebral ischemia were improved (Yousuf et al., 2009). The mitochondrial/glycolytic master switch in hypoxia, HIF1α, is downregulated in hypoxic glioblastoma cells in a SIRT1 dependent manner, which determines mitochondrial metabolism (Yoon et al., 2014). There is also a marked decrease in mitochondrial cytochrome c release leading to less apoptosis (Yousuf et al., 2009).
Apoptosis associated with ischemic brain injury is associated with multiple pathways influenced by SIRT1. Mechanisms in addition to decreased mitochondrial cytochrome c release include but are not limited to Akt:MAPK:CREB:Bcl-2 (Pugazhenthi et al., 2000), poly(ADP-ribose)polymerase activation and depletion of NAD+ (Endres et al., 1997; Meli et al., 2003), and p53 (Hernandez-Jimenez et al., 2013). Resveratrol increases serine/threonine kinase Akt (a downstream effector of phosphatidylinositol 3-kinase) and mitogen-activated protein kinases (extracellular signal-regulated kinase1/2 [ERK1/2] and p38) phosphorylation, which is known to be important for cellular survival after stroke (Irving and Bamford, 2002). Their antiapoptotic activity is associated with the transcription factor cyclic AMP response element-binding protein (CREB), which modulates the anti-apoptotic gene Bcl-2 (Pugazhenthi et al., 2000). Furthermore, direct (Wang et al., 2008) and indirect (Wang et al., 2011b) NAD+ replacement reduces ischemic brain injury. Indirect NAD+ replacement can be accomplished by nicotinamide phosphoribosyltransferase (NAMPT) administration which increases the NAD+ salvage pathway via a SIRT1-dependent adenosine monophosphate-activated kinase (Wang et al., 2011b). This results in a SIRT1 dependent decrease in apoptosis and increase in autophagy through the TSC2-mTOR-S6K1 signaling pathway (Wang et al., 2012). Additional SIRT1 dependent anti-apoptotic effects during brain ischemia are associated with deacetylation and inhibition of p53 (Hernandez-Jimenez et al., 2013), apoptotic FOXO proteins (Yang et al., 2013), as well as decreased pro-apoptotic protein Bax (Li et al., 2012).
Protective effects of resveratrol in ischemic brain tissue are also due to decreases in oxidative stress. PGC-1α, a master regulator of antioxidants and a SIRT1 target protein (St-Pierre et al., 2006), regulation is a key method by which resveratrol modulates oxidative stress (Zhu et al., 2010). Antioxidant enzymes that are downstream mediators of PGC-1α include SOD1, SOD2 (Wang et al., 2014), GPx1, and catalase (St-Pierre et al., 2006). Resveratrol treatment also significantly decreases oxidants such as xanthine oxidase, malondialdehyde, hypoxanthine, and xanthine (Li et al., 2011a). Other studies have demonstrated ischemic neuroprotection against oxidative stress in rodents by resveratrol associated with decreased hydroxyl radicals (Lu et al., 2006), modulation of nitric oxide (Tsai et al., 2007), increased mitochondrial glutathione (GSH) and glucose 6-phosphate dehydrogenase (G6PD) (Yousuf et al., 2009), and decreased mitochondrial lipid peroxidation (LPO), protein carbonyl and intracellular H2O2 content (Yousuf et al., 2009). In contrast, SIRT1 mediated deacetylation of Nrf2 (nuclear factor erythroid 2-related factor 2) inhibits its transcriptional activity of antioxidant genes (Kawai et al., 2011). Resveratrol attenuated neuronal death, decreased the generation of ROS, and brought antioxidant and Na(+)K(+)-ATPase activity in the cortex and hippocampus back to normal levels (Simao et al., 2011). Anti-inflammatory targets decreased by SIRT1 include NFκB (deacetylates the p65 subunit at Lys310) (Hernandez-Jimenez et al., 2013; Yeung et al., 2004).
Not only does SIRT1 activation have acute benefits during cerebral ischemia, it also has delayed benefits. Resveratrol increases angiogenesis after brain ischemia by increasing matrix metalloproteinase-2 (MMP-2) and vascular endothelial growth factor (VEGF) levels (Dong et al., 2008). It also preserves cerebral blood flow (Hattori et al., 2015) by deacetylating endothelial nitric oxide synthase, leading to increased nitric oxide production and vasodilation (Hattori et al., 2014).
Inhibition of SIRT1 with sirtinol abolishes ischemic preconditioning-induced neuroprotection in the CA1 region of the rat hippocampus, while resveratrol pretreatment mimics ischemic preconditioning’s effects (Raval et al., 2008) (Raval et al., 2006). Both resveratrol and ischemic preconditioning increase brain SIRT1 and reduce uncoupling protein 2 levels (Della-Morte et al., 2009). SIRT1 has also been shown to be instrumental in neuroprotection with ischemia afforded by tetrahydroxystilbene glucoside (Wang et al., 2009), leptin (Avraham et al., 2010), and icariin (Zhu et al., 2010).
Traumatic brain injury is another model in which SIRT1 modulation has shown promise which also involves ischemic processes associated with brain edema limiting blood flow. SIRT1 knockdown is associated with increased traumatic brain injury, whereas SIRT1 overexpression reduces neuronal apoptosis also via the MAPK/ERK pathway (Zhao et al., 2012b). Resveratrol also attenuates early brain injury after subarachnoid hemorrhage through inhibition of NF-kappaB-dependent inflammatory/MMP-9 (Shao et al., 2014). Protection against other neurodegenerative diseases such as Alzheimer’s (Kim et al., 2007a), Parkinson’s (Wu et al., 2011), Huntington’s (Jiang et al., 2012), amyotrophic lateral sclerosis (Kim et al., 2007a), and multiple sclerosis (Tegla et al., 2014) has also been shown with SIRT1.
4.1.1.5.2 Cardiac
In the murine heart, ischemia results in decreased levels of SIRT1, and this decrease is accentuated with aging (Jian et al., 2011a; Lu et al., 2014; Tong et al., 2013). Furthermore, overexpression of SIRT1 in mice results in decreased ischemia reperfusion induced myocardial infarction while decreased expression potentiates the degree of infarction (Hsu et al., 2010). This was associated with 1) an increase of prosurvival molecules such as thioredoxin-1 and Bcl-xL; 2) a decrease of the proapoptotic molecules Bax and caspase-3; 3) decreased oxidative stress with FoxO1 dependent increases in manganese superoxide dismutase. Other studies have demonstrated anti-inflammatory mechanisms of SIRT1 after myocardial ischemia. In mice hearts, SIRT1 decreases the transcriptional activity of the inflammatory cytokine NF-κB through deacetylation of RelA/p65, a subunit of NF-κB (Nadtochiy et al., 2011a). Additionally, the Nampt-SIRT1 pathway stimulates autophagy and prevents cell death during myocardial ischemia (Hsu CP, 2009). Through SIRT1 mediated deacetylation of FoxO1, autophagic proteins such as Rab 7 are upregulated (Hariharan et al., 2010). SIRT1 also deacetylates and activates the autophagy proteins Atg 5, 7, and 8 (Lee et al., 2008). However, as excessive autophagy during reperfusion could worsen injury, the degree and length of ischemia and reperfusion may affect SIRT1 related changes in survival associated with increased autophagy. Fructose feeding, an intervention known to decrease myocardial ischemia/reperfusion injury in the rat heart (Jordan et al., 2003) appears to act via a SIRT1 dependent mechanism (Pillai et al., 2008), and also reduces the switch in myosin isoforms from α to β myosin heavy chain leading to less cardiac hypertrophy (Mercadier et al., 1981). Ischemic preconditioning, another intervention known to decrease cardiac injury during ischemia/reperfusion (Yang et al., 2010), is also SIRT1 dependent (Nadtochiy et al., 2011b). In mice hearts, SIRT1 overexpression resulted in cytosolic lysine deacetylation and was protective against ischemia-reperfusion injury, while SIRT1 deficient hearts had increased cytosolic lysine acetylation and could not be preconditioned. The SIRT1 inhibitor splitomycin inhibited cardioprotection (Nadtochiy et al., 2011b). The cardiac protection associated with ischemic preconditioning decreases with age, and while SIRT1 also decreases with age, one study suggested these factors are independent (Adam et al., 2013). The cardiac protection in long term caloric restriction also is associated with an increase in nuclear but not total SIRT1 (Shinmura et al., 2008). Interestingly, in a later study, the authors suggested that deacetylation of mitochondrial electron transport chain proteins is responsible for preparing mitochondria for ischemic stress, a task performed by mitochondrial sirtuins, not nuclear sirtuins like SIRT1. Activation of SIRT1 has also been shown to be associated with cardioprotection during ischemia afforded by insulin like growth factor 1 (Vinciguerra et al., 2010) and sildenafil (Shalwala et al., 2014) in mice. SIRT1 is also a direct target of miR-199a, a microRNA which is acutely downregulated during cardiac hypoxia resulting in rapid upregulation of its target, HIF1-α. miR-199a induced activation of SIRT1 destabilizes HIF-1α, resulting in its degradation and countering its cellular effects and associated injury. These data suggest that sirtuins play a central role in multiple known mechanisms of cardioprotection.
Administration of the sirtuin activator resveratrol in the setting of hemorrhagic shock restores SIRT1 activity and improves left ventricular function, cardiac contractility, and survival in resuscitated animals and improves lifespan in non-resuscitated animals (Ayub et al., 2015; Jian et al., 2012) A shift in cellular energetics from oxidative phosphorylation in the mitochondria to glycolysis in the cytosol associated with increased PDK1 (an inhibitor of pyruvate dehydrogenase complex) after severe hemorrhage is reversed with administration of resveratrol (Jian et al., 2014). Resveratrol also abolished the increase in c-MYC levels (Yuan et al., 2009), and decreased PGC-1α, NRF2, and mitochondrial complex I activity associated with hemorrhage (Jian et al., 2014). It holds promise to reverse cardiovascular decline in aged animals with ischemia (Jian et al., 2011a). Consequently, resveratrol induced increases in SIRT1 activity after hemorrhage appear to improve outcomes by affecting mitochondrial function (Jian et al., 2014). Similar experiments on the effect of hemorrhage on rat myocytes have demonstrated that resveratrol inhibits apoptotic pathways, Akt (protein kinase B) dependent inflammation, and injury (Tsai et al., 2012). Consequently, SIRT1 may be a potential target to alleviate cardiovascular decline due to mitochondrial dysfunction in aged animals.
Resveratrol attenuates hepatocyte (Powell et al., 2014; Yu et al., 2010b), intestinal (Yu et al., 2011), lung (Wu et al., 2008), kidney(Wang et al., 2015a) and endothelial (Yu et al., 2010a) injury during hemorrhage induced ischemia. The accumulation and activity of the mitochondrial/glycolytic master switch in hypoxia, HIF1-α, is regulated by SIRT1 in hypoxic hepatocellular (Laemmle et al., 2012), kidney, colorectal, gastric cancer, cervical, lung, fibrosarcoma, glioblastoma and prostate cancer cells (Yoon et al., 2014). Modulating SIRT1 or downstream targets of SIRT1 may allow us to protect from ischemia, reperfusion, and hypoxic injury (Yamamoto and Sadoshima, 2011).
4.1.1.6 Other Sirtuins
Although the majority of studies have focused on SIRT1, a limited number of other sirtuins have been investigated in the setting of hypoxic, ischemic, and reperfusion injury and/or aging. SIRT2, is upregulated by anoxia-reoxygenation in H9c2 cells, while downregulation is protective by decreasing 14-3-3-zeta and translocating the Bcl-2-associated death promoter (BAD) from the mitochondria to the cytosol (Lynn et al., 2008). SIRT3 knockdown is known to result in increased infarct size in murine hearts associated with decreased Cx1 and SOD activity and increased oxidative damage (Porter et al., 2014). SIRT3 also appears to be an important contributor to the pathway by which exercise training results in cardioprotection (Jiang et al., 2014). Increased SIRT3 levels, AMPK phosphorylation, and mitochondrial biogenesis were found in post myocardial infarction rats exposed to aerobic interval training, which is known to improve outcomes (Godfrey et al., 2013; Wan et al., 2007). Resveratrol has also been shown to upregulate SIRT3 expression in the mitochondria resulting in decreased oxidative stress in human vascular endothelial cells (Zhou et al., 2014) and improved cardiac function (Chen et al., 2015). The mitochondrial sirtuin SIRT4 is down-regulated in hypoxia in H9c2 cardiomyoblast cells, and SIRT4 overexpression decreases their apoptosis (Liu et al., 2013b). SIRT5 has also been shown to be a determinant of apoptosis in heart and lung cells (Liu et al., 2013c; Wang et al., 2015b). SIRT6 overexpression in male mice increases life span with associated lower levels of insulin like growth factor 1 (Kanfi et al., 2012). A few studies in the context of cerebral ischemia have been performed. Decreased expression of sirtuin 6 in rats is associated with release of high mobility group box-1 after cerebral ischemia, resulting in inflammation (Lee et al., 2013b). In brain endothelial cells deprived of oxygen, sodium sulfide has a SIRT6 dependent neuroprotective effect (Hu et al., 2015).
4.1.1.7 Role in Aging
Sirtuins influence the aging process and are involved in age related changes (Tissenbaum and Guarente, 2001). This was initially demonstrated with Sir2 in saccharomyces cerevisiae (Kaeberlein et al., 1999), but was also shown in Caenorhabditis elegans (Tissenbaum and Guarente, 2001), drosophila (Whitaker et al., 2013) and in rodents (Kanfi et al., 2012; Satoh et al., 2013). Rafael de Cabo’s group recently demonstrated that the SIRT1 activator SRT1720 extends lifespan in adult mice fed a standard diet (Mitchell et al., 2014). They observed delayed onset of age-associated metabolic diseases and improved health in these mice, including inhibition of proinflammatory gene expression in liver and muscle. Sir2 loss of function mutations decrease lifespan in yeast, while increasing Sir2 expression increases lifespan (Kaeberlein et al., 1999) by increasing mitochondrial oxidation and respiration (Lin et al., 2002). Sir2’s role in calorie restriction has been questioned by other studies (Kaeberlein et al., 2004; Kaeberlein et al., 2006; Tsuchiya et al., 2006). In C. elegans, a suggested mechanism for Sir2 effect on lifespan is through its impact on the forkhead transcription factor DAF-16, which is typically associated with reduced IGF-1 signaling (Berdichevsky et al., 2006). In this study, overexpression of the Sir2.1 gene extended lifespan during stress in a 14-3-3 protein dependent manner by increasing DAF-16 activation. Increased autophagy has also been suggested to play a primary role as the life span extending effects of SIRT1 activators are lost in autophagy-deficient C. elegans (Morselli et al., 2010). Aged human arteries have decreased SIRT1 expression and activity possibly via cyclin-dependent kinase 5 phosphorylation at the serine 47 residue, suggesting that SIRT1 activators or CDK-5 inhibitors may halt vascular aging (Bai et al., 2014). In the heart, carbonyl modification of SIRT1 is noted to contribute to aging related ischemic intolerance (Gu et al., 2013). SIRT6 in mice affects lifespan by altered phosphorylation levels of major components of IGF1 signaling (Kanfi et al., 2012). Sirtuin activators such as resveratrol have been shown to mimic caloric restriction induced longevity in yeast (Howitz et al., 2003) and animals (Wood et al., 2004). In obese mice on a high calorie diet, resveratrol significantly increased survival and healthspan via increased insulin sensitivity, reduced insulin-like growth factor-1 (IGF-I) levels, increased AMP-activated protein kinase (AMPK), increased peroxisome proliferator-activated receptor-gamma coactivator 1 alpha activity, and increased mitochondrial number (Baur et al., 2006). However, non-obese/standard diet mice do not have increased lifespan but do have improved markers of aging and increased health when treated with resveratrol (Miller et al., 2011; Pearson et al., 2008; Strong et al., 2013). Consequently, given its multiple mechanisms of influencing mitochondrial function in aging and injury, sirtuin activators are a promising way to improve outcomes in elderly individuals sustaining hypoxia or ischemia.
4.1.2 MitoQuinone
MitoQuinone (MitoQ) is a ubiquinone (coenzyme Q10 of the respiratory chain) derivative with a covalent attachment to a lipophilic triphenylphosphonium cation (TPP) through a 10 carbon alkyl chain. Due to significant mitochondrial membrane potential (negative inside), the cationic part of the molecule is directed into mitochondria, while the lipophilic part targets it to the mitochondrial lipid bilayer (Smith et al., 2003). Ubiquinol reduces oxygen radicals, specifically superoxide, and prevents oxidative damage. The respiratory chain then regenerates ubiquinol so it can reduce more radicals. It also has been noted to prevent caspase-independent cell death and prevent lipid peroxidation (McManus et al., 2014). MitoQ accumulates in metabolically active tissues such as the heart, liver, brain, and skeletal muscle (Smith et al., 2003). Fibroblasts pretreated with MitoQ prior to oxidative stress have significantly attenuated free radical production and increased lifespan (Saretzki et al., 2003). They also demonstrated a reversal of telomere shortening. MitoQ prevents hydrogen peroxide-induced apoptosis in mammalian cell culture studies (Kelso et al., 2001) and pretreatment modulates oxidative injury induced by dichlorvos (Wani et al., 2011). Dichlorvos is a synthetic organophosphate insecticide that inhibits neural acetylcholinesterase, mitochondrial complex I, and cytochrome oxidase, resulting in generation of reactive oxygen species. In the study, MitoQ ameliorated dichlorvos induced oxidative stress by decreasing ROS production, increasing MnSOD activity, and increasing glutathione levels. There was also decreased lipid peroxidation, protein oxidation, DNA oxidation, DNA fragmentation, cytochrome c release, and caspase-3 activity. Electron microscopy revealed that MitoQ attenuated dichlorvos induced mitochondrial swelling, loss of cristae, and chromatin condensation. (Wani et al., 2011). Each of these experiments demonstrates good results in the setting of oxidative stress (not induced by ischemia) and pretreatment. Few studies have been performed evaluating it in the context of ischemia, and even fewer with post injury treatment.
One study evaluated MitoQ in the context of obesity and chronic myocardial ischemia via intermittent left anterior descending artery occlusion. Chronic administration increased obese animals’ collateral blood flow and decreased ROS to levels comparable to lean animals (Pung et al., 2012). Another study in a model of cardiac ischemia-reperfusion injury found that feeding MitoQ to rats significantly decreased heart dysfunction, cytochrome c release, caspase 3 upregulation leading to cell death, and mitochondrial damage (Adlam et al., 2005). MitoQ has also been shown to be effective for ischemia/reperfusion injury in the liver (Mukhopadhyay et al., 2012). When animals were pretreated or treated at the time of ischemia, MitoQ dose-dependently attenuated I/R-induced liver dysfunction and damage (manifested by decreased AST, ALT, and histologic changes), but not when given after ischemia and reperfusion. Pretreated animals had significantly improved complex I activity, but unchanged complex II and IV activity. There was less oxidative and nitrative stress response (HNE/carbonyl adducts, malondialdehyde, 8-OHdG, and 3-nitrotyrosine formation), as well as delayed inflammatory cell infiltration, DNA fragmentation, caspase3 activity, and cell death (Mukhopadhyay et al., 2012). MitoQ appears to have significant positive effects for pretreatment of ischemia/reperfusion.
As opposed to pretreatment, one recent study evaluated MitoQ used after ischemic liver injury (Powell et al., 2015). Powell and coworkers used a model of hemorrhagic shock and reperfusion in rats. Arterial blood was withdrawn to a mean arterial pressure of 25 +/− 2 mm Hg for 1 hour before resuscitation and MitoQ was administered intravenously after 30 minutes of ischemia and intraperitoneally after resuscitation. Some positive effects were demonstrated, but results were varied. It decreased markers of inflammation (IL-6 and TNFα) and reduced morbidity. It also increased glutathione activity, but decreased catalase activity, increased liver peroxidation, and did not change superoxide dismutase activity or the degree of liver necrosis. (Powell et al., 2015). MitoQ appears to have varied, but overall beneficial effects when given after hemorrhage.
While no studies of MitoQ’s effect on ischemia in the context of aging have been published, MitoQ appears to be able to reverse some age related changes. It has been studied in a model evaluating arterial endothelial function with age (Gioscia-Ryan et al., 2014). In this experiment, acute and chronic administration of MitoQ to aged mice restored ex vivo carotid artery endothelium-dependent dilation by improving nitric oxide bioavailability. There were increases in antioxidants with normalization of age-related increases in superoxide production and oxidative stress. When arterial cells were treated with rotenone, a compound that inhibits the transfer of electrons from iron-sulfur centers in complex I to ubiquinone, MitoQ reversed the age-related increase in endothelial dysfunction (Gioscia-Ryan et al., 2014). Consequently, via a mitochondria dependent mechanism, it may be able to improve blood flow during ischemia, thereby lessening injury in aged individuals, but has not yet been investigated in this manner. One concern about MitoQ is that at higher concentrations. it may act as a prooxidant (Antonenko et al., 2008). Studies have been reported using other antioxidants such as vitamin E. In a recently published study using mitochondrial targeted vitamin E (Mito-Vit E) and non-targeted vitamin E, Mito-Vit-E exhibited significant efficacy to reduce cytokine and cardiac inflammation in a sepsis model (Zang et al., 2012). Future preclinical and clinical testing of mitochondrial antioxidants may have potential translational significance (Zang et al., 2014).
4.1.3 Estrogens
Rates of ischemic events in women increase after the age of menopause (Rosamond et al., 2007) which raises the question whether increased incidence is due to aging or to hormone status (Cui et al., 2013). At the molecular and cellular level, estrogens appear to decrease mitochondrial-dependent apoptosis (Liou et al., 2010). This study noted that ovariectomied animals that receive 17β-estradiol have decreased ovariectomy-induced cell death. They found that increases in t-Bid, Bax, Bax-to-Bcl2 ratio, Bax-to-BclXL ratio, activated caspase 9, activated caspase 3, anti-apoptotic Bcl2 and Bcl-XL with ovariectomy were countered by estrogen administration (Liou et al., 2010). Mechanistic studies have shown that estrogen receptor beta (ERbeta) is imported into the mitochondria where it affects gene transcription through the interaction with estrogen response elements (ERE) and transcription factors (Simpkins et al., 2008). Conversely, human studies have investigated the influence of estrogen deficiency and/or supplementation on ischemic processes, and there have been conflicting results over the years. Some studies have shown no convincing relationships between postmenopausal status and cardiovascular disease (Atsma et al., 2006), while others have shown a benefit of hormone replacement (Barrett-Connor and Grady, 1998). Another meta-analysis actually showed that hormone replacement therapy increased the risk of coronary artery disease, stroke, and other thromboembolic processes (Nelson et al., 2002). The Women’s Health Initiative among 16,608 women found that estrogen plus progestin increased ischemic stroke risk by 44% (Wassertheil-Smoller et al., 2003). After an initial stroke, estrogen alone increases the rate of recurrent fatal stroke and causes an early rise in overall stroke rate in the first 6 months (Viscoli et al., 2001). Another hormone replacement study showed that treated patients had increased coronary events, strokes, and pulmonary emboli. Consequently, while animal and mechanistic studies have been promising, human trials have demonstrated adverse effects (Prentice, 2014).
4.1.4 Calpain inhibitors
Calpains are calcium-dependent, non-lysosomal cysteine proteases whose activation increases ischemia/reperfusion injury (Yousefi et al., 2006). A review (Neuhof and Neuhof, 2014) recently demonstrated that calpain inhibition in animal experiments showed an enhanced tolerance towards ischemia (Neuhof et al., 2003), a reduction of myocardial infarction and reperfusion injury (Hernando et al., 2010; Khalil et al., 2005; Neuhof et al., 2008; Neuhof et al., 2004), and less remodeling (Ma et al., 2012). Other experiments have also shown neuronal preservation during ischemia with calpain inhibition (Nath et al., 2000). Calpain I and 10 are localized in mitochondria. Calpain activation results in cleavage of several mitochondrial proteins including complex I subunits of the electron transport chain (Arrington et al., 2006) and calpain inhibitors restore respiration during ischemia/reperfusion (Neuhof et al., 2003). Calpain inhibitors also prevent mitochondrial membrane permeability transition (Neuhof et al., 2003), attenuate calpain induced cleavage of the pro-apoptotic Bcl-2 family member Bid to an active state (Chen et al., 2001), and prevent calpain from cleaving and releasing apoptosis inducing factor (AIF) from mitochondria (Chen et al., 2011). It also appears to affect autophagy with aging. When old hepatic cells were pretreated with a calpain inhibitor, there was less Atg4B (autophagy related protein) loss, increased autophagy and mitophagy, and decreased cell death (Wang et al., 2011a). The authors suggested that more mitophagy results in timely clearance of injured or injury prone mitochondria leading to better outcomes with ischemia/reperfusion in aged individuals (Wang et al., 2011a). Given that calpain has multiple targets for cleavage that have many different functions, inhibition of specific downstream targets affecting ischemia/reperfusion tolerance could help prevent the increased injury associated with aging.
4.1.5 Melatonin
Melatonin, the chief secretory product of the pineal gland that functions as a regulator of sleep, circadian rhythm, and immune function, is also a direct free radical scavenger and indirect antioxidant (Poeggeler et al., 1994). It been shown to reduce oxidative stress by quenching the hydroxyl radical (Li et al., 1997), superoxide anion radical, singlet oxygen, peroxyl radical, and the peroxynitrite anion (Mayo et al., 2005). It stimulates superoxide dismutase, glutathione peroxidase, glutathione reductase, and glucose-6-phosphate dehydrogenase and inhibits nitric oxide synthase. It also stabilizes cell membranes (Rodriguez et al., 2004), modulates the gene expression of protective enzymes that reduce apoptosis such as Bcl2 (Ling et al., 1999), inhibits autophagy (Zheng et al., 2014), and decreases lipid peroxidation (Wakatsuki et al., 1999), and prevents mitochondrial inhibition thereby promoting ATP production (Reiter et al., 1998). Melatonin deficient rats have been shown to have increased brain damage after stroke (Manev et al., 1996), while administration of melatonin reduces neuronal injury (Cho et al., 1997) and increases survival (Cuzzocrea et al., 2000a). In neonatal hypoxia/ischemia, melatonin preserves sirtuin 1 expression thereby resulting in neuroprotection. Preadministration of melatonin attenuated renal ischemia/reperfusion injury in rats (Ahmadiasl et al., 2014) and decreases hepatic ischemia/reperfusion injury (Kireev et al., 2012). Melatonin is noted to decrease with age which may be a factor in increased oxidative damage in the elderly (Reiter et al., 1998).
4.1.6 Hydrogen Sulfide (H2S)
Hydrogen sulfide (H2S) is historically known as a toxic environmental pollutant with various deleterious effects on organ systems (Beauchamp et al., 1984). However, H2S along with nitric oxide (NO) and carbon monoxide (CO) is increasingly recognized as an endogenously produced gaseous signaling molecule (Lowicka and Beltowski, 2007). It is now recognized as an antioxidant that modulates glutathione levels (Kimura and Kimura, 2004; Yang et al., 2011) and can antagonize apoptosis in the setting of ischemia and reperfusion (Biermann et al., 2011; Elrod et al., 2007). Endogenous H2S levels are markedly reduced by hypoxia, and over-expression of cystathionine-beta-synthase (CBS), the main producer of hydrogen sulfide in the brain, as well as administration of Na2S prevents hypoxia induced cell apoptosis via the K(ATP)/PKC/ERK1/2/Hsp90 pathway (Tay et al., 2010). In oxygen and cortical neuron glucose deprivation/reperfusion experiments, H2S reduces intracellular ROS, caspase 3 activation, damage of mitochondrial membrane potential, leading to decreased apoptosis (Luo et al., 2013). It has also been shown to be effective in cardiac H9c2 cells exposed to chemical hypoxia by inhibiting ROS-mediated activation of ERK1/2 (Dong et al., 2012). Another study found that aged healthy mice have high levels of CBS and suggested that maintenance (and increase) of CBS levels may sustain cortical function, preclude neuronal loss, and extend survival (Bruintjes et al., 2014). Other studies have corroborated their suggestions, finding that H2S prolonged survival in C. elegans (Qabazard et al., 2013; Qabazard et al., 2014). Consequently, modulation of H2S may be a viable strategy to maintain mitochondrial function and improve outcomes in aging individuals exposed to hypoxic ischemic injury.
Ginkgo biloba extract is a free radical scavenger that may counter the increased oxidative stress with aging.
4.1.7 Methylene Blue
Methylene blue (MB) is a heterocyclic aromatic compound that is FDA-approved for methemoglobinemia and an antidote to cyanide poisoning. MB is known to improve mitochondrial function (Atamna et al., 2008; Tokumitsu and Ui, 1973) by increasing the utilization of oxygen and increasing complex IV activity. It also is an alternative electron carrier that shuttles electrons between NADH and cytochrome c (cyt c) so that electrons are rerouted with complex I and III inhibition thereby attenuating ROS overproduction. Its neuroprotective effects have also been attributed to mitophagy (Di et al., 2015), stimulation of HIF-1α (Ryou et al., 2015), and improved cerebral metabolic and hemodynamic function (Lin et al., 2012). MB significantly decreases infarct volume after middle cerebral artery occlusion in animal models (Di et al., 2015; Shen et al., 2013). Given its current use in clinical settings and known side effects, it may easily be a promising translational treatment for the mitochondria dysfunction seen with aging in ischemia (Wen et al., 2011).
4.2 Other Antioxidants
4.2.1 Tempol
Tempol (4-hydroxy-2,2,6,6-tetramethylpiperidine-1-oxyl) is a nitroxide compound that has been extensively studied as an antioxidant as it functions as a superoxide dismutase mimetic (Wilcox, 2010). Pretreatment with tempol has been shown to attenuate ischemia/reperfusion damage in the kidney (Chatterjee et al., 2000; Patel et al., 2002), heart (McDonald et al., 1999), liver (Sepodes et al., 2004) and brain (Cuzzocrea et al., 2000b). It has also demonstrated protection against shock from hemorrhage (Mota-Filipe et al., 1999). Additionally, it appears to be effective when given intravenously at the time of reperfusion as it reduced lipid peroxidation and the infract size in the brain. However, if given 15 minutes before reperfusion, it was not effective (Kato et al., 2003).
Tempol appears to specifically target mitochondrial reactive oxygen species. When administered orally in an experiment with cardiac cells, tempol prevented O2− production by mitochondrial complex 1 when tumor necrosis factor alpha was administered (Mariappan et al., 2009). It has also been shown to prevent angiotensin II from stimulating mitochondrial ROS (Kimura et al., 2005). Tempol attenuates ROS and prevents hepatic mitochondrial damage and dysfunction associated with increased angiotensin II production in renin-2 transgenic rats (Wei et al., 2009). However, it did not preserve mitochondrial function in a model of spinal cord injury where Ca2+ overload was the major factor contributing to mitochondrial dysfunction rather than ROS (Patel et al., 2009).
Efforts have been made to target tempol to mitochondria by complexing it with ubiquinone and gramicidin S with generally good results although one study showed no difference (Dessolin et al., 2002). Studies have shown increased mitochondrial uptake, less activation of mitochondrial uncoupling proteins (Murphy et al., 2003), less complex I dysfunction (Esplugues et al., 2006), decreased H2O2-induced apoptosis (Kelso et al., 2001), and less ischemia–reperfusion injury (Esplugues et al., 2006) when tempol is conjugated with ubiquinone. Conjugation with gramicidin S also enhances tempol concentration in mitochondria and decreases apoptosis (Jiang et al., 2007; Wipf et al., 2005).
Tempol has been also been effective in reversing changes associated with aging. Tempol increases the lifespan of Drosophila melanogaster (Izmaylov and Obukhova, 1996), and mice fed tempol from birth live significantly longer than controls (Mitchell et al., 2003). By lowering oxidative stress, tempol has been shown to normalize the age-related decline in dopamine receptor function (Fardoun et al., 2006). Additionally, the altered regulation of vascular tone with aging is countered by tempol, as it has been shown to restore the decline in nitric oxide (NO) induced inhibition of cardiac O2 usage (Adler et al., 2003) and restore the decreased arteriolar response to endothelial derived relaxing factor (EDRF) seen with aging (Durrant et al., 2009; Lesniewski et al., 2009; Mayhan et al., 2008; Payne et al., 2003). It appears to function in aged animals by substituting for superoxide dismutase, an antioxidant whose expression is decreased with aging (Lund et al., 2009). Therefore, tempol likely has a role in preventing the increased injury seen with aging during hypoxic ischemic injury (Wilcox, 2010), but its therapeutic value, especially in human studies, remains unclear.
4.2.2 Schisandrin B
Schisandra fruits have been traditionally used in East Asia for numerous diseases, and Schisandrin B, a dibenzocyclooctadiene derivative from Schisandra fruit extract, has been shown to be protective during ischemia to the brain (Lee et al., 2012), liver, and heart (Chiu et al., 2006). During focal cerebral ischemia in rats Schisandrin B administered before and after ischemia reduced infarct volumes up to 53.4% with reduced expression of TNF-α and IL-1β, and degradation of MMP-2 and MMP-9 (Lee et al., 2012). Another study noted that Schisandrin B improved glutathione, alpha-tocopherol and Mn-superoxide dismutase levels, while preserving mitochondrial structure and function (Ca2+ loading, cytochrome c release, and permeability transition) (Chen et al., 2008). Improvement in antioxidant status is noted with chronic administration (Chiu et al., 2006). Following brain anoxia in mice another study noted decreased swelling and disintegration of brain mitochondria with improved membrane fluidity and increased production of cytosol glutathione-peroxidase (Xue et al., 1992). Protection has been demonstrated in both young and aged animals (Chiu et al., 2006). Therefore, by improving cerebral mitochondrial antioxidant status, aged individuals may be protected against ischemia/reperfusion injury with administration of Schisandrin B, although human studies are lacking.
4.2.3 Uric Acid
Uric acid is a well-known antioxidant produced through the metabolic breakdown of purine nucleotides such as ATP. Uric acid protects cultured rat hippocampal neurons against cell death through suppression of oxygen and nitrogen radical accumulation (hydrogen peroxide and peroxynitrite), stabilization of calcium homeostasis, preservation of mitochondrial function, and by decreasing exposure to the excitatory amino acid glutamate and the metabolic poison cyanide (Yu et al., 1998). It is neuroprotective in rats one hour following reperfusion in cerebral ischemia and improves behavioral outcome (Yu et al., 1998). Uric acid has low solubility in water resulting in pathogenic processes such as gout and kidney stones. Consequently, soluble analogs have also been synthesized, studied, and shown to be neuroprotective in ischemic brain injury in mice (Haberman et al., 2007). Dietary restriction, an intervention known to increase ischemic tolerance, has also been shown to increase uric acid levels thereby augmenting the anti-oxidative defense system with aging (Chung and Yu, 2000).
4.2.4 Alpha-phenyl-tert-butyl-nitrone (PBN)
PBN is a free-radical spin trap that also inhibits cyclooxygenase-2 activity and nitric oxide synthase (iNOS) (Durand et al., 2008). In aging rat brain following cardiac arrest and resuscitation, the antioxidant alpha-phenyl-tert-butyl-nitrone (PBN) improves mitochondrial respiratory function and reduces lipid peroxidation (Xu et al., 2008). A follow up study in also in aged rats demonstrated improved overall survival and increased hippocampal CA1 neuron survival with PBN treatment (Xu et al., 2010).
4.2.5 Mannosylated Liposomal Cytidine 5′ Diphosphocholine
Cytidine 5′ diphosphocholine (CDP-Choline), an intermediate in the generation of phosphatidylcholine from choline, has some studies in human trials illustrating its effectiveness in stroke (Warach et al., 2000). Some studies have even reported substantially reduced frequencies of death and disability (Saver, 2008) although a large randomized placebo controlled study failed to show any difference (Davalos et al., 2012). One challenge is drug delivery, as CDP-choline is frequently broken down after administration and must reform. An alternative strategy involves conjugating the drug to mannosylated liposomes. Liposomes are known to be an effective drug carrier especially during ischemia and reperfusion (Fresta and Puglisi, 1997) and mannose receptors expressed on brain cells facilitate endocytosis (Regnier-Vigouroux, 2003). CDP-Choline in a mannosylated liposome delivery system exerts increased protection against global cerebral ischemia and reperfusion induced mitochondrial damage in young and aged rats (Ghosh et al., 2010). Treatment decreased membrane lipid peroxidation, reactive oxygen species, and mitochondrial cytochrome c release, while increasing superoxide dismutase and catalase levels.
4.2.6 Luteolin
Luteolin is a polyphenol compound found in many fruits such as Perilla frutescens, vegetables, and herbs (Zhao et al., 2010) that possesses anti-inflammatory, antiallergic, anticarcinogenic, and immune-modulating properties (Zhao et al., 2012a). It scavenges superoxide radicals using its polyphenolic hydroxyl groups and has been shown to reduce inhibition of mitochondrial function, decrease reactive oxygen species, and increase catalase and GSH levels in primary neurons treated with H2O2 (Zhao et al., 2012a). Liposome-encapsulated luteolin administered after ischemia also prevents ischemia-reperfusion injury with middle cerebral artery occlusion model in rats. Treated animals demonstrated decreased symptom scores, improved balance, and behavioral improvement (Zhao et al., 2011).
4.2.7 Selenium Compounds
Diphenyl diselenide (PhSe)2 is a potent antioxidant that exhibits glutathione peroxidase-like activity and decomposes H2O2 or other radicals. (PhSe)2 administered 30 minutes prior to bilateral common carotid artery occlusion in rats improved cerebral levels of malondialdehyde (MDA), ROS, nitrate/nitrite and antioxidant defenses (catalase and superoxide dismutase), proinflammatory cytokines, tissue damage markers (creatine kinase and α-1-acid glycoprotein), and histological outcomes compared to untreated rats exposed to I/R (Bruning et al., 2012). Another study in which animals were treated one hour after ischemia noted improved neurological testing, decreased collapse of mitochondrial membrane potential, improved mitochondrial complex I (NADH dehydrogenase) activity, decreased mitochondrial swelling, improved GSH/GSSG levels, improved MnSOD activity, improved glutathione peroxidase activity, and decreased Hsp70 gene expression (Dobrachinski et al., 2014). While selenium compounds effect on hypoxia in aged animals is not known, studies have shown that selenium compound supplementation in conjunction with swimming improves pro and anti-inflammatory cytokine profiles, memory and spatial learning in aged rats by increasing phosphorylated cAMP-response element-binding protein (CREB) levels (Cechella et al., 2014; Leite et al., 2015).
5 Conclusions
Mitochondria play critical functions in the response to hypoxia, ischemia and reperfusion. Aging results in increased apoptosis, dysregulated autophagy, increased disruption of the mitochondrial permeability transition pore, and exacerbated injury following hypoxic-ischemic insults. Aging aggravates mitochondrial functional decline following hypoxic-ischemic injury and worsens clinical outcomes. The decreased ATP production, increased ROS and mitochondrial-glycolytic shift in cellular energetics are controlled by master regulators such as HIF-1α, PGC-1α, and c-MYC. Age associated decline in NAD+ contributes to substrate starvation leading to decreased SIRT1 activity and a pseudohypoxic state. Preventative and therapeutic pharmacologic agents such as STACs and resveratrol, tempol, mitoquinone, cationic plastoquinone derivatives, estrogens, as well as apoptosis inhibitors, have been shown to be effective in experimental models of neuronal ischemia. Mitochondrial transfer to cells with damaged mitochondria from donor cells via nanotubular tunnels is an emerging area that require further studies to establish functional relevance in vivo. Translational research is needed for most of these potential therapies to test the feasibility to transition from bench to bedside. Nevertheless, investigations in aging and injury converge on biological mechanisms vital to bioenergetics underlining the significance of mitochondrial functional modulation not only on the effects of ischemia on the nervous system, but also in other organ systems and pathways that determine health and disease.
Highlights.
Nuclear-mitochondrial cross talk is important in maintaining mitochondrial structure and function.
Metabolic master regulators, c-MYC and HIF-1, modulate mitochondrial function and cell fate following hypoxic/ischemic injury.
Aging exacerbates hypoxic/ischemic injury and mitochondrial functional impairment.
AMPK-SIRT1 mediated energy sensing is important in energy homeostasis following hypoxic/ischemic injury.
Post translational regulation of molecular mediators play a critical role in the control of glycolytic-mitochondrial energy axis in response to hypoxic/ischemic conditions.
Acknowledgments
The corresponding author (RR) acknowledges financial support from the National Institute of General Medical Sciences (R01 GM 101927), National Institutes of Health, Bethesda, MD.
Abbreviations (alphabetized list)
- mtDNA
mitochondrial DNA
- PPAR
peroxisome proliferator-activated receptor
- PGC
peroxisome proliferator-activated receptor gamma, coactivator
- TBI
traumatic brain injury
- TFAM
transcription factor A mitochondrial
- ROS
reactive oxygen species
- ERR
estrogen receptor related receptor
- NRF
nuclear respiratory factor
- SRT1
sirtuin 1
- Drp1
Dynamin-related protein 1
- Fis1
Fission 1
- NMN
nicotinamide mononucleotide
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdelmohsen K, Pullmann R, Jr, Lal A, Kim HH, Galban S, Yang X, Blethrow JD, Walker M, Shubert J, Gillespie DA, Furneaux H, Gorospe M. Phosphorylation of HuR by Chk2 regulates SIRT1 expression. Mol Cell. 2007;25:543–557. doi: 10.1016/j.molcel.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adam T, Sharp S, Opie LH, Lecour S. Loss of cardioprotection with ischemic preconditioning in aging hearts: role of sirtuin 1? J Cardiovasc Pharmacol Ther. 2013;18:46–53. doi: 10.1177/1074248412458723. [DOI] [PubMed] [Google Scholar]
- Adlam VJ, Harrison JC, Porteous CM, James AM, Smith RA, Murphy MP, Sammut IA. Targeting an antioxidant to mitochondria decreases cardiac ischemia-reperfusion injury. FASEB J. 2005;19:1088–1095. doi: 10.1096/fj.05-3718com. [DOI] [PubMed] [Google Scholar]
- Adler A, Messina E, Sherman B, Wang Z, Huang H, Linke A, Hintze TH. NAD(P)H oxidase-generated superoxide anion accounts for reduced control of myocardial O2 consumption by NO in old Fischer 344 rats. Am J Physiol Heart Circ Physiol. 2003;285:H1015–1022. doi: 10.1152/ajpheart.01047.2002. [DOI] [PubMed] [Google Scholar]
- Ahmad T, Mukherjee S, Pattnaik B, Kumar M, Singh S, Kumar M, Rehman R, Tiwari BK, Jha KA, Barhanpurkar AP, Wani MR, Roy SS, Mabalirajan U, Ghosh B, Agrawal A. Miro1 regulates intercellular mitochondrial transport & enhances mesenchymal stem cell rescue efficacy. EMBO J. 2014;33:994–1010. doi: 10.1002/embj.201386030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmadiasl N, Banaei S, Alihemmati A, Baradaran B, Azimian E. The anti-inflammatory effect of erythropoietin and melatonin on renal ischemia reperfusion injury in male rats. Adv Pharm Bull. 2014;4:49–54. doi: 10.5681/apb.2014.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberts B. Molecular biology of the cell. Garland Science; New York: 2008. [Google Scholar]
- Alcain FJ, Villalba JM. Sirtuin activators. Expert Opin Ther Pat. 2009;19:403–414. doi: 10.1517/13543770902762893. [DOI] [PubMed] [Google Scholar]
- Alcendor RR, Kirshenbaum LA, Imai S, Vatner SF, Sadoshima J. Silent information regulator 2alpha, a longevity factor and class III histone deacetylase, is an essential endogenous apoptosis inhibitor in cardiac myocytes. Circ Res. 2004;95:971–980. doi: 10.1161/01.RES.0000147557.75257.ff. [DOI] [PubMed] [Google Scholar]
- Aliev G, Smith MA, Seyidov D, Neal ML, Lamb BT, Nunomura A, Gasimov EK, Vinters HV, Perry G, LaManna JC, Friedland RP. The role of oxidative stress in the pathophysiology of cerebrovascular lesions in Alzheimer’s disease. Brain Pathol. 2002;12:21–35. doi: 10.1111/j.1750-3639.2002.tb00419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson R, Prolla T. PGC-1alpha in aging and anti-aging interventions. Biochim Biophys Acta. 2009;1790:1059–1066. doi: 10.1016/j.bbagen.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andres-Lacueva C, Macarulla MT, Rotches-Ribalta M, Boto-Ordonez M, Urpi-Sarda M, Rodriguez VM, Portillo MP. Distribution of resveratrol metabolites in liver, adipose tissue, and skeletal muscle in rats fed different doses of this polyphenol. J Agric Food Chem. 2012;60:4833–4840. doi: 10.1021/jf3001108. [DOI] [PubMed] [Google Scholar]
- Antonenko YN, Avetisyan AV, Bakeeva LE, Chernyak BV, Chertkov VA, Domnina LV, Ivanova OY, Izyumov DS, Khailova LS, Klishin SS, Korshunova GA, Lyamzaev KG, Muntyan MS, Nepryakhina OK, Pashkovskaya AA, Pletjushkina OY, Pustovidko AV, Roginsky VA, Rokitskaya TI, Ruuge EK, Saprunova VB, Severina II, Simonyan RA, Skulachev IV, Skulachev MV, Sumbatyan NV, Sviryaeva IV, Tashlitsky VN, Vassiliev JM, Vyssokikh MY, Yaguzhinsky LS, Zamyatnin AA, Jr, Skulachev VP. Mitochondria-targeted plastoquinone derivatives as tools to interrupt execution of the aging program. 1. Cationic plastoquinone derivatives: synthesis and in vitro studies. Biochemistry (Mosc) 2008;73:1273–1287. doi: 10.1134/s0006297908120018. [DOI] [PubMed] [Google Scholar]
- Aon MA, Cortassa S, O’Rourke B. Percolation and criticality in a mitochondrial network. Proc Natl Acad Sci U S A. 2004;101:4447–4452. doi: 10.1073/pnas.0307156101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrington DD, Van Vleet TR, Schnellmann RG. Calpain 10: a mitochondrial calpain and its role in calcium-induced mitochondrial dysfunction. Am J Physiol Cell Physiol. 2006;291:C1159–1171. doi: 10.1152/ajpcell.00207.2006. [DOI] [PubMed] [Google Scholar]
- Atamna H, Nguyen A, Schultz C, Boyle K, Newberry J, Kato H, Ames BN. Methylene blue delays cellular senescence and enhances key mitochondrial biochemical pathways. FASEB J. 2008;22:703–712. doi: 10.1096/fj.07-9610com. [DOI] [PubMed] [Google Scholar]
- Atsma F, Bartelink ML, Grobbee DE, van der Schouw YT. Postmenopausal status and early menopause as independent risk factors for cardiovascular disease: a meta-analysis. Menopause. 2006;13:265–279. doi: 10.1097/01.gme.0000218683.97338.ea. [DOI] [PubMed] [Google Scholar]
- Avraham Y, Davidi N, Porat M, Chernoguz D, Magen I, Vorobeiv L, Berry EM, Leker RR. Leptin reduces infarct size in association with enhanced expression of CB2, TRPV1, SIRT-1 and leptin receptor. Curr Neurovasc Res. 2010;7:136–143. doi: 10.2174/156720210791184943. [DOI] [PubMed] [Google Scholar]
- Ayub A, Poulose N, Raju R. Resveratrol Improves Survival and Prolongs Life Following Hemorrhagic Shock. Mol Med. 2015;21:305–312. doi: 10.2119/molmed.2015.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baarine M, Thandapilly SJ, Louis XL, Mazue F, Yu L, Delmas D, Netticadan T, Lizard G, Latruffe N. Pro-apoptotic versus anti-apoptotic properties of dietary resveratrol on tumoral and normal cardiac cells. Genes Nutr. 2011;6:161–169. doi: 10.1007/s12263-011-0232-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai B, Vanhoutte PM, Wang Y. Loss-of-SIRT1 function during vascular ageing: hyperphosphorylation mediated by cyclin-dependent kinase 5. Trends Cardiovasc Med. 2014;24:81–84. doi: 10.1016/j.tcm.2013.07.001. [DOI] [PubMed] [Google Scholar]
- Ballinger SW. Mitochondrial dysfunction in cardiovascular disease. Free Radic Biol Med. 2005;38:1278–1295. doi: 10.1016/j.freeradbiomed.2005.02.014. [DOI] [PubMed] [Google Scholar]
- Barrett-Connor E, Grady D. Hormone replacement therapy, heart disease, and other considerations. Annu Rev Public Health. 1998;19:55–72. doi: 10.1146/annurev.publhealth.19.1.55. [DOI] [PubMed] [Google Scholar]
- Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R, Sinclair DA. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bavaresco L. Role of viticultural factors on stilbene concentrations of grapes and wine. Drugs Exp Clin Res. 2003;29:181–187. [PubMed] [Google Scholar]
- Beauchamp RO, Jr, Bus JS, Popp JA, Boreiko CJ, Andjelkovich DA. A critical review of the literature on hydrogen sulfide toxicity. Crit Rev Toxicol. 1984;13:25–97. doi: 10.3109/10408448409029321. [DOI] [PubMed] [Google Scholar]
- Berdichevsky A, Viswanathan M, Horvitz HR, Guarente L. C. elegans SIR-2.1 interacts with 14-3-3 proteins to activate DAF-16 and extend life span. Cell. 2006;125:1165–1177. doi: 10.1016/j.cell.2006.04.036. [DOI] [PubMed] [Google Scholar]
- Bhat AH, Dar KB, Anees S, Zargar MA, Masood A, Sofi MA, Ganie SA. Oxidative stress, mitochondrial dysfunction and neurodegenerative diseases; a mechanistic insight. Biomed Pharmacother. 2015;74:101–110. doi: 10.1016/j.biopha.2015.07.025. [DOI] [PubMed] [Google Scholar]
- Biala AK, Dhingra R, Kirshenbaum LA. Mitochondrial dynamics: Orchestrating the journey to advanced age. J Mol Cell Cardiol. 2015;83:37–43. doi: 10.1016/j.yjmcc.2015.04.015. [DOI] [PubMed] [Google Scholar]
- Biermann J, Lagreze WA, Schallner N, Schwer CI, Goebel U. Inhalative preconditioning with hydrogen sulfide attenuated apoptosis after retinal ischemia/reperfusion injury. Mol Vis. 2011;17:1275–1286. [PMC free article] [PubMed] [Google Scholar]
- Bonanni L, Chachar M, Jover-Mengual T, Li H, Jones A, Yokota H, Ofengeim D, Flannery RJ, Miyawaki T, Cho CH, Polster BM, Pypaert M, Hardwick JM, Sensi SL, Zukin RS, Jonas EA. Zinc-dependent multi-conductance channel activity in mitochondria isolated from ischemic brain. J Neurosci. 2006;26:6851–6862. doi: 10.1523/JNEUROSCI.5444-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boocock DJ, Patel KR, Faust GE, Normolle DP, Marczylo TH, Crowell JA, Brenner DE, Booth TD, Gescher A, Steward WP. Quantitation of trans-resveratrol and detection of its metabolites in human plasma and urine by high performance liquid chromatography. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;848:182–187. doi: 10.1016/j.jchromb.2006.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowling AC, Mutisya EM, Walker LC, Price DL, Cork LC, Beal MF. Age-dependent impairment of mitochondrial function in primate brain. J Neurochem. 1993;60:1964–1967. doi: 10.1111/j.1471-4159.1993.tb13430.x. [DOI] [PubMed] [Google Scholar]
- Boyer PD. The ATP synthase--a splendid molecular machine. Annu Rev Biochem. 1997;66:717–749. doi: 10.1146/annurev.biochem.66.1.717. [DOI] [PubMed] [Google Scholar]
- Braunstein M, Rose AB, Holmes SG, Allis CD, Broach JR. Transcriptional silencing in yeast is associated with reduced nucleosome acetylation. Genes Dev. 1993;7:592–604. doi: 10.1101/gad.7.4.592. [DOI] [PubMed] [Google Scholar]
- Brenmoehl J, Hoeflich A. Dual control of mitochondrial biogenesis by sirtuin 1 and sirtuin 3. Mitochondrion. 2013;13:755–761. doi: 10.1016/j.mito.2013.04.002. [DOI] [PubMed] [Google Scholar]
- Brookes PS, Yoon Y, Robotham JL, Anders MW, Sheu SS. Calcium, ATP, and ROS: a mitochondrial love-hate triangle. Am J Physiol Cell Physiol. 2004;287:C817–833. doi: 10.1152/ajpcell.00139.2004. [DOI] [PubMed] [Google Scholar]
- Bruintjes JJ, Henning RH, Douwenga W, van der Zee EA. Hippocampal cystathionine beta synthase in young and aged mice. Neurosci Lett. 2014;563:135–139. doi: 10.1016/j.neulet.2014.01.049. [DOI] [PubMed] [Google Scholar]
- Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, Tran H, Ross SE, Mostoslavsky R, Cohen HY, Hu LS, Cheng HL, Jedrychowski MP, Gygi SP, Sinclair DA, Alt FW, Greenberg ME. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004;303:2011–2015. doi: 10.1126/science.1094637. [DOI] [PubMed] [Google Scholar]
- Bruning CA, Prigol M, Luchese C, Jesse CR, Duarte MM, Roman SS, Nogueira CW. Protective effect of diphenyl diselenide on ischemia and reperfusion-induced cerebral injury: involvement of oxidative stress and pro-inflammatory cytokines. Neurochem Res. 2012;37:2249–2258. doi: 10.1007/s11064-012-0853-7. [DOI] [PubMed] [Google Scholar]
- Buga AM, Dunoiu C, Balseanu A, Popa-Wagner A. Cellular and molecular mechanisms underlying neurorehabilitation after stroke in aged subjects. Rom J Morphol Embryol. 2008;49:279–302. [PubMed] [Google Scholar]
- Bukoreshtliev NV, Wang X, Hodneland E, Gurke S, Barroso JF, Gerdes HH. Selective block of tunneling nanotube (TNT) formation inhibits intercellular organelle transfer between PC12 cells. FEBS Lett. 2009;583:1481–1488. doi: 10.1016/j.febslet.2009.03.065. [DOI] [PubMed] [Google Scholar]
- Burkon A, Somoza V. Quantification of free and protein-bound trans-resveratrol metabolites and identification of trans-resveratrol-C/O-conjugated diglucuronides - two novel resveratrol metabolites in human plasma. Mol Nutr Food Res. 2008;52:549–557. doi: 10.1002/mnfr.200700290. [DOI] [PubMed] [Google Scholar]
- Cairns CB, Moore FA, Haenel JB, Gallea BL, Ortner JP, Rose SJ, Moore EE. Evidence for early supply independent mitochondrial dysfunction in patients developing multiple organ failure after trauma. J Trauma. 1997;42:532–536. doi: 10.1097/00005373-199703000-00023. [DOI] [PubMed] [Google Scholar]
- Calvo SE, Mootha VK. The mitochondrial proteome and human disease. Annu Rev Genomics Hum Genet. 2010;11:25–44. doi: 10.1146/annurev-genom-082509-141720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camins A, Sureda FX, Junyent F, Verdaguer E, Folch J, Pelegri C, Vilaplana J, Beas-Zarate C, Pallas M. Sirtuin activators: designing molecules to extend life span. Biochim Biophys Acta. 2010;1799:740–749. doi: 10.1016/j.bbagrm.2010.06.005. [DOI] [PubMed] [Google Scholar]
- Canto C, Auwerx J. PGC-1alpha, SIRT1 and AMPK, an energy sensing network that controls energy expenditure. Curr Opin Lipidol. 2009;20:98–105. doi: 10.1097/MOL.0b013e328328d0a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canto C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, Elliott PJ, Puigserver P, Auwerx J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature. 2009;458:1056–1060. doi: 10.1038/nature07813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao L, Liu C, Wang F, Wang H. SIRT1 negatively regulates amyloid-beta-induced inflammation via the NF-kappaB pathway. Braz J Med Biol Res. 2013;46:659–669. doi: 10.1590/1414-431X20132903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecchini G. Function and structure of complex II of the respiratory chain. Annu Rev Biochem. 2003;72:77–109. doi: 10.1146/annurev.biochem.72.121801.161700. [DOI] [PubMed] [Google Scholar]
- Cechella JL, Leite MR, Rosario AR, Sampaio TB, Zeni G. Diphenyl diselenide-supplemented diet and swimming exercise enhance novel object recognition memory in old rats. Age (Dordr) 2014;36:9666. doi: 10.1007/s11357-014-9666-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan PH. Reactive oxygen radicals in signaling and damage in the ischemic brain. J Cereb Blood Flow Metab. 2001;21:2–14. doi: 10.1097/00004647-200101000-00002. [DOI] [PubMed] [Google Scholar]
- Chang CC, Chang CY, Huang JP, Hung LM. Effect of resveratrol on oxidative and inflammatory stress in liver and spleen of streptozotocin-induced type 1 diabetic rats. Chin J Physiol. 2012;55:192–201. doi: 10.4077/CJP.2012.BAA012. [DOI] [PubMed] [Google Scholar]
- Chang DT, Reynolds IJ. Mitochondrial trafficking and morphology in healthy and injured neurons. Prog Neurobiol. 2006;80:241–268. doi: 10.1016/j.pneurobio.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Chatterjee PK, Cuzzocrea S, Brown PA, Zacharowski K, Stewart KN, Mota-Filipe H, Thiemermann C. Tempol, a membrane-permeable radical scavenger, reduces oxidant stress-mediated renal dysfunction and injury in the rat. Kidney Int. 2000;58:658–673. doi: 10.1046/j.1523-1755.2000.00212.x. [DOI] [PubMed] [Google Scholar]
- Chen G, Shan W, Wu Y, Ren L, Dong J, Ji Z. Synthesis and anti-inflammatory activity of resveratrol analogs. Chem Pharm Bull (Tokyo) 2005a;53:1587–1590. doi: 10.1248/cpb.53.1587. [DOI] [PubMed] [Google Scholar]
- Chen M, He H, Zhan S, Krajewski S, Reed JC, Gottlieb RA. Bid is cleaved by calpain to an active fragment in vitro and during myocardial ischemia/reperfusion. J Biol Chem. 2001;276:30724–30728. doi: 10.1074/jbc.M103701200. [DOI] [PubMed] [Google Scholar]
- Chen N, Chiu PY, Ko KM. Schisandrin B enhances cerebral mitochondrial antioxidant status and structural integrity, and protects against cerebral ischemia/reperfusion injury in rats. Biol Pharm Bull. 2008;31:1387–1391. doi: 10.1248/bpb.31.1387. [DOI] [PubMed] [Google Scholar]
- Chen Q, Camara AK, Stowe DF, Hoppel CL, Lesnefsky EJ. Modulation of electron transport protects cardiac mitochondria and decreases myocardial injury during ischemia and reperfusion. Am J Physiol Cell Physiol. 2007;292:C137–147. doi: 10.1152/ajpcell.00270.2006. [DOI] [PubMed] [Google Scholar]
- Chen Q, Paillard M, Gomez L, Ross T, Hu Y, Xu A, Lesnefsky EJ. Activation of mitochondrial mu-calpain increases AIF cleavage in cardiac mitochondria during ischemia-reperfusion. Biochem Biophys Res Commun. 2011;415:533–538. doi: 10.1016/j.bbrc.2011.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T, Li J, Liu J, Li N, Wang S, Liu H, Zeng M, Zhang Y, Bu P. Activation of SIRT3 by resveratrol ameliorates cardiac fibrosis and improves cardiac function via the TGF-beta/Smad3 pathway. Am J Physiol Heart Circ Physiol. 2015;308:H424–434. doi: 10.1152/ajpheart.00454.2014. [DOI] [PubMed] [Google Scholar]
- Chen WY, Wang DH, Yen RC, Luo J, Gu W, Baylin SB. Tumor suppressor HIC1 directly regulates SIRT1 to modulate p53-dependent DNA-damage responses. Cell. 2005b;123:437–448. doi: 10.1016/j.cell.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Cheng G, Zhang X, Gao D, Jiang X, Dong W. Resveratrol inhibits MMP-9 expression by up-regulating PPAR alpha expression in an oxygen glucose deprivation-exposed neuron model. Neurosci Lett. 2009;451:105–108. doi: 10.1016/j.neulet.2008.12.045. [DOI] [PubMed] [Google Scholar]
- Cheng HL, Mostoslavsky R, Saito S, Manis JP, Gu Y, Patel P, Bronson R, Appella E, Alt FW, Chua KF. Developmental defects and p53 hyperacetylation in Sir2 homolog (SIRT1)-deficient mice. Proc Natl Acad Sci U S A. 2003;100:10794–10799. doi: 10.1073/pnas.1934713100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu PY, Leung HY, Poon MK, Ko KM. Chronic schisandrin B treatment improves mitochondrial antioxidant status and tissue heat shock protein production in various tissues of young adult and middle-aged rats. Biogerontology. 2006;7:199–210. doi: 10.1007/s10522-006-9017-y. [DOI] [PubMed] [Google Scholar]
- Cho S, Joh TH, Baik HH, Dibinis C, Volpe BT. Melatonin administration protects CA1 hippocampal neurons after transient forebrain ischemia in rats. Brain Res. 1997;755:335–338. doi: 10.1016/s0006-8993(97)00188-1. [DOI] [PubMed] [Google Scholar]
- Christofk HR, Vander Heiden MG, Harris MH, Ramanathan A, Gerszten RE, Wei R, Fleming MD, Schreiber SL, Cantley LC. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature. 2008;452:230–233. doi: 10.1038/nature06734. [DOI] [PubMed] [Google Scholar]
- Chung HY, Yu BP. Significance of hepatic xanthine oxidase and uric acid in aged and dietary restricted rats. J Am Aging Assoc. 2000;23:123–128. doi: 10.1007/s11357-000-0013-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung JH, Manganiello V, Dyck JR. Resveratrol as a calorie restriction mimetic: therapeutic implications. Trends Cell Biol. 2012;22:546–554. doi: 10.1016/j.tcb.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark D, Tuor UI, Thompson R, Institoris A, Kulynych A, Zhang X, Kinniburgh DW, Bari F, Busija DW, Barber PA. Protection against recurrent stroke with resveratrol: endothelial protection. PLoS One. 2012;7:e47792. doi: 10.1371/journal.pone.0047792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clouser CL, Chauhan J, Bess MA, van Oploo JL, Zhou D, Dimick-Gray S, Mansky LM, Patterson SE. Anti-HIV-1 activity of resveratrol derivatives and synergistic inhibition of HIV-1 by the combination of resveratrol and decitabine. Bioorg Med Chem Lett. 2012;22:6642–6646. doi: 10.1016/j.bmcl.2012.08.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen A, Lerner-Yardeni J, Meridor D, Kasher R, Nathan I, Parola AH. Humanin-derivatives Inhibit Necrotic Cell Death in Neurons. Mol Med. 2015 doi: 10.2119/molmed.2015.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corral-Debrinski M, Shoffner JM, Lott MT, Wallace DC. Association of mitochondrial DNA damage with aging and coronary atherosclerotic heart disease. Mutat Res. 1992;275:169–180. doi: 10.1016/0921-8734(92)90021-g. [DOI] [PubMed] [Google Scholar]
- Correia-Melo C, Passos JF. Mitochondria: Are they causal players in cellular senescence? Biochim Biophys Acta. 2015 doi: 10.1016/j.bbabio.2015.05.017. [DOI] [PubMed] [Google Scholar]
- Corsini F, Scaglione A, Iacomino M, Mascia G, Melorio S, Riccio C, Romano S, Vetrano A, Celardo S, Corsini G, Chieffo C. Acute myocardial infarction in the elderly. A case-control study with a younger population and review of literature. Monaldi Arch Chest Dis. 2006;66:13–19. doi: 10.4081/monaldi.2006.537. [DOI] [PubMed] [Google Scholar]
- Cui J, Shen Y, Li R. Estrogen synthesis and signaling pathways during aging: from periphery to brain. Trends Mol Med. 2013;19:197–209. doi: 10.1016/j.molmed.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curry N, Hopewell S, Doree C, Hyde C, Brohi K, Stanworth S. The acute management of trauma hemorrhage: a systematic review of randomized controlled trials. Crit Care. 2011;15:R92. doi: 10.1186/cc10096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuzzocrea S, Costantino G, Gitto E, Mazzon E, Fulia F, Serraino I, Cordaro S, Barberi I, De Sarro A, Caputi AP. Protective effects of melatonin in ischemic brain injury. J Pineal Res. 2000a;29:217–227. doi: 10.1034/j.1600-0633.2002.290404.x. [DOI] [PubMed] [Google Scholar]
- Cuzzocrea S, McDonald MC, Mazzon E, Siriwardena D, Costantino G, Fulia F, Cucinotta G, Gitto E, Cordaro S, Barberi I, De Sarro A, Caputi AP, Thiemermann C. Effects of tempol, a membrane-permeable radical scavenger, in a gerbil model of brain injury. Brain Res. 2000b;875:96–106. doi: 10.1016/s0006-8993(00)02582-8. [DOI] [PubMed] [Google Scholar]
- Dai H, Kustigian L, Carney D, Case A, Considine T, Hubbard BP, Perni RB, Riera TV, Szczepankiewicz B, Vlasuk GP, Stein RL. SIRT1 activation by small molecules: kinetic and biophysical evidence for direct interaction of enzyme and activator. J Biol Chem. 2010;285:32695–32703. doi: 10.1074/jbc.M110.133892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai SH, Chen T, Wang YH, Zhu J, Luo P, Rao W, Yang YF, Fei Z, Jiang XF. Sirt3 attenuates hydrogen peroxide-induced oxidative stress through the preservation of mitochondrial function in HT22 cells. Int J Mol Med. 2014;34:1159–1168. doi: 10.3892/ijmm.2014.1876. [DOI] [PubMed] [Google Scholar]
- Davalos A, Alvarez-Sabin J, Castillo J, Diez-Tejedor E, Ferro J, Martinez-Vila E, Serena J, Segura T, Cruz VT, Masjuan J, Cobo E, Secades JJ International Citicoline Trial on acUte Stroke trial i. Citicoline in the treatment of acute ischaemic stroke: an international, randomised, multicentre, placebo-controlled study (ICTUS trial) Lancet. 2012;380:349–357. doi: 10.1016/S0140-6736(12)60813-7. [DOI] [PubMed] [Google Scholar]
- Davis M, Whitely T, Turnbull DM, Mendelow AD. Selective impairments of mitochondrial respiratory chain activity during aging and ischemic brain damage. Acta Neurochir Suppl. 1997;70:56–58. doi: 10.1007/978-3-7091-6837-0_17. [DOI] [PubMed] [Google Scholar]
- Day JJ, Bayer AJ, Pathy MS, Chadha JS. Acute myocardial infarction: diagnostic difficulties and outcome in advanced old age. Age Ageing. 1987;16:239–243. doi: 10.1093/ageing/16.4.239. [DOI] [PubMed] [Google Scholar]
- Della-Morte D, Dave KR, DeFazio RA, Bao YC, Raval AP, Perez-Pinzon MA. Resveratrol pretreatment protects rat brain from cerebral ischemic damage via a sirtuin 1-uncoupling protein 2 pathway. Neuroscience. 2009;159:993–1002. doi: 10.1016/j.neuroscience.2009.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dessolin J, Schuler M, Quinart A, De Giorgi F, Ghosez L, Ichas F. Selective targeting of synthetic antioxidants to mitochondria: towards a mitochondrial medicine for neurodegenerative diseases? Eur J Pharmacol. 2002;447:155–161. doi: 10.1016/s0014-2999(02)01839-3. [DOI] [PubMed] [Google Scholar]
- Di Y, He YL, Zhao T, Huang X, Wu KW, Liu SH, Zhao YQ, Fan M, Wu LY, Zhu LL. Methylene Blue Reduces Acute Cerebral Ischemic Injury via the Induction of Mitophagy. Mol Med. 2015 doi: 10.2119/molmed.2015.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding WX, Yin XM. Mitophagy: mechanisms, pathophysiological roles, and analysis. Biol Chem. 2012;393:547–564. doi: 10.1515/hsz-2012-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrachinski F, da Silva MH, Tassi CL, de Carvalho NR, Dias GR, Golombieski RM, da Silva Loreto EL, da Rocha JB, Fighera MR, Soares FA. Neuroprotective effect of diphenyl diselenide in a experimental stroke model: maintenance of redox system in mitochondria of brain regions. Neurotox Res. 2014;26:317–330. doi: 10.1007/s12640-014-9463-2. [DOI] [PubMed] [Google Scholar]
- Dong W, Li N, Gao D, Zhen H, Zhang X, Li F. Resveratrol attenuates ischemic brain damage in the delayed phase after stroke and induces messenger RNA and protein express for angiogenic factors. J Vasc Surg. 2008;48:709–714. doi: 10.1016/j.jvs.2008.04.007. [DOI] [PubMed] [Google Scholar]
- Dong XB, Yang CT, Zheng DD, Mo LQ, Wang XY, Lan AP, Hu F, Chen PX, Feng JQ, Zhang MF, Liao XX. Inhibition of ROS-activated ERK1/2 pathway contributes to the protection of H2S against chemical hypoxia-induced injury in H9c2 cells. Mol Cell Biochem. 2012;362:149–157. doi: 10.1007/s11010-011-1137-2. [DOI] [PubMed] [Google Scholar]
- Du J, Zhou Y, Su X, Yu JJ, Khan S, Jiang H, Kim J, Woo J, Kim JH, Choi BH, He B, Chen W, Zhang S, Cerione RA, Auwerx J, Hao Q, Lin H. Sirt5 is a NAD-dependent protein lysine demalonylase and desuccinylase. Science. 2011;334:806–809. doi: 10.1126/science.1207861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand G, Choteau F, Pucci B, Villamena FA. Reactivity of superoxide radical anion and hydroperoxyl radical with alpha-phenyl-N-tert-butylnitrone (PBN) derivatives. J Phys Chem A. 2008;112:12498–12509. doi: 10.1021/jp804929d. [DOI] [PubMed] [Google Scholar]
- Durieux J, Wolff S, Dillin A. The cell-non-autonomous nature of electron transport chain-mediated longevity. Cell. 2011;144:79–91. doi: 10.1016/j.cell.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durrant JR, Seals DR, Connell ML, Russell MJ, Lawson BR, Folian BJ, Donato AJ, Lesniewski LA. Voluntary wheel running restores endothelial function in conduit arteries of old mice: direct evidence for reduced oxidative stress, increased superoxide dismutase activity and down-regulation of NADPH oxidase. J Physiol. 2009;587:3271–3285. doi: 10.1113/jphysiol.2009.169771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehninger D, Neff F, Xie K. Longevity, aging and rapamycin. Cell Mol Life Sci. 2014;71:4325–4346. doi: 10.1007/s00018-014-1677-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elrod JW, Calvert JW, Morrison J, Doeller JE, Kraus DW, Tao L, Jiao X, Scalia R, Kiss L, Szabo C, Kimura H, Chow CW, Lefer DJ. Hydrogen sulfide attenuates myocardial ischemia-reperfusion injury by preservation of mitochondrial function. Proc Natl Acad Sci U S A. 2007;104:15560–15565. doi: 10.1073/pnas.0705891104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elson JL, Samuels DC, Turnbull DM, Chinnery PF. Random intracellular drift explains the clonal expansion of mitochondrial DNA mutations with age. Am J Hum Genet. 2001;68:802–806. doi: 10.1086/318801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endres M, Wang ZQ, Namura S, Waeber C, Moskowitz MA. Ischemic brain injury is mediated by the activation of poly(ADP-ribose)polymerase. J Cereb Blood Flow Metab. 1997;17:1143–1151. doi: 10.1097/00004647-199711000-00002. [DOI] [PubMed] [Google Scholar]
- Erfani S, Khaksari M, Oryan S, Shamsaei N, Aboutaleb N, Nikbakht F. Nampt/PBEF/Visfatin Exerts Neuroprotective Effects Against Ischemia/Reperfusion Injury via Modulation of Bax/Bcl-2 Ratio and Prevention of Caspase-3 Activation. J Mol Neurosci. 2015;56:237–243. doi: 10.1007/s12031-014-0486-1. [DOI] [PubMed] [Google Scholar]
- Esplugues JV, Rocha M, Nunez C, Bosca I, Ibiza S, Herance JR, Ortega A, Serrador JM, D’Ocon P, Victor VM. Complex I dysfunction and tolerance to nitroglycerin: an approach based on mitochondrial-targeted antioxidants. Circ Res. 2006;99:1067–1075. doi: 10.1161/01.RES.0000250430.62775.99. [DOI] [PubMed] [Google Scholar]
- Fardoun RZ, Asghar M, Lokhandwala M. Role of oxidative stress in defective renal dopamine D1 receptor-G protein coupling and function in old Fischer 344 rats. Am J Physiol Renal Physiol. 2006;291:F945–951. doi: 10.1152/ajprenal.00111.2006. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Rinaldi B, Corbi G, Conti V, Stiuso P, Boccuti S, Rengo G, Rossi F, Filippelli A. Exercise training promotes SIRT1 activity in aged rats. Rejuvenation Res. 2008;11:139–150. doi: 10.1089/rej.2007.0576. [DOI] [PubMed] [Google Scholar]
- Fillingame RH, Steed PR. Half channels mediating H(+) transport and the mechanism of gating in the Fo sector of Escherichia coli F1Fo ATP synthase. Biochim Biophys Acta. 2014;1837:1063–1068. doi: 10.1016/j.bbabio.2014.03.005. [DOI] [PubMed] [Google Scholar]
- Finck BN, Kelly DP. Peroxisome proliferator-activated receptor gamma coactivator-1 (PGC-1) regulatory cascade in cardiac physiology and disease. Circulation. 2007;115:2540–2548. doi: 10.1161/CIRCULATIONAHA.107.670588. [DOI] [PubMed] [Google Scholar]
- Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- Fischer H. A guide to U.S. Military casulaty statistics: Operation inherent resolve, Operation New Dawn, Operation Iraqu Freedom, and Operation Enduring Freedom. In. Congressional Research Service Report 2014 [Google Scholar]
- Flamm ES, Demopoulos HB, Seligman ML, Poser RG, Ransohoff J. Free radicals in cerebral ischemia. Stroke. 1978;9:445–447. doi: 10.1161/01.str.9.5.445. [DOI] [PubMed] [Google Scholar]
- Folbergrova J, Zhao Q, Katsura K, Siesjo BK. N-tert-butyl-alpha-phenylnitrone improves recovery of brain energy state in rats following transient focal ischemia. Proc Natl Acad Sci U S A. 1995;92:5057–5061. doi: 10.1073/pnas.92.11.5057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forester BP, Berlow YA, Harper DG, Jensen JE, Lange N, Froimowitz MP, Ravichandran C, Iosifescu DV, Lukas SE, Renshaw PF, Cohen BM. Age-related changes in brain energetics and phospholipid metabolism. NMR Biomed. 2010;23:242–250. doi: 10.1002/nbm.1444. [DOI] [PubMed] [Google Scholar]
- Fox PT, Raichle ME. Focal physiological uncoupling of cerebral blood flow and oxidative metabolism during somatosensory stimulation in human subjects. Proc Natl Acad Sci U S A. 1986;83:1140–1144. doi: 10.1073/pnas.83.4.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox PT, Raichle ME, Mintun MA, Dence C. Nonoxidative glucose consumption during focal physiologic neural activity. Science. 1988;241:462–464. doi: 10.1126/science.3260686. [DOI] [PubMed] [Google Scholar]
- Fresta M, Puglisi G. Survival rate improvement in a rat ischemia model by long circulating liposomes containing cytidine-5I-diphosphate choline. Life Sci. 1997;61:1227–1235. doi: 10.1016/s0024-3205(97)00667-x. [DOI] [PubMed] [Google Scholar]
- Frye RA. Characterization of five human cDNAs with homology to the yeast SIR2 gene: Sir2-like proteins (sirtuins) metabolize NAD and may have protein ADP-ribosyltransferase activity. Biochem Biophys Res Commun. 1999;260:273–279. doi: 10.1006/bbrc.1999.0897. [DOI] [PubMed] [Google Scholar]
- Funahashi T, Floyd RA, Carney JM. Age effect on brain pH during ischemia/reperfusion and pH influence on peroxidation. Neurobiol Aging. 1994;15:161–167. doi: 10.1016/0197-4580(94)90107-4. [DOI] [PubMed] [Google Scholar]
- Gao D, Zhang X, Jiang X, Peng Y, Huang W, Cheng G, Song L. Resveratrol reduces the elevated level of MMP-9 induced by cerebral ischemia-reperfusion in mice. Life Sci. 2006;78:2564–2570. doi: 10.1016/j.lfs.2005.10.030. [DOI] [PubMed] [Google Scholar]
- Gao J, Wang WY, Mao YW, Graff J, Guan JS, Pan L, Mak G, Kim D, Su SC, Tsai LH. A novel pathway regulates memory and plasticity via SIRT1 and miR-134. Nature. 2010;466:1105–1109. doi: 10.1038/nature09271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao L, Laude K, Cai H. Mitochondrial pathophysiology, reactive oxygen species, and cardiovascular diseases. Vet Clin North Am Small Anim Pract. 2008;38:137–155. vi. doi: 10.1016/j.cvsm.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellerich FN. The role of adenylate kinase in dynamic compartmentation of adenine nucleotides in the mitochondrial intermembrane space. FEBS Lett. 1992;297:55–58. doi: 10.1016/0014-5793(92)80326-c. [DOI] [PubMed] [Google Scholar]
- Gerdes HH, Bukoreshtliev NV, Barroso JF. Tunneling nanotubes: a new route for the exchange of components between animal cells. FEBS Lett. 2007;581:2194–2201. doi: 10.1016/j.febslet.2007.03.071. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Das N, Mandal AK, Dungdung SR, Sarkar S. Mannosylated liposomal cytidine 5′ diphosphocholine prevent age related global moderate cerebral ischemia reperfusion induced mitochondrial cytochrome c release in aged rat brain. Neuroscience. 2010;171:1287–1299. doi: 10.1016/j.neuroscience.2010.09.049. [DOI] [PubMed] [Google Scholar]
- Gioscia-Ryan RA, LaRocca TJ, Sindler AL, Zigler MC, Murphy MP, Seals DR. Mitochondria-targeted antioxidant (MitoQ) ameliorates age-related arterial endothelial dysfunction in mice. J Physiol. 2014;592:2549–2561. doi: 10.1113/jphysiol.2013.268680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey R, Theologou T, Dellegrottaglie S, Binukrishnan S, Wright J, Whyte G, Ellison G. The effect of high-intensity aerobic interval training on postinfarction left ventricular remodelling. BMJ Case Rep. 2013;2013 doi: 10.1136/bcr-2012-007668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes AP, Price NL, Ling AJ, Moslehi JJ, Montgomery MK, Rajman L, White JP, Teodoro JS, Wrann CD, Hubbard BP, Mercken EM, Palmeira CM, de Cabo R, Rolo AP, Turner N, Bell EL, Sinclair DA. Declining NAD(+) induces a pseudohypoxic state disrupting nuclear-mitochondrial communication during aging. Cell. 2013;155:1624–1638. doi: 10.1016/j.cell.2013.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes LC, Di Benedetto G, Scorrano L. During autophagy mitochondria elongate, are spared from degradation and sustain cell viability. Nat Cell Biol. 2011;13:589–598. doi: 10.1038/ncb2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Y, Hua Y, Keep RF, Hoff JT, Xi G. Intracerebral hemorrhage: effects of aging on brain edema and neurological deficits. Stroke. 2004;35:2571–2575. doi: 10.1161/01.STR.0000145485.67827.d0. [DOI] [PubMed] [Google Scholar]
- Gong Y, Xi GH, Keep RF, Hoff JT, Hua Y. Aging enhances intracerebral hemorrhage-induced brain injury in rats. Acta Neurochir Suppl. 2005;95:425–427. doi: 10.1007/3-211-32318-x_87. [DOI] [PubMed] [Google Scholar]
- Gong Z, Tas E, Muzumdar R. Humanin and age-related diseases: a new link? Front Endocrinol (Lausanne) 2014;5:210. doi: 10.3389/fendo.2014.00210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal MS, Hawrylycz M, Miller JA, Snyder AZ, Raichle ME. Aerobic glycolysis in the human brain is associated with development and neotenous gene expression. Cell Metab. 2014;19:49–57. doi: 10.1016/j.cmet.2013.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DR, Kroemer G. The pathophysiology of mitochondrial cell death. Science. 2004;305:626–629. doi: 10.1126/science.1099320. [DOI] [PubMed] [Google Scholar]
- Gu C, Xing Y, Jiang L, Chen M, Xu M, Yin Y, Li C, Yang Z, Yu L, Ma H. Impaired cardiac SIRT1 activity by carbonyl stress contributes to aging-related ischemic intolerance. PLoS One. 2013;8:e74050. doi: 10.1371/journal.pone.0074050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A, Manhas N, Raghubir R. Energy metabolism during cutaneous wound healing in immunocompromised and aged rats. Mol Cell Biochem. 2004;259:9–14. doi: 10.1023/b:mcbi.0000021339.34784.81. [DOI] [PubMed] [Google Scholar]
- Gurke S, Barroso JF, Hodneland E, Bukoreshtliev NV, Schlicker O, Gerdes HH. Tunneling nanotube (TNT)-like structures facilitate a constitutive, actomyosin-dependent exchange of endocytic organelles between normal rat kidney cells. Exp Cell Res. 2008;314:3669–3683. doi: 10.1016/j.yexcr.2008.08.022. [DOI] [PubMed] [Google Scholar]
- Haberman F, Tang SC, Arumugam TV, Hyun DH, Yu QS, Cutler RG, Guo Z, Holloway HW, Greig NH, Mattson MP. Soluble neuroprotective antioxidant uric acid analogs ameliorate ischemic brain injury in mice. Neuromolecular Med. 2007;9:315–323. doi: 10.1007/s12017-007-8010-1. [DOI] [PubMed] [Google Scholar]
- Haigis MC, Guarente LP. Mammalian sirtuins--emerging roles in physiology, aging, and calorie restriction. Genes Dev. 2006;20:2913–2921. doi: 10.1101/gad.1467506. [DOI] [PubMed] [Google Scholar]
- Haigis MC, Mostoslavsky R, Haigis KM, Fahie K, Christodoulou DC, Murphy AJ, Valenzuela DM, Yancopoulos GD, Karow M, Blander G, Wolberger C, Prolla TA, Weindruch R, Alt FW, Guarente L. SIRT4 inhibits glutamate dehydrogenase and opposes the effects of calorie restriction in pancreatic beta cells. Cell. 2006;126:941–954. doi: 10.1016/j.cell.2006.06.057. [DOI] [PubMed] [Google Scholar]
- Hallberg BM, Larsson NG. Making proteins in the powerhouse. Cell Metab. 2014;20:226–240. doi: 10.1016/j.cmet.2014.07.001. [DOI] [PubMed] [Google Scholar]
- Hansford RG, Castro F. Effect of senescence on Ca2+-ion transport by heart mitochondria. Mech Ageing Dev. 1982;19:5–13. doi: 10.1016/0047-6374(82)90044-6. [DOI] [PubMed] [Google Scholar]
- Hariharan N, Maejima Y, Nakae J, Paik J, Depinho RA, Sadoshima J. Deacetylation of FoxO by Sirt1 Plays an Essential Role in Mediating Starvation-Induced Autophagy in Cardiac Myocytes. Circ Res. 2010;107:1470–1482. doi: 10.1161/CIRCRESAHA.110.227371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- Harman D. The biologic clock: the mitochondria? J Am Geriatr Soc. 1972;20:145–147. doi: 10.1111/j.1532-5415.1972.tb00787.x. [DOI] [PubMed] [Google Scholar]
- Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, Pahor M, Javors MA, Fernandez E, Miller RA. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori Y, Okamoto Y, Maki T, Yamamoto Y, Oishi N, Yamahara K, Nagatsuka K, Takahashi R, Kalaria RN, Fukuyama H, Kinoshita M, Ihara M. Silent information regulator 2 homolog 1 counters cerebral hypoperfusion injury by deacetylating endothelial nitric oxide synthase. Stroke. 2014;45:3403–3411. doi: 10.1161/STROKEAHA.114.006265. [DOI] [PubMed] [Google Scholar]
- Hattori Y, Okamoto Y, Nagatsuka K, Takahashi R, Kalaria RN, Kinoshita M, Ihara M. SIRT1 attenuates severe ischemic damage by preserving cerebral blood flow. Neuroreport. 2015;26:113–117. doi: 10.1097/WNR.0000000000000308. [DOI] [PubMed] [Google Scholar]
- Hernandez-Jimenez M, Hurtado O, Cuartero MI, Ballesteros I, Moraga A, Pradillo JM, McBurney MW, Lizasoain I, Moro MA. Silent information regulator 1 protects the brain against cerebral ischemic damage. Stroke. 2013;44:2333–2337. doi: 10.1161/STROKEAHA.113.001715. [DOI] [PubMed] [Google Scholar]
- Hernando V, Inserte J, Sartorio CL, Parra VM, Poncelas-Nozal M, Garcia-Dorado D. Calpain translocation and activation as pharmacological targets during myocardial ischemia/reperfusion. J Mol Cell Cardiol. 2010;49:271–279. doi: 10.1016/j.yjmcc.2010.02.024. [DOI] [PubMed] [Google Scholar]
- Herrmann JM, Riemer J. The intermembrane space of mitochondria. Antioxid Redox Signal. 2010;13:1341–1358. doi: 10.1089/ars.2009.3063. [DOI] [PubMed] [Google Scholar]
- Hirschey MD, Shimazu T, Goetzman E, Jing E, Schwer B, Lombard DB, Grueter CA, Harris C, Biddinger S, Ilkayeva OR, Stevens RD, Li Y, Saha AK, Ruderman NB, Bain JR, Newgard CB, Farese RV, Jr, Alt FW, Kahn CR, Verdin E. SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature. 2010;464:121–125. doi: 10.1038/nature08778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst J. Towards the molecular mechanism of respiratory complex I. Biochem J. 2010;425:327–339. doi: 10.1042/BJ20091382. [DOI] [PubMed] [Google Scholar]
- Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, Scherer B, Sinclair DA. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- Hsu CP, Oka S, Shao D, Hariharan N, Sadoshima J. Nicotinamide phosphoribosyltransferase regulates cell survival through NAD+ synthesis in cardiac myocytes. Circ Res. 2009;105:481–491. doi: 10.1161/CIRCRESAHA.109.203703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu CPOS, Shao D, Hariharan N, Sadoshima J. Nicotinamide phosphoribosyltransferase regulates cell survival through NAD+ synthesis in cardiac myocytes. Circulation Research. 2009;105:481–491. doi: 10.1161/CIRCRESAHA.109.203703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu CP, Zhai P, Yamamoto T, Maejima Y, Matsushima S, Hariharan N, Shao D, Takagi H, Oka S, Sadoshima J. Silent information regulator 1 protects the heart from ischemia/reperfusion. Circulation. 2010;122:2170–2182. doi: 10.1161/CIRCULATIONAHA.110.958033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Li R, Yang H, Luo H, Chen Z. Sirtuin 6 is essential for sodium sulfide-mediated cytoprotective effect in ischemia/reperfusion-stimulated brain endothelial cells. J Stroke Cerebrovasc Dis. 2015;24:601–609. doi: 10.1016/j.jstrokecerebrovasdis.2014.10.006. [DOI] [PubMed] [Google Scholar]
- Huang SS, Tsai MC, Chih CL, Hung LM, Tsai SK. Resveratrol reduction of infarct size in Long-Evans rats subjected to focal cerebral ischemia. Life Sci. 2001;69:1057–1065. doi: 10.1016/s0024-3205(01)01195-x. [DOI] [PubMed] [Google Scholar]
- Ide T, Tsutsui H, Hayashidani S, Kang D, Suematsu N, Nakamura K, Utsumi H, Hamasaki N, Takeshita A. Mitochondrial DNA damage and dysfunction associated with oxidative stress in failing hearts after myocardial infarction. Circ Res. 2001;88:529–535. doi: 10.1161/01.res.88.5.529. [DOI] [PubMed] [Google Scholar]
- Imai S. Dissecting systemic control of metabolism and aging in the NAD World: the importance of SIRT1 and NAMPT-mediated NAD biosynthesis. FEBS Lett. 2011;585:1657–1662. doi: 10.1016/j.febslet.2011.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai S, Armstrong CM, Kaeberlein M, Guarente L. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature. 2000;403:795–800. doi: 10.1038/35001622. [DOI] [PubMed] [Google Scholar]
- Irving EA, Bamford M. Role of mitogen- and stress-activated kinases in ischemic injury. J Cereb Blood Flow Metab. 2002;22:631–647. doi: 10.1097/00004647-200206000-00001. [DOI] [PubMed] [Google Scholar]
- Islam MN, Das SR, Emin MT, Wei M, Sun L, Westphalen K, Rowlands DJ, Quadri SK, Bhattacharya S, Bhattacharya J. Mitochondrial transfer from bone-marrow-derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat Med. 2012;18:759–765. doi: 10.1038/nm.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh Y, Oyake T, Hayashi N, Satoh K, Hatayama I, Yamamoto M, Nabeshima Y. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun. 1997;236:313–322. doi: 10.1006/bbrc.1997.6943. [DOI] [PubMed] [Google Scholar]
- Itoh T, Imano M, Nishida S, Tsubaki M, Mizuguchi N, Hashimoto S, Ito A, Satou T. Increased apoptotic neuronal cell death and cognitive impairment at early phase after traumatic brain injury in aged rats. Brain Struct Funct. 2013;218:209–220. doi: 10.1007/s00429-012-0394-5. [DOI] [PubMed] [Google Scholar]
- Iwata S, Lee JW, Okada K, Lee JK, Iwata M, Rasmussen B, Link TA, Ramaswamy S, Jap BK. Complete structure of the 11-subunit bovine mitochondrial cytochrome bc1 complex. Science. 1998;281:64–71. doi: 10.1126/science.281.5373.64. [DOI] [PubMed] [Google Scholar]
- Izmaylov DM, Obukhova LK. Geroprotector efficiency depends on viability of control population: life span investigation in D. melanogaster. Mech Ageing Dev. 1996;91:155–164. doi: 10.1016/s0047-6374(96)01776-9. [DOI] [PubMed] [Google Scholar]
- Jannin B, Menzel M, Berlot JP, Delmas D, Lancon A, Latruffe N. Transport of resveratrol, a cancer chemopreventive agent, to cellular targets: plasmatic protein binding and cell uptake. Biochem Pharmacol. 2004;68:1113–1118. doi: 10.1016/j.bcp.2004.04.028. [DOI] [PubMed] [Google Scholar]
- Jastroch M, Divakaruni AS, Mookerjee S, Treberg JR, Brand MD. Mitochondrial proton and electron leaks. Essays Biochem. 2010;47:53–67. doi: 10.1042/bse0470053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji J, Kline AE, Amoscato A, Samhan-Arias AK, Sparvero LJ, Tyurin VA, Tyurina YY, Fink B, Manole MD, Puccio AM, Okonkwo DO, Cheng JP, Alexander H, Clark RS, Kochanek PM, Wipf P, Kagan VE, Bayir H. Lipidomics identifies cardiolipin oxidation as a mitochondrial target for redox therapy of brain injury. Nat Neurosci. 2012;15:1407–1413. doi: 10.1038/nn.3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jian B, Yang S, Chaudry IH, Raju R. Resveratrol improves cardiac contractility following trauma-hemorrhage by modulating Sirt1. Mol Med. 2012;18:209–214. doi: 10.2119/molmed.2011.00365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jian B, Yang S, Chaudry IH, Raju R. Resveratrol restores sirtuin 1 (SIRT1) activity and pyruvate dehydrogenase kinase 1 (PDK1) expression after hemorrhagic injury in a rat model. Mol Med. 2014;20:10–16. doi: 10.2119/molmed.2013.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jian B, Yang S, Chen D, Chaudry I, Raju R. Influence of aging and hemorrhage injury on Sirt1 expression: possible role of myc-Sirt1 regulation in mitochondrial function. Biochim Biophys Acta. 2011a;1812:1446–1451. doi: 10.1016/j.bbadis.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jian B, Yang S, Chen D, Zou L, Chatham JC, Chaudry I, Raju R. Aging influences cardiac mitochondrial gene expression and cardiovascular function following hemorrhage injury. Mol Med. 2011b;17:542–549. doi: 10.2119/molmed.2010.00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang HK, Miao Y, Wang YH, Zhao M, Feng ZH, Yu XJ, Liu JK, Zang WJ. Aerobic interval training protects against myocardial infarction-induced oxidative injury by enhancing antioxidase system and mitochondrial biosynthesis. Clin Exp Pharmacol Physiol. 2014;41:192–201. doi: 10.1111/1440-1681.12211. [DOI] [PubMed] [Google Scholar]
- Jiang J, Kurnikov I, Belikova NA, Xiao J, Zhao Q, Amoscato AA, Braslau R, Studer A, Fink MP, Greenberger JS, Wipf P, Kagan VE. Structural requirements for optimized delivery, inhibition of oxidative stress, and antiapoptotic activity of targeted nitroxides. J Pharmacol Exp Ther. 2007;320:1050–1060. doi: 10.1124/jpet.106.114769. [DOI] [PubMed] [Google Scholar]
- Jiang M, Wang J, Fu J, Du L, Jeong H, West T, Xiang L, Peng Q, Hou Z, Cai H, Seredenina T, Arbez N, Zhu S, Sommers K, Qian J, Zhang J, Mori S, Yang XW, Tamashiro KL, Aja S, Moran TH, Luthi-Carter R, Martin B, Maudsley S, Mattson MP, Cichewicz RH, Ross CA, Holtzman DM, Krainc D, Duan W. Neuroprotective role of Sirt1 in mammalian models of Huntington’s disease through activation of multiple Sirt1 targets. Nat Med. 2012;18:153–158. doi: 10.1038/nm.2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan JE, Simandle SA, Tulbert CD, Busija DW, Miller AW. Fructose-fed rats are protected against ischemia/reperfusion injury. J Pharmacol Exp Ther. 2003;307:1007–1011. doi: 10.1124/jpet.103.055970. [DOI] [PubMed] [Google Scholar]
- Judge S, Jang YM, Smith A, Hagen T, Leeuwenburgh C. Age-associated increases in oxidative stress and antioxidant enzyme activities in cardiac interfibrillar mitochondria: implications for the mitochondrial theory of aging. FASEB J. 2005;19:419–421. doi: 10.1096/fj.04-2622fje. [DOI] [PubMed] [Google Scholar]
- Judge S, Leeuwenburgh C. Cardiac mitochondrial bioenergetics, oxidative stress, and aging. Am J Physiol Cell Physiol. 2007;292:C1983–1992. doi: 10.1152/ajpcell.00285.2006. [DOI] [PubMed] [Google Scholar]
- Kaeberlein M, Kirkland KT, Fields S, Kennedy BK. Sir2-independent life span extension by calorie restriction in yeast. PLoS Biol. 2004;2:E296. doi: 10.1371/journal.pbio.0020296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeberlein M, McVey M, Guarente L. The SIR2/3/4 complex and SIR2 alone promote longevity in Saccharomyces cerevisiae by two different mechanisms. Genes Dev. 1999;13:2570–2580. doi: 10.1101/gad.13.19.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeberlein M, Steffen KK, Hu D, Dang N, Kerr EO, Tsuchiya M, Fields S, Kennedy BK. Comment on “HST2 mediates SIR2-independent life-span extension by calorie restriction”. Science. 2006;312:1312. doi: 10.1126/science.1124608. author reply 1312. [DOI] [PubMed] [Google Scholar]
- Kane LA, Youle RJ. Mitochondrial fission and fusion and their roles in the heart. J Mol Med (Berl) 2010;88:971–979. doi: 10.1007/s00109-010-0674-6. [DOI] [PubMed] [Google Scholar]
- Kanfi Y, Naiman S, Amir G, Peshti V, Zinman G, Nahum L, Bar-Joseph Z, Cohen HY. The sirtuin SIRT6 regulates lifespan in male mice. Nature. 2012;483:218–221. doi: 10.1038/nature10815. [DOI] [PubMed] [Google Scholar]
- Kang D, Hamasaki N. Mitochondrial transcription factor A in the maintenance of mitochondrial DNA: overview of its multiple roles. Ann N Y Acad Sci. 2005;1042:101–108. doi: 10.1196/annals.1338.010. [DOI] [PubMed] [Google Scholar]
- Kato N, Yanaka K, Hyodo K, Homma K, Nagase S, Nose T. Stable nitroxide Tempol ameliorates brain injury by inhibiting lipid peroxidation in a rat model of transient focal cerebral ischemia. Brain Res. 2003;979:188–193. doi: 10.1016/s0006-8993(03)02918-4. [DOI] [PubMed] [Google Scholar]
- Kauppinen A, Suuronen T, Ojala J, Kaarniranta K, Salminen A. Antagonistic crosstalk between NF-kappaB and SIRT1 in the regulation of inflammation and metabolic disorders. Cell Signal. 2013;25:1939–1948. doi: 10.1016/j.cellsig.2013.06.007. [DOI] [PubMed] [Google Scholar]
- Kawai Y, Garduno L, Theodore M, Yang J, Arinze IJ. Acetylation-deacetylation of the transcription factor Nrf2 (nuclear factor erythroid 2-related factor 2) regulates its transcriptional activity and nucleocytoplasmic localization. J Biol Chem. 2011;286:7629–7640. doi: 10.1074/jbc.M110.208173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke Q, Costa M. Hypoxia-inducible factor-1 (HIF-1) Mol Pharmacol. 2006;70:1469–1480. doi: 10.1124/mol.106.027029. [DOI] [PubMed] [Google Scholar]
- Kelso GF, Porteous CM, Coulter CV, Hughes G, Porteous WK, Ledgerwood EC, Smith RA, Murphy MP. Selective targeting of a redox-active ubiquinone to mitochondria within cells: antioxidant and antiapoptotic properties. J Biol Chem. 2001;276:4588–4596. doi: 10.1074/jbc.M009093200. [DOI] [PubMed] [Google Scholar]
- Khalil PN, Neuhof C, Huss R, Pollhammer M, Khalil MN, Neuhof H, Fritz H, Siebeck M. Calpain inhibition reduces infarct size and improves global hemodynamics and left ventricular contractility in a porcine myocardial ischemia/reperfusion model. Eur J Pharmacol. 2005;528:124–131. doi: 10.1016/j.ejphar.2005.10.032. [DOI] [PubMed] [Google Scholar]
- Kim D, Nguyen MD, Dobbin MM, Fischer A, Sananbenesi F, Rodgers JT, Delalle I, Baur JA, Sui G, Armour SM, Puigserver P, Sinclair DA, Tsai LH. SIRT1 deacetylase protects against neurodegeneration in models for Alzheimer’s disease and amyotrophic lateral sclerosis. EMBO J. 2007a;26:3169–3179. doi: 10.1038/sj.emboj.7601758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim EJ, Kho JH, Kang MR, Um SJ. Active regulator of SIRT1 cooperates with SIRT1 and facilitates suppression of p53 activity. Mol Cell. 2007b;28:277–290. doi: 10.1016/j.molcel.2007.08.030. [DOI] [PubMed] [Google Scholar]
- Kim J, Lee JH, Iyer VR. Global identification of Myc target genes reveals its direct role in mitochondrial biogenesis and its E-box usage in vivo. PLoS One. 2008;3:e1798. doi: 10.1371/journal.pone.0001798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Shrago E, Elson CE. Age-related changes in respiration coupled to phosphorylation. II. Cardiac mitochondria. Mech Ageing Dev. 1988;46:279–290. doi: 10.1016/0047-6374(88)90130-3. [DOI] [PubMed] [Google Scholar]
- Kim JW, Tchernyshyov I, Semenza GL, Dang CV. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 2006;3:177–185. doi: 10.1016/j.cmet.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Kim TH, Vemuganti R. Effect of sex and age interactions on functional outcome after stroke. CNS Neurosci Ther. 2015;21:327–336. doi: 10.1111/cns.12346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura S, Zhang GX, Nishiyama A, Shokoji T, Yao L, Fan YY, Rahman M, Abe Y. Mitochondria-derived reactive oxygen species and vascular MAP kinases: comparison of angiotensin II and diazoxide. Hypertension. 2005;45:438–444. doi: 10.1161/01.HYP.0000157169.27818.ae. [DOI] [PubMed] [Google Scholar]
- Kimura Y, Kimura H. Hydrogen sulfide protects neurons from oxidative stress. FASEB J. 2004;18:1165–1167. doi: 10.1096/fj.04-1815fje. [DOI] [PubMed] [Google Scholar]
- King RW, Plewa MC, Buderer NM, Knotts FB. Shock index as a marker for significant injury in trauma patients. Acad Emerg Med. 1996;3:1041–1045. doi: 10.1111/j.1553-2712.1996.tb03351.x. [DOI] [PubMed] [Google Scholar]
- Kireev RA, Cuesta S, Ibarrola C, Bela T, Moreno Gonzalez E, Vara E, Tresguerres JA. Age-related differences in hepatic ischemia/reperfusion: gene activation, liver injury, and protective effect of melatonin. J Surg Res. 2012;178:922–934. doi: 10.1016/j.jss.2012.04.060. [DOI] [PubMed] [Google Scholar]
- Klar AJ, Fogel S, Macleod K. MAR1-a Regulator of the HMa and HMalpha Loci in SACCHAROMYCES CEREVISIAE. Genetics. 1979;93:37–50. doi: 10.1093/genetics/93.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korzick DH, Lancaster TS. Age-related differences in cardiac ischemia-reperfusion injury: effects of estrogen deficiency. Pflugers Arch. 2013;465:669–685. doi: 10.1007/s00424-013-1255-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowald A, Kirkwood TB. Mitochondrial mutations and aging: random drift is insufficient to explain the accumulation of mitochondrial deletion mutants in short-lived animals. Aging Cell. 2013;12:728–731. doi: 10.1111/acel.12098. [DOI] [PubMed] [Google Scholar]
- Kowald A, Kirkwood TB. Transcription could be the key to the selection advantage of mitochondrial deletion mutants in aging. Proc Natl Acad Sci U S A. 2014;111:2972–2977. doi: 10.1073/pnas.1314970111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyanagi M, Brandes RP, Haendeler J, Zeiher AM, Dimmeler S. Cell-to-cell connection of endothelial progenitor cells with cardiac myocytes by nanotubes: a novel mechanism for cell fate changes? Circ Res. 2005;96:1039–1041. doi: 10.1161/01.RES.0000168650.23479.0c. [DOI] [PubMed] [Google Scholar]
- Laemmle A, Lechleiter A, Roh V, Schwarz C, Portmann S, Furer C, Keogh A, Tschan MP, Candinas D, Vorburger SA, Stroka D. Inhibition of SIRT1 impairs the accumulation and transcriptional activity of HIF-1alpha protein under hypoxic conditions. PLoS One. 2012;7:e33433. doi: 10.1371/journal.pone.0033433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landry J, Sutton A, Tafrov ST, Heller RC, Stebbins J, Pillus L, Sternglanz R. The silencing protein SIR2 and its homologs are NAD-dependent protein deacetylases. Proc Natl Acad Sci U S A. 2000;97:5807–5811. doi: 10.1073/pnas.110148297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapointe J, Hekimi S. When a theory of aging ages badly. Cell Mol Life Sci. 2010;67:1–8. doi: 10.1007/s00018-009-0138-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Las G, Shirihai OS. Miro1: new wheels for transferring mitochondria. EMBO J. 2014;33:939–941. doi: 10.1002/embj.201488441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavu S, Boss O, Elliott PJ, Lambert PD. Sirtuins--novel therapeutic targets to treat age-associated diseases. Nat Rev Drug Discov. 2008;7:841–853. doi: 10.1038/nrd2665. [DOI] [PubMed] [Google Scholar]
- Lee C, Yen K, Cohen P. Humanin: a harbinger of mitochondrial-derived peptides? Trends Endocrinol Metab. 2013a;24:222–228. doi: 10.1016/j.tem.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee IH, Cao L, Mostoslavsky R, Lombard DB, Liu J, Bruns NE, Tsokos M, Alt FW, Finkel T. A role for the NAD-dependent deacetylase Sirt1 in the regulation of autophagy. Proc Natl Acad Sci U S A. 2008;105:3374–3379. doi: 10.1073/pnas.0712145105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee OH, Kim J, Kim JM, Lee H, Kim EH, Bae SK, Choi Y, Nam HS, Heo JH. Decreased expression of sirtuin 6 is associated with release of high mobility group box-1 after cerebral ischemia. Biochem Biophys Res Commun. 2013b;438:388–394. doi: 10.1016/j.bbrc.2013.07.085. [DOI] [PubMed] [Google Scholar]
- Lee TH, Jung CH, Lee DH. Neuroprotective effects of Schisandrin B against transient focal cerebral ischemia in Sprague-Dawley rats. Food Chem Toxicol. 2012;50:4239–4245. doi: 10.1016/j.fct.2012.08.047. [DOI] [PubMed] [Google Scholar]
- Leiser SF, Kaeberlein M. The hypoxia-inducible factor HIF-1 functions as both a positive and negative modulator of aging. Biol Chem. 2010;391:1131–1137. doi: 10.1515/BC.2010.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leite MR, Cechella JL, Mantovani AC, Duarte MM, Nogueira CW, Zeni G. Swimming exercise and diphenyl diselenide-supplemented diet affect the serum levels of pro- and anti-inflammatory cytokines differently depending on the age of rats. Cytokine. 2015;71:119–123. doi: 10.1016/j.cyto.2014.09.006. [DOI] [PubMed] [Google Scholar]
- Lemasters JJ, Theruvath TP, Zhong Z, Nieminen AL. Mitochondrial calcium and the permeability transition in cell death. Biochim Biophys Acta. 2009;1787:1395–1401. doi: 10.1016/j.bbabio.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesnefsky EJ, Gallo DS, Ye J, Whittingham TS, Lust WD. Aging increases ischemia-reperfusion injury in the isolated, buffer-perfused heart. J Lab Clin Med. 1994;124:843–851. [PubMed] [Google Scholar]
- Lesnefsky EJ, Minkler P, Hoppel CL. Enhanced modification of cardiolipin during ischemia in the aged heart. J Mol Cell Cardiol. 2009;46:1008–1015. doi: 10.1016/j.yjmcc.2009.03.007. [DOI] [PubMed] [Google Scholar]
- Lesniewski LA, Connell ML, Durrant JR, Folian BJ, Anderson MC, Donato AJ, Seals DR. B6D2F1 Mice are a suitable model of oxidative stress-mediated impaired endothelium-dependent dilation with aging. J Gerontol A Biol Sci Med Sci. 2009;64:9–20. doi: 10.1093/gerona/gln049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Wang Y, Zeller KI, Potter JJ, Wonsey DR, O’Donnell KA, Kim JW, Yustein JT, Lee LA, Dang CV. Myc stimulates nuclearly encoded mitochondrial genes and mitochondrial biogenesis. Mol Cell Biol. 2005;25:6225–6234. doi: 10.1128/MCB.25.14.6225-6234.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Yan Z, Zhu J, Yang J, He J. Neuroprotective effects of resveratrol on ischemic injury mediated by improving brain energy metabolism and alleviating oxidative stress in rats. Neuropharmacology. 2011a;60:252–258. doi: 10.1016/j.neuropharm.2010.09.005. [DOI] [PubMed] [Google Scholar]
- Li N, Kong X, Ye R, Yang Q, Han J, Xiong L. Age-related differences in experimental stroke: possible involvement of mitochondrial dysfunction and oxidative damage. Rejuvenation Res. 2011b;14:261–273. doi: 10.1089/rej.2010.1115. [DOI] [PubMed] [Google Scholar]
- Li X, Zhang Y, Yeung SC, Liang Y, Liang X, Ding Y, Ip MS, Tse HF, Mak JC, Lian Q. Mitochondrial transfer of induced pluripotent stem cell-derived mesenchymal stem cells to airway epithelial cells attenuates cigarette smoke-induced damage. Am J Respir Cell Mol Biol. 2014;51:455–465. doi: 10.1165/rcmb.2013-0529OC. [DOI] [PubMed] [Google Scholar]
- Li XJ, Zhang LM, Gu J, Zhang AZ, Sun FY. Melatonin decreases production of hydroxyl radical during cerebral ischemia-reperfusion. Zhongguo Yao Li Xue Bao. 1997;18:394–396. [PubMed] [Google Scholar]
- Li Z, Pang L, Fang F, Zhang G, Zhang J, Xie M, Wang L. Resveratrol attenuates brain damage in a rat model of focal cerebral ischemia via up-regulation of hippocampal Bcl-2. Brain Res. 2012;1450:116–124. doi: 10.1016/j.brainres.2012.02.019. [DOI] [PubMed] [Google Scholar]
- Lim JH, Lee YM, Chun YS, Chen J, Kim JE, Park JW. Sirtuin 1 modulates cellular responses to hypoxia by deacetylating hypoxia-inducible factor 1alpha. Mol Cell. 2010;38:864–878. doi: 10.1016/j.molcel.2010.05.023. [DOI] [PubMed] [Google Scholar]
- Lin AL, Poteet E, Du F, Gourav RC, Liu R, Wen Y, Bresnen A, Huang S, Fox PT, Yang SH, Duong TQ. Methylene blue as a cerebral metabolic and hemodynamic enhancer. PLoS One. 2012;7:e46585. doi: 10.1371/journal.pone.0046585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin MY, Sheng ZH. Regulation of mitochondrial transport in neurons. Exp Cell Res. 2015;334:35–44. doi: 10.1016/j.yexcr.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SJ, Kaeberlein M, Andalis AA, Sturtz LA, Defossez PA, Culotta VC, Fink GR, Guarente L. Calorie restriction extends Saccharomyces cerevisiae lifespan by increasing respiration. Nature. 2002;418:344–348. doi: 10.1038/nature00829. [DOI] [PubMed] [Google Scholar]
- Ling X, Zhang LM, Lu SD, Li XJ, Sun FY. Protective effect of melatonin on injuried cerebral neurons is associated with bcl-2 protein over-expression. Zhongguo Yao Li Xue Bao. 1999;20:409–414. [PubMed] [Google Scholar]
- Liou CM, Yang AL, Kuo CH, Tin H, Huang CY, Lee SD. Effects of 17beta-estradiol on cardiac apoptosis in ovariectomized rats. Cell Biochem Funct. 2010;28:521–528. doi: 10.1002/cbf.1687. [DOI] [PubMed] [Google Scholar]
- Liu AJ, Guo JM, Liu W, Su FY, Zhai QW, Mehta JL, Wang WZ, Su DF. Involvement of arterial baroreflex in the protective effect of dietary restriction against stroke. J Cereb Blood Flow Metab. 2013a;33:906–913. doi: 10.1038/jcbfm.2013.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Che W, Xue J, Zheng C, Tang K, Zhang J, Wen J, Xu Y. SIRT4 prevents hypoxia-induced apoptosis in H9c2 cardiomyoblast cells. Cell Physiol Biochem. 2013b;32:655–662. doi: 10.1159/000354469. [DOI] [PubMed] [Google Scholar]
- Liu B, Che W, Zheng C, Liu W, Wen J, Fu H, Tang K, Zhang J, Xu Y. SIRT5: a safeguard against oxidative stress-induced apoptosis in cardiomyocytes. Cell Physiol Biochem. 2013c;32:1050–1059. doi: 10.1159/000354505. [DOI] [PubMed] [Google Scholar]
- Liu TF, McCall CE. Deacetylation by SIRT1 Reprograms Inflammation and Cancer. Genes Cancer. 2013;4:135–147. doi: 10.1177/1947601913476948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowicka E, Beltowski J. Hydrogen sulfide (H2S) - the third gas of interest for pharmacologists. Pharmacol Rep. 2007;59:4–24. [PubMed] [Google Scholar]
- Lu KT, Chiou RY, Chen LG, Chen MH, Tseng WT, Hsieh HT, Yang YL. Neuroprotective effects of resveratrol on cerebral ischemia-induced neuron loss mediated by free radical scavenging and cerebral blood flow elevation. J Agric Food Chem. 2006;54:3126–3131. doi: 10.1021/jf053011q. [DOI] [PubMed] [Google Scholar]
- Lu TM, Tsai JY, Chen YC, Huang CY, Hsu HL, Weng CF, Shih CC, Hsu CP. Downregulation of Sirt1 as aging change in advanced heart failure. J Biomed Sci. 2014;21:57. doi: 10.1186/1423-0127-21-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund DD, Chu Y, Miller JD, Heistad DD. Protective effect of extracellular superoxide dismutase on endothelial function during aging. Am J Physiol Heart Circ Physiol. 2009;296:H1920–1925. doi: 10.1152/ajpheart.01342.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y, Yang X, Zhao S, Wei C, Yin Y, Liu T, Jiang S, Xie J, Wan X, Mao M, Wu J. Hydrogen sulfide prevents OGD/R-induced apoptosis via improving mitochondrial dysfunction and suppressing an ROS-mediated caspase-3 pathway in cortical neurons. Neurochem Int. 2013;63:826–831. doi: 10.1016/j.neuint.2013.06.004. [DOI] [PubMed] [Google Scholar]
- Lynn EG, McLeod CJ, Gordon JP, Bao J, Sack MN. SIRT2 is a negative regulator of anoxia-reoxygenation tolerance via regulation of 14-3-3 zeta and BAD in H9c2 cells. FEBS Lett. 2008;582:2857–2862. doi: 10.1016/j.febslet.2008.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H, Cai Q, Lu W, Sheng ZH, Mochida S. KIF5B motor adaptor syntabulin maintains synaptic transmission in sympathetic neurons. J Neurosci. 2009;29:13019–13029. doi: 10.1523/JNEUROSCI.2517-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Wei M, Wang Q, Li J, Wang H, Liu W, Lacefield JC, Greer PA, Karmazyn M, Fan GC, Peng T. Deficiency of Capn4 gene inhibits nuclear factor-kappaB (NF-kappaB) protein signaling/inflammation and reduces remodeling after myocardial infarction. J Biol Chem. 2012;287:27480–27489. doi: 10.1074/jbc.M112.358929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maksin-Matveev A, Kanfi Y, Hochhauser E, Isak A, Cohen HY, Shainberg A. Sirtuin 6 protects the heart from hypoxic damage. Exp Cell Res. 2015;330:81–90. doi: 10.1016/j.yexcr.2014.07.013. [DOI] [PubMed] [Google Scholar]
- Manev H, Uz T, Kharlamov A, Joo JY. Increased brain damage after stroke or excitotoxic seizures in melatonin-deficient rats. FASEB J. 1996;10:1546–1551. doi: 10.1096/fasebj.10.13.8940301. [DOI] [PubMed] [Google Scholar]
- Mariappan N, Elks CM, Fink B, Francis J. TNF-induced mitochondrial damage: a link between mitochondrial complex I activity and left ventricular dysfunction. Free Radic Biol Med. 2009;46:462–470. doi: 10.1016/j.freeradbiomed.2008.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzetti E, Csiszar A, Dutta D, Balagopal G, Calvani R, Leeuwenburgh C. Role of mitochondrial dysfunction and altered autophagy in cardiovascular aging and disease: from mechanisms to therapeutics. Am J Physiol Heart Circ Physiol. 2013;305:H459–476. doi: 10.1152/ajpheart.00936.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Culmsee C, Yu ZF. Apoptotic and antiapoptotic mechanisms in stroke. Cell Tissue Res. 2000;301:173–187. doi: 10.1007/s004419900154. [DOI] [PubMed] [Google Scholar]
- Mayhan WG, Arrick DM, Sharpe GM, Sun H. Age-related alterations in reactivity of cerebral arterioles: role of oxidative stress. Microcirculation. 2008;15:225–236. doi: 10.1080/10739680701641421. [DOI] [PubMed] [Google Scholar]
- Mayo JC, Sainz RM, Tan DX, Hardeland R, Leon J, Rodriguez C, Reiter RJ. Anti-inflammatory actions of melatonin and its metabolites, N1-acetyl-N2-formyl-5-methoxykynuramine (AFMK) and N1-acetyl-5-methoxykynuramine (AMK), in macrophages. J Neuroimmunol. 2005;165:139–149. doi: 10.1016/j.jneuroim.2005.05.002. [DOI] [PubMed] [Google Scholar]
- McDonald MC, Zacharowski K, Bowes J, Cuzzocrea S, Thiemermann C. Tempol reduces infarct size in rodent models of regional myocardial ischemia and reperfusion. Free Radic Biol Med. 1999;27:493–503. doi: 10.1016/s0891-5849(99)00100-8. [DOI] [PubMed] [Google Scholar]
- McManus MJ, Murphy MP, Franklin JL. Mitochondria-derived reactive oxygen species mediate caspase-dependent and -independent neuronal deaths. Mol Cell Neurosci. 2014;63:13–23. doi: 10.1016/j.mcn.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mees ST, Gwinner M, Marx K, Faendrich F, Schroeder J, Haier J, Kahlke V. Influence of sex and age on morphological organ damage after hemorrhagic shock. Shock. 2008;29:670–674. doi: 10.1097/shk.0b013e31815c3ea0. [DOI] [PubMed] [Google Scholar]
- Meli E, Pangallo M, Baronti R, Chiarugi A, Cozzi A, Pellegrini-Giampietro DE, Moroni F. Poly(ADP-ribose) polymerase as a key player in excitotoxicity and post-ischemic brain damage. Toxicol Lett. 2003;139:153–162. doi: 10.1016/s0378-4274(02)00429-0. [DOI] [PubMed] [Google Scholar]
- Menghini R, Casagrande V, Cardellini M, Martelli E, Terrinoni A, Amati F, Vasa-Nicotera M, Ippoliti A, Novelli G, Melino G, Lauro R, Federici M. MicroRNA 217 modulates endothelial cell senescence via silent information regulator 1. Circulation. 2009;120:1524–1532. doi: 10.1161/CIRCULATIONAHA.109.864629. [DOI] [PubMed] [Google Scholar]
- Mercadier JJ, Lompre AM, Wisnewsky C, Samuel JL, Bercovici J, Swynghedauw B, Schwartz K. Myosin isoenzyme changes in several models of rat cardiac hypertrophy. Circ Res. 1981;49:525–532. doi: 10.1161/01.res.49.2.525. [DOI] [PubMed] [Google Scholar]
- Michishita E, McCord RA, Berber E, Kioi M, Padilla-Nash H, Damian M, Cheung P, Kusumoto R, Kawahara TL, Barrett JC, Chang HY, Bohr VA, Ried T, Gozani O, Chua KF. SIRT6 is a histone H3 lysine 9 deacetylase that modulates telomeric chromatin. Nature. 2008;452:492–496. doi: 10.1038/nature06736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michishita E, Park JY, Burneskis JM, Barrett JC, Horikawa I. Evolutionarily conserved and nonconserved cellular localizations and functions of human SIRT proteins. Mol Biol Cell. 2005;16:4623–4635. doi: 10.1091/mbc.E05-01-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KE, Sheetz MP. Axonal mitochondrial transport and potential are correlated. J Cell Sci. 2004;117:2791–2804. doi: 10.1242/jcs.01130. [DOI] [PubMed] [Google Scholar]
- Miller RA, Harrison DE, Astle CM, Baur JA, Boyd AR, de Cabo R, Fernandez E, Flurkey K, Javors MA, Nelson JF, Orihuela CJ, Pletcher S, Sharp ZD, Sinclair D, Starnes JW, Wilkinson JE, Nadon NL, Strong R. Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice. J Gerontol A Biol Sci Med Sci. 2011;66:191–201. doi: 10.1093/gerona/glq178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JB, Xavier S, DeLuca AM, Sowers AL, Cook JA, Krishna MC, Hahn SM, Russo A. A low molecular weight antioxidant decreases weight and lowers tumor incidence. Free Radic Biol Med. 2003;34:93–102. doi: 10.1016/s0891-5849(02)01193-0. [DOI] [PubMed] [Google Scholar]
- Mitchell P. Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature. 1961;191:144–148. doi: 10.1038/191144a0. [DOI] [PubMed] [Google Scholar]
- Mitchell SJ, Martin-Montalvo A, Mercken EM, Palacios HH, Ward TM, Abulwerdi G, Minor RK, Vlasuk GP, Ellis JL, Sinclair DA, Dawson J, Allison DB, Zhang Y, Becker KG, Bernier M, de Cabo R. The SIRT1 activator SRT1720 extends lifespan and improves health of mice fed a standard diet. Cell Rep. 2014;6:836–843. doi: 10.1016/j.celrep.2014.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooradian AD. Effect of aging on the blood-brain barrier. Neurobiol Aging. 1988;9:31–39. doi: 10.1016/s0197-4580(88)80013-7. [DOI] [PubMed] [Google Scholar]
- Morselli E, Maiuri MC, Markaki M, Megalou E, Pasparaki A, Palikaras K, Criollo A, Galluzzi L, Malik SA, Vitale I, Michaud M, Madeo F, Tavernarakis N, Kroemer G. The life span-prolonging effect of sirtuin-1 is mediated by autophagy. Autophagy. 2010;6:186–188. doi: 10.4161/auto.6.1.10817. [DOI] [PubMed] [Google Scholar]
- Mota-Filipe H, McDonald MC, Cuzzocrea S, Thiemermann C. A membrane-permeable radical scavenger reduces the organ injury in hemorrhagic shock. Shock. 1999;12:255–261. doi: 10.1097/00024382-199910000-00002. [DOI] [PubMed] [Google Scholar]
- Motta MC, Divecha N, Lemieux M, Kamel C, Chen D, Gu W, Bultsma Y, McBurney M, Guarente L. Mammalian SIRT1 represses forkhead transcription factors. Cell. 2004;116:551–563. doi: 10.1016/s0092-8674(04)00126-6. [DOI] [PubMed] [Google Scholar]
- Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, McGuire DK, Mohler ER, 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, Turner MB American Heart Association Statistics C., Stroke Statistics S. Heart disease and stroke statistics--2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay P, Horvath B, Zsengeller Z, Batkai S, Cao Z, Kechrid M, Holovac E, Erdelyi K, Tanchian G, Liaudet L, Stillman IE, Joseph J, Kalyanaraman B, Pacher P. Mitochondrial reactive oxygen species generation triggers inflammatory response and tissue injury associated with hepatic ischemia-reperfusion: therapeutic potential of mitochondrially targeted antioxidants. Free Radic Biol Med. 2012;53:1123–1138. doi: 10.1016/j.freeradbiomed.2012.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy AN, Fiskum G, Beal MF. Mitochondria in neurodegeneration: bioenergetic function in cell life and death. J Cereb Blood Flow Metab. 1999;19:231–245. doi: 10.1097/00004647-199903000-00001. [DOI] [PubMed] [Google Scholar]
- Murphy E, Steenbergen C. Mechanisms underlying acute protection from cardiac ischemia-reperfusion injury. Physiol Rev. 2008;88:581–609. doi: 10.1152/physrev.00024.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy MP, Echtay KS, Blaikie FH, Asin-Cayuela J, Cocheme HM, Green K, Buckingham JA, Taylor ER, Hurrell F, Hughes G, Miwa S, Cooper CE, Svistunenko DA, Smith RA, Brand MD. Superoxide activates uncoupling proteins by generating carbon-centered radicals and initiating lipid peroxidation: studies using a mitochondria-targeted spin trap derived from alpha-phenyl-N-tert-butylnitrone. J Biol Chem. 2003;278:48534–48545. doi: 10.1074/jbc.M308529200. [DOI] [PubMed] [Google Scholar]
- Nadtochiy SM, Redman E, Rahman I, Brookes PS. Lysine deacetylation in ischaemic preconditioning: the role of SIRT1. Cardiovasc Res. 2011a;89:643–649. doi: 10.1093/cvr/cvq287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadtochiy SM, Yao H, McBurney MW, Gu W, Guarente L, Rahman I, Brookes PS. SIRT1-mediated acute cardioprotection. Am J Physiol Heart Circ Physiol. 2011b;301:H1506–1512. doi: 10.1152/ajpheart.00587.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasrin N, Kaushik VK, Fortier E, Wall D, Pearson KJ, de Cabo R, Bordone L. JNK1 phosphorylates SIRT1 and promotes its enzymatic activity. PLoS One. 2009;4:e8414. doi: 10.1371/journal.pone.0008414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath R, Davis M, Probert AW, Kupina NC, Ren X, Schielke GP, Wang KK. Processing of cdk5 activator p35 to its truncated form (p25) by calpain in acutely injured neuronal cells. Biochem Biophys Res Commun. 2000;274:16–21. doi: 10.1006/bbrc.2000.3070. [DOI] [PubMed] [Google Scholar]
- Nelson HD, Humphrey LL, Nygren P, Teutsch SM, Allan JD. Postmenopausal hormone replacement therapy: scientific review. JAMA. 2002;288:872–881. doi: 10.1001/jama.288.7.872. [DOI] [PubMed] [Google Scholar]
- Nemoto S, Fergusson MM, Finkel T. SIRT1 functionally interacts with the metabolic regulator and transcriptional coactivator PGC-1{alpha} J Biol Chem. 2005;280:16456–16460. doi: 10.1074/jbc.M501485200. [DOI] [PubMed] [Google Scholar]
- Neuhof C, Fabiunk V, Speth M, Moller A, Fritz F, Tillmanns H, Neuhof H, Erdogan A. Reduction of myocardial infarction by postischemic administration of the calpain inhibitor A-705253 in comparison to the Na(+)/H(+) exchange inhibitor Cariporide in isolated perfused rabbit hearts. Biol Chem. 2008;389:1505–1512. doi: 10.1515/BC.2008.172. [DOI] [PubMed] [Google Scholar]
- Neuhof C, Fabiunke V, Deibele K, Speth M, Moller A, Lubisch W, Fritz H, Tillmanns H, Neuhof H. Reduction of myocardial infarction by calpain inhibitors A-705239 and A-705253 in isolated perfused rabbit hearts. Biol Chem. 2004;385:1077–1082. doi: 10.1515/BC.2004.139. [DOI] [PubMed] [Google Scholar]
- Neuhof C, Gotte O, Trumbeckaite S, Attenberger M, Kuzkaya N, Gellerich F, Moller A, Lubisch W, Speth M, Tillmanns H, Neuhof H. A novel water-soluble and cell-permeable calpain inhibitor protects myocardial and mitochondrial function in postischemic reperfusion. Biol Chem. 2003;384:1597–1603. doi: 10.1515/BC.2003.177. [DOI] [PubMed] [Google Scholar]
- Neuhof C, Neuhof H. Calpain system and its involvement in myocardial ischemia and reperfusion injury. World J Cardiol. 2014;6:638–652. doi: 10.4330/wjc.v6.i7.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng LF, Gruber J, Cheah IK, Goo CK, Cheong WF, Shui G, Sit KP, Wenk MR, Halliwell B. The mitochondria-targeted antioxidant MitoQ extends lifespan and improves healthspan of a transgenic Caenorhabditis elegans model of Alzheimer disease. Free Radic Biol Med. 2014;71:390–401. doi: 10.1016/j.freeradbiomed.2014.03.003. [DOI] [PubMed] [Google Scholar]
- Nicolini G, Rigolio R, Miloso M, Bertelli AA, Tredici G. Anti-apoptotic effect of trans-resveratrol on paclitaxel-induced apoptosis in the human neuroblastoma SH-SY5Y cell line. Neurosci Lett. 2001;302:41–44. doi: 10.1016/s0304-3940(01)01654-8. [DOI] [PubMed] [Google Scholar]
- Nohl H, Kramer R. Molecular basis of age-dependent changes in the activity of adenine nucleotide translocase. Mech Ageing Dev. 1980;14:137–144. doi: 10.1016/0047-6374(80)90112-8. [DOI] [PubMed] [Google Scholar]
- Nohl H, Staniek K, Gille L. Imbalance of oxygen activation and energy metabolism as a consequence or mediator of aging. Exp Gerontol. 1997;32:485–500. doi: 10.1016/s0531-5565(96)00169-6. [DOI] [PubMed] [Google Scholar]
- Ohsawa S, Miura M. Caspase-mediated changes in Sir2alpha during apoptosis. FEBS Lett. 2006;580:5875–5879. doi: 10.1016/j.febslet.2006.09.051. [DOI] [PubMed] [Google Scholar]
- Ohtsuji M, Katsuoka F, Kobayashi A, Aburatani H, Hayes JD, Yamamoto M. Nrf1 and Nrf2 play distinct roles in activation of antioxidant response element-dependent genes. J Biol Chem. 2008;283:33554–33562. doi: 10.1074/jbc.M804597200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onodera H, Iijima K, Kogure K. Mononucleotide metabolism in the rat brain after transient ischemia. J Neurochem. 1986;46:1704–1710. doi: 10.1111/j.1471-4159.1986.tb08487.x. [DOI] [PubMed] [Google Scholar]
- Onyszchuk G, He YY, Berman NE, Brooks WM. Detrimental effects of aging on outcome from traumatic brain injury: a behavioral, magnetic resonance imaging, and histological study in mice. J Neurotrauma. 2008;25:153–171. doi: 10.1089/neu.2007.0430. [DOI] [PubMed] [Google Scholar]
- Palacios HH, Yendluri BB, Parvathaneni K, Shadlinski VB, Obrenovich ME, Leszek J, Gokhman D, Gasiorowski K, Bragin V, Aliev G. Mitochondrion-specific antioxidants as drug treatments for Alzheimer disease. CNS Neurol Disord Drug Targets. 2011;10:149–162. doi: 10.2174/187152711794480474. [DOI] [PubMed] [Google Scholar]
- Palade GE. An electron microscope study of the mitochondrial structure. J Histochem Cytochem. 1953;1:188–211. doi: 10.1177/1.4.188. [DOI] [PubMed] [Google Scholar]
- Papandreou I, Cairns RA, Fontana L, Lim AL, Denko NC. HIF-1 mediates adaptation to hypoxia by actively downregulating mitochondrial oxygen consumption. Cell Metab. 2006;3:187–197. doi: 10.1016/j.cmet.2006.01.012. [DOI] [PubMed] [Google Scholar]
- Paradies G, Ruggiero FM. Age-related changes in the activity of the pyruvate carrier and in the lipid composition in rat-heart mitochondria. Biochim Biophys Acta. 1990;1016:207–212. doi: 10.1016/0005-2728(90)90060-h. [DOI] [PubMed] [Google Scholar]
- Paradies G, Ruggiero FM. Effect of aging on the activity of the phosphate carrier and on the lipid composition in rat liver mitochondria. Arch Biochem Biophys. 1991;284:332–337. doi: 10.1016/0003-9861(91)90304-2. [DOI] [PubMed] [Google Scholar]
- Park SJ, Ahmad F, Philp A, Baar K, Williams T, Luo H, Ke H, Rehmann H, Taussig R, Brown AL, Kim MK, Beaven MA, Burgin AB, Manganiello V, Chung JH. Resveratrol ameliorates aging-related metabolic phenotypes by inhibiting cAMP phosphodiesterases. Cell. 2012;148:421–433. doi: 10.1016/j.cell.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel NS, Chatterjee PK, Chatterjee BE, Cuzzocrea S, Serraino I, Brown PA, Stewart KN, Mota-Filipe H, Thiemermann C. TEMPONE reduces renal dysfunction and injury mediated by oxidative stress of the rat kidney. Free Radic Biol Med. 2002;33:1575–1589. doi: 10.1016/s0891-5849(02)01116-4. [DOI] [PubMed] [Google Scholar]
- Patel SP, Sullivan PG, Pandya JD, Rabchevsky AG. Differential effects of the mitochondrial uncoupling agent, 2,4-dinitrophenol, or the nitroxide antioxidant, Tempol, on synaptic or nonsynaptic mitochondria after spinal cord injury. J Neurosci Res. 2009;87:130–140. doi: 10.1002/jnr.21814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne JA, Reckelhoff JF, Khalil RA. Role of oxidative stress in age-related reduction of NO-cGMP-mediated vascular relaxation in SHR. Am J Physiol Regul Integr Comp Physiol. 2003;285:R542–551. doi: 10.1152/ajpregu.00056.2003. [DOI] [PubMed] [Google Scholar]
- Pearce FJ, Connett RJ, Drucker WR. Phase-related changes in tissue energy reserves during hemorrhagic shock. J Surg Res. 1985;39:390–398. doi: 10.1016/0022-4804(85)90092-7. [DOI] [PubMed] [Google Scholar]
- Pearson KJ, Baur JA, Lewis KN, Peshkin L, Price NL, Labinskyy N, Swindell WR, Kamara D, Minor RK, Perez E, Jamieson HA, Zhang Y, Dunn SR, Sharma K, Pleshko N, Woollett LA, Csiszar A, Ikeno Y, Le Couteur D, Elliott PJ, Becker KG, Navas P, Ingram DK, Wolf NS, Ungvari Z, Sinclair DA, de Cabo R. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab. 2008;8:157–168. doi: 10.1016/j.cmet.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pervaiz S. Resveratrol: from grapevines to mammalian biology. FASEB J. 2003;17:1975–1985. doi: 10.1096/fj.03-0168rev. [DOI] [PubMed] [Google Scholar]
- Pervaiz S, Holme AL. Resveratrol: its biologic targets and functional activity. Antioxid Redox Signal. 2009;11:2851–2897. doi: 10.1089/ars.2008.2412. [DOI] [PubMed] [Google Scholar]
- Picard F, Kurtev M, Chung N, Topark-Ngarm A, Senawong T, Machado De Oliveira R, Leid M, McBurney MW, Guarente L. Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-gamma. Nature. 2004;429:771–776. doi: 10.1038/nature02583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai JB, Chen M, Rajamohan SB, Samant S, Pillai VB, Gupta M, Gupta MP. Activation of SIRT1, a class III histone deacetylase, contributes to fructose feeding-mediated induction of the alpha-myosin heavy chain expression. Am J Physiol Heart Circ Physiol. 2008;294:H1388–1397. doi: 10.1152/ajpheart.01339.2007. [DOI] [PubMed] [Google Scholar]
- Plotnikov EY, Khryapenkova TG, Vasileva AK, Marey MV, Galkina SI, Isaev NK, Sheval EV, Polyakov VY, Sukhikh GT, Zorov DB. Cell-to-cell cross-talk between mesenchymal stem cells and cardiomyocytes in co-culture. J Cell Mol Med. 2008;12:1622–1631. doi: 10.1111/j.1582-4934.2007.00205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poeggeler B, Saarela S, Reiter RJ, Tan DX, Chen LD, Manchester LC, Barlow-Walden LR. Melatonin--a highly potent endogenous radical scavenger and electron donor: new aspects of the oxidation chemistry of this indole accessed in vitro. Ann N Y Acad Sci. 1994;738:419–420. doi: 10.1111/j.1749-6632.1994.tb21831.x. [DOI] [PubMed] [Google Scholar]
- Polster BM. AIF, reactive oxygen species, and neurodegeneration: a “complex” problem. Neurochem Int. 2013;62:695–702. doi: 10.1016/j.neuint.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porat-Shliom N, Chen Y, Tora M, Shitara A, Masedunskas A, Weigert R. In vivo tissue-wide synchronization of mitochondrial metabolic oscillations. Cell Rep. 2014;9:514–521. doi: 10.1016/j.celrep.2014.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter GA, Urciuoli WR, Brookes PS, Nadtochiy SM. SIRT3 deficiency exacerbates ischemia-reperfusion injury: implication for aged hearts. Am J Physiol Heart Circ Physiol. 2014;306:H1602–1609. doi: 10.1152/ajpheart.00027.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulose N, Raju R. Aging and injury: alterations in cellular energetics and organ function. Aging Dis. 2014;5:101–108. doi: 10.14336/AD.2014.0500101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulose N, Raju R. Sirtuin regulation in aging and injury. Biochim Biophys Acta. 2015;1852:2442–2455. doi: 10.1016/j.bbadis.2015.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell RD, Swet JH, Kennedy KL, Huynh TT, McKillop IH, Evans SL. Resveratrol attenuates hypoxic injury in a primary hepatocyte model of hemorrhagic shock and resuscitation. J Trauma Acute Care Surg. 2014;76:409–417. doi: 10.1097/TA.0000000000000096. [DOI] [PubMed] [Google Scholar]
- Powell RD, Swet JH, Kennedy KL, Huynh TT, Murphy MP, McKillop IH, Evans SL. MitoQ modulates oxidative stress and decreases inflammation following hemorrhage. J Trauma Acute Care Surg. 2015;78:573–579. doi: 10.1097/TA.0000000000000533. [DOI] [PubMed] [Google Scholar]
- Prentice RL. Postmenopausal hormone therapy and the risks of coronary heart disease, breast cancer, and stroke. Semin Reprod Med. 2014;32:419–425. doi: 10.1055/s-0034-1384624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price NL, Gomes AP, Ling AJ, Duarte FV, Martin-Montalvo A, North BJ, Agarwal B, Ye L, Ramadori G, Teodoro JS, Hubbard BP, Varela AT, Davis JG, Varamini B, Hafner A, Moaddel R, Rolo AP, Coppari R, Palmeira CM, de Cabo R, Baur JA, Sinclair DA. SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab. 2012;15:675–690. doi: 10.1016/j.cmet.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugazhenthi S, Nesterova A, Sable C, Heidenreich KA, Boxer LM, Heasley LE, Reusch JE. Akt/protein kinase B up-regulates Bcl-2 expression through cAMP-response element-binding protein. J Biol Chem. 2000;275:10761–10766. doi: 10.1074/jbc.275.15.10761. [DOI] [PubMed] [Google Scholar]
- Puigserver P, Spiegelman BM. Peroxisome proliferator-activated receptor-gamma coactivator 1 alpha (PGC-1 alpha): transcriptional coactivator and metabolic regulator. Endocr Rev. 2003;24:78–90. doi: 10.1210/er.2002-0012. [DOI] [PubMed] [Google Scholar]
- Pung YF, Rocic P, Murphy MP, Smith RA, Hafemeister J, Ohanyan V, Guarini G, Yin L, Chilian WM. Resolution of mitochondrial oxidative stress rescues coronary collateral growth in Zucker obese fatty rats. Arterioscler Thromb Vasc Biol. 2012;32:325–334. doi: 10.1161/ATVBAHA.111.241802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purushotham A, Schug TT, Xu Q, Surapureddi S, Guo X, Li X. Hepatocyte-specific deletion of SIRT1 alters fatty acid metabolism and results in hepatic steatosis and inflammation. Cell Metab. 2009;9:327–338. doi: 10.1016/j.cmet.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qabazard B, Ahmed S, Li L, Arlt VM, Moore PK, Sturzenbaum SR. C. elegans aging is modulated by hydrogen sulfide and the sulfhydrylase/cysteine synthase cysl-2. PLoS One. 2013;8:e80135. doi: 10.1371/journal.pone.0080135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qabazard B, Li L, Gruber J, Peh MT, Ng LF, Kumar SD, Rose P, Tan CH, Dymock BW, Wei F, Swain SC, Halliwell B, Sturzenbaum SR, Moore PK. Hydrogen sulfide is an endogenous regulator of aging in Caenorhabditis elegans. Antioxid Redox Signal. 2014;20:2621–2630. doi: 10.1089/ars.2013.5448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raju R, Jian B, Hubbard W, Chaudry I. The Mitoscriptome in Aging and Disease. Aging Dis. 2011;2:174–180. [PMC free article] [PubMed] [Google Scholar]
- Rambold AS, Kostelecky B, Elia N, Lippincott-Schwartz J. Tubular network formation protects mitochondria from autophagosomal degradation during nutrient starvation. Proc Natl Acad Sci U S A. 2011;108:10190–10195. doi: 10.1073/pnas.1107402108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey KM, Yoshino J, Brace CS, Abrassart D, Kobayashi Y, Marcheva B, Hong HK, Chong JL, Buhr ED, Lee C, Takahashi JS, Imai S, Bass J. Circadian clock feedback cycle through NAMPT-mediated NAD+ biosynthesis. Science. 2009;324:651–654. doi: 10.1126/science.1171641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rane S, He M, Sayed D, Vashistha H, Malhotra A, Sadoshima J, Vatner DE, Vatner SF, Abdellatif M. Downregulation of miR-199a derepresses hypoxia-inducible factor-1alpha and Sirtuin 1 and recapitulates hypoxia preconditioning in cardiac myocytes. Circ Res. 2009;104:879–886. doi: 10.1161/CIRCRESAHA.108.193102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raval AP, Dave KR, Perez-Pinzon MA. Resveratrol mimics ischemic preconditioning in the brain. J Cereb Blood Flow Metab. 2006;26:1141–1147. doi: 10.1038/sj.jcbfm.9600262. [DOI] [PubMed] [Google Scholar]
- Raval AP, Lin HW, Dave KR, Defazio RA, Della Morte D, Kim EJ, Perez-Pinzon MA. Resveratrol and ischemic preconditioning in the brain. Curr Med Chem. 2008;15:1545–1551. doi: 10.2174/092986708784638861. [DOI] [PubMed] [Google Scholar]
- Regnier-Vigouroux A. The mannose receptor in the brain. Int Rev Cytol. 2003;226:321–342. doi: 10.1016/s0074-7696(03)01006-4. [DOI] [PubMed] [Google Scholar]
- Reiter RJ, Guerrero JM, Garcia JJ, Acuna-Castroviejo D. Reactive oxygen intermediates, molecular damage, and aging. Relation to melatonin. Ann N Y Acad Sci. 1998;854:410–424. doi: 10.1111/j.1749-6632.1998.tb09920.x. [DOI] [PubMed] [Google Scholar]
- Renaud S, de Lorgeril M. Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet. 1992;339:1523–1526. doi: 10.1016/0140-6736(92)91277-f. [DOI] [PubMed] [Google Scholar]
- Riera CE, Dillin A. Tipping the metabolic scales towards increased longevity in mammals. Nat Cell Biol. 2015;17:196–203. doi: 10.1038/ncb3107. [DOI] [PubMed] [Google Scholar]
- Rine J, Herskowitz I. Four genes responsible for a position effect on expression from HML and HMR in Saccharomyces cerevisiae. Genetics. 1987;116:9–22. doi: 10.1093/genetics/116.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez C, Mayo JC, Sainz RM, Antolin I, Herrera F, Martin V, Reiter RJ. Regulation of antioxidant enzymes: a significant role for melatonin. J Pineal Res. 2004;36:1–9. doi: 10.1046/j.1600-079x.2003.00092.x. [DOI] [PubMed] [Google Scholar]
- Rogers RS, Bhattacharya J. When cells become organelle donors. Physiology (Bethesda) 2013;28:414–422. doi: 10.1152/physiol.00032.2013. [DOI] [PubMed] [Google Scholar]
- Rosamond W, Flegal K, Friday G, Furie K, Go A, Greenlund K, Haase N, Ho M, Howard V, Kissela B, Kittner S, Lloyd-Jones D, McDermott M, Meigs J, Moy C, Nichol G, O’Donnell CJ, Roger V, Rumsfeld J, Sorlie P, Steinberger J, Thom T, Wasserthiel-Smoller S, Hong Y American Heart Association Statistics C, Stroke Statistics S. Heart disease and stroke statistics--2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115:e69–171. doi: 10.1161/CIRCULATIONAHA.106.179918. [DOI] [PubMed] [Google Scholar]
- Rustom A, Saffrich R, Markovic I, Walther P, Gerdes HH. Nanotubular highways for intercellular organelle transport. Science. 2004;303:1007–1010. doi: 10.1126/science.1093133. [DOI] [PubMed] [Google Scholar]
- Ryou MG, Choudhury GR, Li W, Winters A, Yuan F, Liu R, Yang SH. Methylene blue-induced neuronal protective mechanism against hypoxia-reoxygenation stress. Neuroscience. 2015 doi: 10.1016/j.neuroscience.2015.05.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salceda S, Caro J. Hypoxia-inducible factor 1alpha (HIF-1alpha) protein is rapidly degraded by the ubiquitin-proteasome system under normoxic conditions. Its stabilization by hypoxia depends on redox-induced changes. J Biol Chem. 1997;272:22642–22647. doi: 10.1074/jbc.272.36.22642. [DOI] [PubMed] [Google Scholar]
- Saretzki G, Murphy MP, von Zglinicki T. MitoQ counteracts telomere shortening and elongates lifespan of fibroblasts under mild oxidative stress. Aging Cell. 2003;2:141–143. doi: 10.1046/j.1474-9728.2003.00040.x. [DOI] [PubMed] [Google Scholar]
- Sasaki T, Maier B, Koclega KD, Chruszcz M, Gluba W, Stukenberg PT, Minor W, Scrable H. Phosphorylation regulates SIRT1 function. PLoS One. 2008a;3:e4020. doi: 10.1371/journal.pone.0004020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki T, Unno K, Tahara S, Shimada A, Chiba Y, Hoshino M, Kaneko T. Age-related increase of superoxide generation in the brains of mammals and birds. Aging Cell. 2008b;7:459–469. doi: 10.1111/j.1474-9726.2008.00394.x. [DOI] [PubMed] [Google Scholar]
- Satoh A, Brace CS, Rensing N, Cliften P, Wozniak DF, Herzog ED, Yamada KA, Imai S. Sirt1 extends life span and delays aging in mice through the regulation of Nk2 homeobox 1 in the DMH and LH. Cell Metab. 2013;18:416–430. doi: 10.1016/j.cmet.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saver JL. Citicoline: update on a promising and widely available agent for neuroprotection and neurorepair. Rev Neurol Dis. 2008;5:167–177. [PubMed] [Google Scholar]
- Sazanov LA, Hinchliffe P. Structure of the hydrophilic domain of respiratory complex I from Thermus thermophilus. Science. 2006;311:1430–1436. doi: 10.1126/science.1123809. [DOI] [PubMed] [Google Scholar]
- Scarpulla RC. Nuclear control of respiratory chain expression by nuclear respiratory factors and PGC-1-related coactivator. Ann N Y Acad Sci. 2008;1147:321–334. doi: 10.1196/annals.1427.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmeisser S, Priebe S, Groth M, Monajembashi S, Hemmerich P, Guthke R, Platzer M, Ristow M. Neuronal ROS signaling rather than AMPK/sirtuin-mediated energy sensing links dietary restriction to lifespan extension. Mol Metab. 2013;2:92–102. doi: 10.1016/j.molmet.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonfeld P, Wieckowski MR, Lebiedzinska M, Wojtczak L. Mitochondrial fatty acid oxidation and oxidative stress: lack of reverse electron transfer-associated production of reactive oxygen species. Biochim Biophys Acta. 2010;1797:929–938. doi: 10.1016/j.bbabio.2010.01.010. [DOI] [PubMed] [Google Scholar]
- Sebastian D, Hernandez-Alvarez MI, Segales J, Sorianello E, Munoz JP, Sala D, Waget A, Liesa M, Paz JC, Gopalacharyulu P, Oresic M, Pich S, Burcelin R, Palacin M, Zorzano A. Mitofusin 2 (Mfn2) links mitochondrial and endoplasmic reticulum function with insulin signaling and is essential for normal glucose homeostasis. Proc Natl Acad Sci U S A. 2012;109:5523–5528. doi: 10.1073/pnas.1108220109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza GL. Transcriptional regulation by hypoxia-inducible factor 1 molecular mechanisms of oxygen homeostasis. Trends Cardiovasc Med. 1996;6:151–157. doi: 10.1016/1050-1738(96)00039-4. [DOI] [PubMed] [Google Scholar]
- Semenza GL. Hypoxia-inducible factor 1 (HIF-1) pathway. Sci STKE. 2007;2007:cm8. doi: 10.1126/stke.4072007cm8. [DOI] [PubMed] [Google Scholar]
- Semenza GL. Hypoxia-inducible factor 1: regulator of mitochondrial metabolism and mediator of ischemic preconditioning. Biochim Biophys Acta. 2011;1813:1263–1268. doi: 10.1016/j.bbamcr.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepodes B, Maio R, Pinto R, Marques C, Mendes-do-Vale J, McDonald MC, Thiemermann C, Mota-Filipe H. Tempol, an intracelullar free radical scavenger, reduces liver injury in hepatic ischemia-reperfusion in the rat. Transplant Proc. 2004;36:849–853. doi: 10.1016/j.transproceed.2004.03.050. [DOI] [PubMed] [Google Scholar]
- Shalwala M, Zhu SG, Das A, Salloum FN, Xi L, Kukreja RC. Sirtuin 1 (SIRT1) activation mediates sildenafil induced delayed cardioprotection against ischemia-reperfusion injury in mice. PLoS One. 2014;9:e86977. doi: 10.1371/journal.pone.0086977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao AW, Wu HJ, Chen S, Ammar AB, Zhang JM, Hong Y. Resveratrol attenuates early brain injury after subarachnoid hemorrhage through inhibition of NF-kappaB-dependent inflammatory/MMP-9 pathway. CNS Neurosci Ther. 2014;20:182–185. doi: 10.1111/cns.12194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Q, Du F, Huang S, Rodriguez P, Watts LT, Duong TQ. Neuroprotective efficacy of methylene blue in ischemic stroke: an MRI study. PLoS One. 2013;8:e79833. doi: 10.1371/journal.pone.0079833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin JA, Lee KE, Kim HS, Park EM. Acute resveratrol treatment modulates multiple signaling pathways in the ischemic brain. Neurochem Res. 2012;37:2686–2696. doi: 10.1007/s11064-012-0858-2. [DOI] [PubMed] [Google Scholar]
- Shinmura K, Tamaki K, Bolli R. Impact of 6-mo caloric restriction on myocardial ischemic tolerance: possible involvement of nitric oxide-dependent increase in nuclear Sirt1. Am J Physiol Heart Circ Physiol. 2008;295:H2348–2355. doi: 10.1152/ajpheart.00602.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinozaki S, Chang K, Sakai M, Shimizu N, Yamada M, Tanaka T, Nakazawa H, Ichinose F, Yamada Y, Ishigami A, Ito H, Ouchi Y, Starr ME, Saito H, Shimokado K, Stamler JS, Kaneki M. Inflammatory stimuli induce inhibitory S-nitrosylation of the deacetylase SIRT1 to increase acetylation and activation of p53 and p65. Sci Signal. 2014;7:ra106. doi: 10.1126/scisignal.2005375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simao F, Matte A, Matte C, Soares FM, Wyse AT, Netto CA, Salbego CG. Resveratrol prevents oxidative stress and inhibition of Na(+)K(+)-ATPase activity induced by transient global cerebral ischemia in rats. J Nutr Biochem. 2011;22:921–928. doi: 10.1016/j.jnutbio.2010.07.013. [DOI] [PubMed] [Google Scholar]
- Simpkins JW, Yang SH, Sarkar SN, Pearce V. Estrogen actions on mitochondria--physiological and pathological implications. Mol Cell Endocrinol. 2008;290:51–59. doi: 10.1016/j.mce.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims NR, Muyderman H. Mitochondria, oxidative metabolism and cell death in stroke. Biochim Biophys Acta. 2010;1802:80–91. doi: 10.1016/j.bbadis.2009.09.003. [DOI] [PubMed] [Google Scholar]
- Smith JS, Brachmann CB, Celic I, Kenna MA, Muhammad S, Starai VJ, Avalos JL, Escalante-Semerena JC, Grubmeyer C, Wolberger C, Boeke JD. A phylogenetically conserved NAD+-dependent protein deacetylase activity in the Sir2 protein family. Proc Natl Acad Sci U S A. 2000;97:6658–6663. doi: 10.1073/pnas.97.12.6658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RA, Porteous CM, Gane AM, Murphy MP. Delivery of bioactive molecules to mitochondria in vivo. Proc Natl Acad Sci U S A. 2003;100:5407–5412. doi: 10.1073/pnas.0931245100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow BJ, Rolfe FL, Lockhart MM, Frampton CM, O’Sullivan JD, Fung V, Smith RA, Murphy MP, Taylor KM Protect Study G. A double-blind, placebo-controlled study to assess the mitochondria-targeted antioxidant MitoQ as a disease-modifying therapy in Parkinson’s disease. Mov Disord. 2010;25:1670–1674. doi: 10.1002/mds.23148. [DOI] [PubMed] [Google Scholar]
- Solaini G, Baracca A, Lenaz G, Sgarbi G. Hypoxia and mitochondrial oxidative metabolism. Biochim Biophys Acta. 2010;1797:1171–1177. doi: 10.1016/j.bbabio.2010.02.011. [DOI] [PubMed] [Google Scholar]
- Spanier G, Xu H, Xia N, Tobias S, Deng S, Wojnowski L, Forstermann U, Li H. Resveratrol reduces endothelial oxidative stress by modulating the gene expression of superoxide dismutase 1 (SOD1), glutathione peroxidase 1 (GPx1) and NADPH oxidase subunit (Nox4) J Physiol Pharmacol. 2009;60(Suppl 4):111–116. [PubMed] [Google Scholar]
- Spees JL, Olson SD, Whitney MJ, Prockop DJ. Mitochondrial transfer between cells can rescue aerobic respiration. Proc Natl Acad Sci U S A. 2006;103:1283–1288. doi: 10.1073/pnas.0510511103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-Pierre J, Drori S, Uldry M, Silvaggi JM, Rhee J, Jager S, Handschin C, Zheng K, Lin J, Yang W, Simon DK, Bachoo R, Spiegelman BM. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell. 2006;127:397–408. doi: 10.1016/j.cell.2006.09.024. [DOI] [PubMed] [Google Scholar]
- Stowe DF, Camara AK. Mitochondrial reactive oxygen species production in excitable cells: modulators of mitochondrial and cell function. Antioxid Redox Signal. 2009;11:1373–1414. doi: 10.1089/ars.2008.2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong R, Miller RA, Astle CM, Baur JA, de Cabo R, Fernandez E, Guo W, Javors M, Kirkland JL, Nelson JF, Sinclair DA, Teter B, Williams D, Zaveri N, Nadon NL, Harrison DE. Evaluation of resveratrol, green tea extract, curcumin, oxaloacetic acid, and medium-chain triglyceride oil on life span of genetically heterogeneous mice. J Gerontol A Biol Sci Med Sci. 2013;68:6–16. doi: 10.1093/gerona/gls070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun AY, Wang Q, Simonyi A, Sun GY. Resveratrol as a therapeutic agent for neurodegenerative diseases. Mol Neurobiol. 2010;41:375–383. doi: 10.1007/s12035-010-8111-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D, Colello RJ, Daugherty WP, Kwon TH, McGinn MJ, Harvey HB, Bullock MR. Cell proliferation and neuronal differentiation in the dentate gyrus in juvenile and adult rats following traumatic brain injury. J Neurotrauma. 2005;22:95–105. doi: 10.1089/neu.2005.22.95. [DOI] [PubMed] [Google Scholar]
- Sun D, McGinn M, Hankins JE, Mays KM, Rolfe A, Colello RJ. Aging- and injury-related differential apoptotic response in the dentate gyrus of the hippocampus in rats following brain trauma. Front Aging Neurosci. 2013a;5:95. doi: 10.3389/fnagi.2013.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun T, Qiao H, Pan PY, Chen Y, Sheng ZH. Motile axonal mitochondria contribute to the variability of presynaptic strength. Cell Rep. 2013b;4:413–419. doi: 10.1016/j.celrep.2013.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzman R, Beard J. N.I.o. Aging, N.I.o. Health, W.H. Organization, editor . Global Health and Aging. 2011. [Google Scholar]
- Tani M, Suganuma Y, Hasegawa H, Shinmura K, Ebihara Y, Hayashi Y, Guo X, Takayama M. Decrease in ischemic tolerance with aging in isolated perfused Fischer 344 rat hearts: relation to increases in intracellular Na+ after ischemia. J Mol Cell Cardiol. 1997;29:3081–3089. doi: 10.1006/jmcc.1997.0533. [DOI] [PubMed] [Google Scholar]
- Tanno M, Sakamoto J, Miura T, Shimamoto K, Horio Y. Nucleocytoplasmic shuttling of the NAD+-dependent histone deacetylase SIRT1. J Biol Chem. 2007;282:6823–6832. doi: 10.1074/jbc.M609554200. [DOI] [PubMed] [Google Scholar]
- Tatarkova Z, Kuka S, Racay P, Lehotsky J, Dobrota D, Mistuna D, Kaplan P. Effects of aging on activities of mitochondrial electron transport chain complexes and oxidative damage in rat heart. Physiol Res. 2011;60:281–289. doi: 10.33549/physiolres.932019. [DOI] [PubMed] [Google Scholar]
- Tay AS, Hu LF, Lu M, Wong PT, Bian JS. Hydrogen sulfide protects neurons against hypoxic injury via stimulation of ATP-sensitive potassium channel/protein kinase C/extracellular signal-regulated kinase/heat shock protein 90 pathway. Neuroscience. 2010;167:277–286. doi: 10.1016/j.neuroscience.2010.02.006. [DOI] [PubMed] [Google Scholar]
- Tegla CA, Azimzadeh P, Andrian-Albescu M, Martin A, Cudrici CD, Trippe R, 3rd, Sugarman A, Chen H, Boodhoo D, Vlaicu SI, Royal W, 3rd, Bever C, Rus V, Rus H. SIRT1 is decreased during relapses in patients with multiple sclerosis. Exp Mol Pathol. 2014;96:139–148. doi: 10.1016/j.yexmp.2013.12.010. [DOI] [PubMed] [Google Scholar]
- Thompson HJ, McCormick WC, Kagan SH. Traumatic brain injury in older adults: epidemiology, outcomes, and future implications. J Am Geriatr Soc. 2006;54:1590–1595. doi: 10.1111/j.1532-5415.2006.00894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tissenbaum HA, Guarente L. Increased dosage of a sir-2 gene extends lifespan in Caenorhabditis elegans. Nature. 2001;410:227–230. doi: 10.1038/35065638. [DOI] [PubMed] [Google Scholar]
- Tokumitsu Y, Ui M. The incorporation of 32 P i into intramitochondrial ADP fraction dependent on the substrate-level phosphorylation. Biochim Biophys Acta. 1973;292:310–324. doi: 10.1016/0005-2728(73)90038-8. [DOI] [PubMed] [Google Scholar]
- Tomasello F, Messina A, Lartigue L, Schembri L, Medina C, Reina S, Thoraval D, Crouzet M, Ichas F, De Pinto V, De Giorgi F. Outer membrane VDAC1 controls permeability transition of the inner mitochondrial membrane in cellulo during stress-induced apoptosis. Cell Res. 2009;19:1363–1376. doi: 10.1038/cr.2009.98. [DOI] [PubMed] [Google Scholar]
- Tong C, Morrison A, Mattison S, Qian S, Bryniarski M, Rankin B, Wang J, Thomas DP, Li J. Impaired SIRT1 nucleocytoplasmic shuttling in the senescent heart during ischemic stress. FASEB J. 2013;27:4332–4342. doi: 10.1096/fj.12-216473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trumpower BL. The protonmotive Q cycle. Energy transduction by coupling of proton translocation to electron transfer by the cytochrome bc1 complex. J Biol Chem. 1990;265:11409–11412. [PubMed] [Google Scholar]
- Tsai SK, Hung LM, Fu YT, Cheng H, Nien MW, Liu HY, Zhang FB, Huang SS. Resveratrol neuroprotective effects during focal cerebral ischemia injury via nitric oxide mechanism in rats. J Vasc Surg. 2007;46:346–353. doi: 10.1016/j.jvs.2007.04.044. [DOI] [PubMed] [Google Scholar]
- Tsai YF, Liu FC, Lau YT, Yu HP. Role of Akt-dependent pathway in resveratrol-mediated cardioprotection after trauma-hemorrhage. J Surg Res. 2012;176:171–177. doi: 10.1016/j.jss.2011.05.039. [DOI] [PubMed] [Google Scholar]
- Tsuchiya M, Dang N, Kerr EO, Hu D, Steffen KK, Oakes JA, Kennedy BK, Kaeberlein M. Sirtuin-independent effects of nicotinamide on lifespan extension from calorie restriction in yeast. Aging Cell. 2006;5:505–514. doi: 10.1111/j.1474-9726.2006.00240.x. [DOI] [PubMed] [Google Scholar]
- Tsukihara T, Aoyama H, Yamashita E, Tomizaki T, Yamaguchi H, Shinzawa-Itoh K, Nakashima R, Yaono R, Yoshikawa S. The whole structure of the 13-subunit oxidized cytochrome c oxidase at 2.8 A. Science. 1996;272:1136–1144. doi: 10.1126/science.272.5265.1136. [DOI] [PubMed] [Google Scholar]
- Tummino PJ, Gafni A. A comparative study of succinate-supported respiration and ATP/ADP translocation in liver mitochondria from adult and old rats. Mech Ageing Dev. 1991;59:177–188. doi: 10.1016/0047-6374(91)90083-c. [DOI] [PubMed] [Google Scholar]
- Turrens JF. Mitochondrial formation of reactive oxygen species. J Physiol. 2003;552:335–344. doi: 10.1113/jphysiol.2003.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udenigwe CC, Ramprasath VR, Aluko RE, Jones PJ. Potential of resveratrol in anticancer and anti-inflammatory therapy. Nutr Rev. 2008;66:445–454. doi: 10.1111/j.1753-4887.2008.00076.x. [DOI] [PubMed] [Google Scholar]
- Udomphorn Y, Armstead WM, Vavilala MS. Cerebral blood flow and autoregulation after pediatric traumatic brain injury. Pediatr Neurol. 2008;38:225–234. doi: 10.1016/j.pediatrneurol.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaishnavi SN, Vlassenko AG, Rundle MM, Snyder AZ, Mintun MA, Raichle ME. Regional aerobic glycolysis in the human brain. Proc Natl Acad Sci U S A. 2010;107:17757–17762. doi: 10.1073/pnas.1010459107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaquero A, Scher M, Lee D, Erdjument-Bromage H, Tempst P, Reinberg D. Human SirT1 interacts with histone H1 and promotes formation of facultative heterochromatin. Mol Cell. 2004;16:93–105. doi: 10.1016/j.molcel.2004.08.031. [DOI] [PubMed] [Google Scholar]
- Vaquero A, Sternglanz R, Reinberg D. NAD+-dependent deacetylation of H4 lysine 16 by class III HDACs. Oncogene. 2007;26:5505–5520. doi: 10.1038/sj.onc.1210617. [DOI] [PubMed] [Google Scholar]
- Vaziri H, Dessain SK, Ng Eaton E, Imai SI, Frye RA, Pandita TK, Guarente L, Weinberg RA. hSIR2(SIRT1) functions as an NAD-dependent p53 deacetylase. Cell. 2001;107:149–159. doi: 10.1016/s0092-8674(01)00527-x. [DOI] [PubMed] [Google Scholar]
- Vemuganti R. The MicroRNAs and Stroke: No Need to be Coded to be Counted. Transl Stroke Res. 2010;1:158–160. doi: 10.1007/s12975-010-0030-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinciguerra M, Santini MP, Claycomb WC, Ladurner AG, Rosenthal N. Local IGF-1 isoform protects cardiomyocytes from hypertrophic and oxidative stresses via SirT1 activity. Aging (Albany NY) 2010;2:43–62. doi: 10.18632/aging.100107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viscoli CM, Brass LM, Kernan WN, Sarrel PM, Suissa S, Horwitz RI. A clinical trial of estrogen-replacement therapy after ischemic stroke. N Engl J Med. 2001;345:1243–1249. doi: 10.1056/NEJMoa010534. [DOI] [PubMed] [Google Scholar]
- Wakatsuki A, Okatani Y, Izumiya C, Ikenoue N. Melatonin protects against ischemia and reperfusion-induced oxidative lipid and DNA damage in fetal rat brain. J Pineal Res. 1999;26:147–152. doi: 10.1111/j.1600-079x.1999.tb00576.x. [DOI] [PubMed] [Google Scholar]
- Walle T. Bioavailability of resveratrol. Ann N Y Acad Sci. 2011;1215:9–15. doi: 10.1111/j.1749-6632.2010.05842.x. [DOI] [PubMed] [Google Scholar]
- Walle T, Hsieh F, DeLegge MH, Oatis JE, Jr, Walle UK. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab Dispos. 2004;32:1377–1382. doi: 10.1124/dmd.104.000885. [DOI] [PubMed] [Google Scholar]
- Walters JW, Amos D, Ray K, Santanam N. Mitochondrial redox status as a target for cardiovascular disease. Curr Opin Pharmacol. 2016;27:50–55. doi: 10.1016/j.coph.2016.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan W, Powers AS, Li J, Ji L, Erikson JM, Zhang JQ. Effect of post-myocardial infarction exercise training on the renin-angiotensin-aldosterone system and cardiac function. Am J Med Sci. 2007;334:265–273. doi: 10.1097/MAJ.0b013e318068b5ed. [DOI] [PubMed] [Google Scholar]
- Wang C, Chen L, Hou X, Li Z, Kabra N, Ma Y, Nemoto S, Finkel T, Gu W, Cress WD, Chen J. Interactions between E2F1 and SirT1 regulate apoptotic response to DNA damage. Nat Cell Biol. 2006;8:1025–1031. doi: 10.1038/ncb1468. [DOI] [PubMed] [Google Scholar]
- Wang H, Guan Y, Karamercan MA, Ye L, Bhatti T, Becker LB, Baur JA, Sims CA. Resveratrol Rescues Kidney Mitochondrial Function Following Hemorrhagic Shock. Shock. 2015a;44:173–180. doi: 10.1097/SHK.0000000000000390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JH, Ahn IS, Fischer TD, Byeon JI, Dunn WA, Jr, Behrns KE, Leeuwenburgh C, Kim JS. Autophagy suppresses age-dependent ischemia and reperfusion injury in livers of mice. Gastroenterology. 2011a;141:2188–2199. e2186. doi: 10.1053/j.gastro.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Guan YF, Du H, Zhai QW, Su DF, Miao CY. Induction of autophagy contributes to the neuroprotection of nicotinamide phosphoribosyltransferase in cerebral ischemia. Autophagy. 2012;8:77–87. doi: 10.4161/auto.8.1.18274. [DOI] [PubMed] [Google Scholar]
- Wang P, Xu TY, Guan YF, Tian WW, Viollet B, Rui YC, Zhai QW, Su DF, Miao CY. Nicotinamide phosphoribosyltransferase protects against ischemic stroke through SIRT1-dependent adenosine monophosphate-activated kinase pathway. Ann Neurol. 2011b;69:360–374. doi: 10.1002/ana.22236. [DOI] [PubMed] [Google Scholar]
- Wang R, Liu YY, Liu XY, Jia SW, Zhao J, Cui D, Wang L. Resveratrol protects neurons and the myocardium by reducing oxidative stress and ameliorating mitochondria damage in a cerebral ischemia rat model. Cell Physiol Biochem. 2014;34:854–864. doi: 10.1159/000366304. [DOI] [PubMed] [Google Scholar]
- Wang S, Xing Z, Vosler PS, Yin H, Li W, Zhang F, Signore AP, Stetler RA, Gao Y, Chen J. Cellular NAD replenishment confers marked neuroprotection against ischemic cell death: role of enhanced DNA repair. Stroke. 2008;39:2587–2595. doi: 10.1161/STROKEAHA.107.509158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Gu J, Wu PF, Wang F, Xiong Z, Yang YJ, Wu WN, Dong LD, Chen JG. Protection by tetrahydroxystilbene glucoside against cerebral ischemia: involvement of JNK, SIRT1, and NF-kappaB pathways and inhibition of intracellular ROS/RNS generation. Free Radic Biol Med. 2009;47:229–240. doi: 10.1016/j.freeradbiomed.2009.02.027. [DOI] [PubMed] [Google Scholar]
- Wang X, Gerdes HH. Transfer of mitochondria via tunneling nanotubes rescues apoptotic PC12 cells. Cell Death Differ. 2015;22:1181–1191. doi: 10.1038/cdd.2014.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Michaelis EK. Selective neuronal vulnerability to oxidative stress in the brain. Front Aging Neurosci. 2010;2:12. doi: 10.3389/fnagi.2010.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Cui J, Sun X, Zhang Y. Tunneling-nanotube development in astrocytes depends on p53 activation. Cell Death Differ. 2011c;18:732–742. doi: 10.1038/cdd.2010.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Hekimi S. Mitochondrial dysfunction and longevity in animals: Untangling the knot. Science. 2015;350:1204–1207. doi: 10.1126/science.aac4357. [DOI] [PubMed] [Google Scholar]
- Wang Y, Zhu Y, Xing S, Ma P, Lin D. SIRT5 prevents cigarette smoke extract-induced apoptosis in lung epithelial cells via deacetylation of FOXO. Cell Stress Chaperones. 2015b doi: 10.1007/s12192-015-0599-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wani WY, Gudup S, Sunkaria A, Bal A, Singh PP, Kandimalla RJ, Sharma DR, Gill KD. Protective efficacy of mitochondrial targeted antioxidant MitoQ against dichlorvos induced oxidative stress and cell death in rat brain. Neuropharmacology. 2011;61:1193–1201. doi: 10.1016/j.neuropharm.2011.07.008. [DOI] [PubMed] [Google Scholar]
- Warach S, Pettigrew LC, Dashe JF, Pullicino P, Lefkowitz DM, Sabounjian L, Harnett K, Schwiderski U, Gammans R. Effect of citicoline on ischemic lesions as measured by diffusion-weighted magnetic resonance imaging. Citicoline 010 Investigators. Ann Neurol. 2000;48:713–722. [PubMed] [Google Scholar]
- Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- Wassertheil-Smoller S, Hendrix SL, Limacher M, Heiss G, Kooperberg C, Baird A, Kotchen T, Curb JD, Black H, Rossouw JE, Aragaki A, Safford M, Stein E, Laowattana S, Mysiw WJ Investigators WHI. Effect of estrogen plus progestin on stroke in postmenopausal women: the Women’s Health Initiative: a randomized trial. JAMA. 2003;289:2673–2684. doi: 10.1001/jama.289.20.2673. [DOI] [PubMed] [Google Scholar]
- Wei Y, Clark SE, Thyfault JP, Uptergrove GM, Li W, Whaley-Connell AT, Ferrario CM, Sowers JR, Ibdah JA. Oxidative stress-mediated mitochondrial dysfunction contributes to angiotensin II-induced nonalcoholic fatty liver disease in transgenic Ren2 rats. Am J Pathol. 2009;174:1329–1337. doi: 10.2353/ajpath.2009.080697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen Y, Li W, Poteet EC, Xie L, Tan C, Yan LJ, Ju X, Liu R, Qian H, Marvin MA, Goldberg MS, She H, Mao Z, Simpkins JW, Yang SH. Alternative mitochondrial electron transfer as a novel strategy for neuroprotection. J Biol Chem. 2011;286:16504–16515. doi: 10.1074/jbc.M110.208447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenz T. Mitochondria and PGC-1alpha in Aging and Age-Associated Diseases. J Aging Res. 2011;2011:810619. doi: 10.4061/2011/810619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheaton WW, Chandel NS. Hypoxia. 2. Hypoxia regulates cellular metabolism. Am J Physiol Cell Physiol. 2011;300:C385–393. doi: 10.1152/ajpcell.00485.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker R, Faulkner S, Miyokawa R, Burhenn L, Henriksen M, Wood JG, Helfand SL. Increased expression of Drosophila Sir2 extends life span in a dose-dependent manner. Aging (Albany NY) 2013;5:682–691. doi: 10.18632/aging.100599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox CS. Effects of tempol and redox-cycling nitroxides in models of oxidative stress. Pharmacol Ther. 2010;126:119–145. doi: 10.1016/j.pharmthera.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterbourn CC. The biological chemistry of hydrogen peroxide. Methods Enzymol. 2013;528:3–25. doi: 10.1016/B978-0-12-405881-1.00001-X. [DOI] [PubMed] [Google Scholar]
- Wipf P, Xiao J, Jiang J, Belikova NA, Tyurin VA, Fink MP, Kagan VE. Mitochondrial targeting of selective electron scavengers: synthesis and biological analysis of hemigramicidin-TEMPO conjugates. J Am Chem Soc. 2005;127:12460–12461. doi: 10.1021/ja053679l. [DOI] [PubMed] [Google Scholar]
- Wood JG, Rogina B, Lavu S, Howitz K, Helfand SL, Tatar M, Sinclair D. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature. 2004;430:686–689. doi: 10.1038/nature02789. [DOI] [PubMed] [Google Scholar]
- Wu CC, Bratton SB. Regulation of the intrinsic apoptosis pathway by reactive oxygen species. Antioxid Redox Signal. 2013;19:546–558. doi: 10.1089/ars.2012.4905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CT, Yu HP, Chung CY, Lau YT, Liao SK. Attenuation of lung inflammation and pro-inflammatory cytokine production by resveratrol following trauma-hemorrhage. Chin J Physiol. 2008;51:363–368. [PubMed] [Google Scholar]
- Wu Y, Li X, Zhu JX, Xie W, Le W, Fan Z, Jankovic J, Pan T. Resveratrol-activated AMPK/SIRT1/autophagy in cellular models of Parkinson’s disease. Neurosignals. 2011;19:163–174. doi: 10.1159/000328516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing G, Barry ES, Benford B, Grunberg NE, Li H, Watson WD, Sharma P. Impact of repeated stress on traumatic brain injury-induced mitochondrial electron transport chain expression and behavioral responses in rats. Front Neurol. 2013;4:196. doi: 10.3389/fneur.2013.00196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu K, Puchowicz MA, Sun X, LaManna JC. Mitochondrial dysfunction in aging rat brain following transient global ischemia. Adv Exp Med Biol. 2008;614:379–386. doi: 10.1007/978-0-387-74911-2_42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu K, Puchowicz MA, Sun X, LaManna JC. Decreased brainstem function following cardiac arrest and resuscitation in aged rat. Brain Res. 2010;1328:181–189. doi: 10.1016/j.brainres.2010.02.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue JY, Liu GT, Wei HL, Pan Y. Antioxidant activity of two dibenzocyclooctene lignans on the aged and ischemic brain in rats. Free Radic Biol Med. 1992;12:127–135. doi: 10.1016/0891-5849(92)90006-3. [DOI] [PubMed] [Google Scholar]
- Yamakuchi M, Ferlito M, Lowenstein CJ. miR-34a repression of SIRT1 regulates apoptosis. Proc Natl Acad Sci U S A. 2008;105:13421–13426. doi: 10.1073/pnas.0801613105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T, Sadoshima J. Protection of the heart against ischemia/reperfusion by silent information regulator 1. Trends Cardiovasc Med. 2011;21:27–32. doi: 10.1016/j.tcm.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Yang Z, Zhang M, Dong Q, Wang X, Lan A, Zeng F, Chen P, Wang C, Feng J. Hydrogen sulfide protects against chemical hypoxia-induced cytotoxicity and inflammation in HaCaT cells through inhibition of ROS/NF-kappaB/COX-2 pathway. PLoS One. 2011;6:e21971. doi: 10.1371/journal.pone.0021971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Cohen MV, Downey JM. Mechanism of cardioprotection by early ischemic preconditioning. Cardiovasc Drugs Ther. 2010;24:225–234. doi: 10.1007/s10557-010-6236-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Duan W, Li Y, Yan J, Yi W, Liang Z, Wang N, Yi D, Jin Z. New role of silent information regulator 1 in cerebral ischemia. Neurobiol Aging. 2013;34:2879–2888. doi: 10.1016/j.neurobiolaging.2013.06.008. [DOI] [PubMed] [Google Scholar]
- Yang Y, Fu W, Chen J, Olashaw N, Zhang X, Nicosia SV, Bhalla K, Bai W. SIRT1 sumoylation regulates its deacetylase activity and cellular response to genotoxic stress. Nat Cell Biol. 2007;9:1253–1262. doi: 10.1038/ncb1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yankovskaya V, Horsefield R, Tornroth S, Luna-Chavez C, Miyoshi H, Leger C, Byrne B, Cecchini G, Iwata S. Architecture of succinate dehydrogenase and reactive oxygen species generation. Science. 2003;299:700–704. doi: 10.1126/science.1079605. [DOI] [PubMed] [Google Scholar]
- Yeung F, Hoberg JE, Ramsey CS, Keller MD, Jones DR, Frye RA, Mayo MW. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004;23:2369–2380. doi: 10.1038/sj.emboj.7600244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi JH, Hazell AS. Excitotoxic mechanisms and the role of astrocytic glutamate transporters in traumatic brain injury. Neurochem Int. 2006;48:394–403. doi: 10.1016/j.neuint.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Yoon H, Shin SH, Shin DH, Chun YS, Park JW. Differential roles of Sirt1 in HIF-1alpha and HIF-2alpha mediated hypoxic responses. Biochem Biophys Res Commun. 2014;444:36–43. doi: 10.1016/j.bbrc.2014.01.001. [DOI] [PubMed] [Google Scholar]
- Yousefi S, Perozzo R, Schmid I, Ziemiecki A, Schaffner T, Scapozza L, Brunner T, Simon HU. Calpain-mediated cleavage of Atg5 switches autophagy to apoptosis. Nat Cell Biol. 2006;8:1124–1132. doi: 10.1038/ncb1482. [DOI] [PubMed] [Google Scholar]
- Yousuf S, Atif F, Ahmad M, Hoda N, Ishrat T, Khan B, Islam F. Resveratrol exerts its neuroprotective effect by modulating mitochondrial dysfunctions and associated cell death during cerebral ischemia. Brain Res. 2009;1250:242–253. doi: 10.1016/j.brainres.2008.10.068. [DOI] [PubMed] [Google Scholar]
- Yu HP, Hwang TL, Hsieh PW, Lau YT. Role of estrogen receptor-dependent upregulation of P38 MAPK/heme oxygenase 1 in resveratrol-mediated attenuation of intestinal injury after trauma-hemorrhage. Shock. 2011;35:517–523. doi: 10.1097/SHK.0b013e318209e931. [DOI] [PubMed] [Google Scholar]
- Yu HP, Hwang TL, Hwang TL, Yen CH, Lau YT. Resveratrol prevents endothelial dysfunction and aortic superoxide production after trauma hemorrhage through estrogen receptor-dependent hemeoxygenase-1 pathway. Crit Care Med. 2010a;38:1147–1154. doi: 10.1097/CCM.0b013e3181cd124e. [DOI] [PubMed] [Google Scholar]
- Yu HP, Yang SC, Lau YT, Hwang TL. Role of Akt-dependent up-regulation of hemeoxygenase-1 in resveratrol-mediated attenuation of hepatic injury after trauma hemorrhage. Surgery. 2010b;148:103–109. doi: 10.1016/j.surg.2009.12.008. [DOI] [PubMed] [Google Scholar]
- Yu ZF, Bruce-Keller AJ, Goodman Y, Mattson MP. Uric acid protects neurons against excitotoxic and metabolic insults in cell culture, and against focal ischemic brain injury in vivo. J Neurosci Res. 1998;53:613–625. doi: 10.1002/(SICI)1097-4547(19980901)53:5<613::AID-JNR11>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Yuan J, Minter-Dykhouse K, Lou Z. A c-Myc-SIRT1 feedback loop regulates cell growth and transformation. J Cell Biol. 2009;185:203–211. doi: 10.1083/jcb.200809167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang QS, Sadek H, Maass DL, Martinez B, Ma L, Kilgore JA, Williams NS, Frantz DE, Wigginton JG, Nwariaku FE, Wolf SE, Minei JP. Specific inhibition of mitochondrial oxidative stress suppresses inflammation and improves cardiac function in a rat pneumonia-related sepsis model. Am J Physiol Heart Circ Physiol. 2012;302:H1847–1859. doi: 10.1152/ajpheart.00203.2011. [DOI] [PubMed] [Google Scholar]
- Zang QS, Wolf SE, Minei JP. Sepsis-induced Cardiac Mitochondrial Damage and Potential Therapeutic Interventions in the Elderly. Aging Dis. 2014;5:137–149. doi: 10.14336/AD.2014.0500137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarzaur BL, Croce MA, Fischer PE, Magnotti LJ, Fabian TC. New vitals after injury: shock index for the young and age x shock index for the old. J Surg Res. 2008;147:229–236. doi: 10.1016/j.jss.2008.03.025. [DOI] [PubMed] [Google Scholar]
- Zhang F, Wang S, Gan L, Vosler PS, Gao Y, Zigmond MJ, Chen J. Protective effects and mechanisms of sirtuins in the nervous system. Prog Neurobiol. 2011;95:373–395. doi: 10.1016/j.pneurobio.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao G, Qin GW, Wang J, Chu WJ, Guo LH. Functional activation of monoamine transporters by luteolin and apigenin isolated from the fruit of Perilla frutescens (L.) Britt. Neurochem Int. 2010;56:168–176. doi: 10.1016/j.neuint.2009.09.015. [DOI] [PubMed] [Google Scholar]
- Zhao G, Yao-Yue C, Qin GW, Guo LH. Luteolin from Purple Perilla mitigates ROS insult particularly in primary neurons. Neurobiol Aging. 2012a;33:176–186. doi: 10.1016/j.neurobiolaging.2010.02.013. [DOI] [PubMed] [Google Scholar]
- Zhao G, Zang SY, Jiang ZH, Chen YY, Ji XH, Lu BF, Wu JH, Qin GW, Guo LH. Postischemic administration of liposome-encapsulated luteolin prevents against ischemia-reperfusion injury in a rat middle cerebral artery occlusion model. J Nutr Biochem. 2011;22:929–936. doi: 10.1016/j.jnutbio.2010.07.014. [DOI] [PubMed] [Google Scholar]
- Zhao W, Kruse JP, Tang Y, Jung SY, Qin J, Gu W. Negative regulation of the deacetylase SIRT1 by DBC1. Nature. 2008;451:587–590. doi: 10.1038/nature06515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Luo P, Guo Q, Li S, Zhang L, Zhao M, Xu H, Yang Y, Poon W, Fei Z. Interactions between SIRT1 and MAPK/ERK regulate neuronal apoptosis induced by traumatic brain injury in vitro and in vivo. Exp Neurol. 2012b;237:489–498. doi: 10.1016/j.expneurol.2012.07.004. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Hou J, Liu J, Yao M, Li L, Zhang B, Zhu H, Wang Z. Inhibition of autophagy contributes to melatonin-mediated neuroprotection against transient focal cerebral ischemia in rats. J Pharmacol Sci. 2014;124:354–364. doi: 10.1254/jphs.13220fp. [DOI] [PubMed] [Google Scholar]
- Zhou X, Chen M, Zeng X, Yang J, Deng H, Yi L, Mi MT. Resveratrol regulates mitochondrial reactive oxygen species homeostasis through Sirt3 signaling pathway in human vascular endothelial cells. Cell Death Dis. 2014;5:e1576. doi: 10.1038/cddis.2014.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu HR, Wang ZY, Zhu XL, Wu XX, Li EG, Xu Y. Icariin protects against brain injury by enhancing SIRT1-dependent PGC-1alpha expression in experimental stroke. Neuropharmacology. 2010;59:70–76. doi: 10.1016/j.neuropharm.2010.03.017. [DOI] [PubMed] [Google Scholar]
- Zou H, Henzel WJ, Liu X, Lutschg A, Wang X. Apaf-1, a human protein homologous to C. elegans CED-4, participates in cytochrome c-dependent activation of caspase-3. Cell. 1997;90:405–413. doi: 10.1016/s0092-8674(00)80501-2. [DOI] [PubMed] [Google Scholar]